Abstract
Objective
The intent of the study is to describe an unusual pattern of intramuscular migration of calcific deposits related to hydroxyapatite deposition disease (HADD) involving the rotator cuff, to illustrate the characteristic imaging features of this phenomenon, and to discuss the clinical significance of such migration.
Materials and methods
A series of cases of intramuscular accumulation of calcium hydroxyapatite crystals collected over a 7-year period at multiple hospitals within the same academic institution were retrospectively reviewed.
Results
The patient group was composed of seven men and four women, ranging in age from 51 to 79 years, with a mean age of 63 years. All subjects presented with acute shoulder pain. The majority of subjects reported the spontaneous onset of the symptoms (64 %), while others reported weight lifting (27 %) and a fall on the arm (9 %) as the mechanisms of injury. The right shoulder was affected in 73 % of the subjects. The supraspinatus was the most commonly affected muscle (82 %), followed by the infraspinatus muscle (36 %).
Conclusions
Knowledge of the imaging features of intramuscular migration of hydroxyapatite deposits is important in order to avoid the erroneous diagnosis of other causes of muscle edema and inflammation such as myotendinous injury, myositis, subacute denervation, and neoplasm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium hydroxyapatite is the most common form of calcium in the human body, representing the major crystal constituent of bone. It is also the crystal type seen in the majority of pathologic calcifications in the body, particularly those calcifications found in tendons and ligaments. Soft-tissue deposition of hydroxyapatite may occur in an isolated location or it may be widespread, related to a number of systemic disorders such as idiopathic tumoral calcinosis, autoimmune rheumatologic disorders, and metabolic conditions such as renal failure, hypervitaminosis D, and other disorders that alter calcium homeostasis [1].
Hydroxyapatite deposition disease (HADD) has been extensively described in the literature over the years. Asymptomatic hydroxyapatite deposits are common, and may be found in the periarticular soft tissues of virtually every joint [2]. Symptomatic HADD typically affects middle-aged persons and represents a common cause of joint pain, related primarily to periarticular deposition of calcific material within tendons [3]. Clinical symptoms range from chronic or recurrent joint pain associated with a limited range of motion to acute severe pain and tenderness [4]. It is estimated that HADD afflicts 3 % of the asymptomatic adult population and 7 % of those with shoulder pain [5]. The shoulder is the most commonly affected region, where symptomatic HADD is usually referred to as “calcific tendinitis”, “calcific periarthritis” or “calcific bursitis”. The critical zone of the supraspinatus tendon (approximately 1 cm from its insertion in the greater tuberosity of the humerus) is, by far, the most frequently affected site. Less frequent sites of symptomatic tendon deposition include the hip, elbow, wrist, and knee [1].
It is common for hydroxyapatite deposits within tendons to migrate to adjacent tissues such as bursae and, less frequently, bones. However, movement of hydroxyapatite crystals along the course of the tendon toward the myotendinous junction, to our knowledge, has been described in only one case report in the English literature [6]. The purpose of this study was to perform a review of patients that presented with intramuscular migration of rotator cuff hydroxyapatite deposits, noting clinical manifestations, imaging features, and differential diagnosis.
Materials and method
This study was complied according to HIPAA guidelines, and approval, along with exemption status for informed consent, was obtained from the Institutional Review Board. An electronic search was performed in the radiology report databases of three affiliated institutions for shoulder magnetic resonance (MR) imaging examinations obtained between January 2007 and January 2015. Cases were identified by searching a combination of terms, including “intramuscular or intratendinous”, “hydroxyapatite”, “HADD”, “calcific tendinitis”, “calcification” and the name of each rotator cuff muscle (supraspinatus, infraspinatus, subscapularis and teres minor). The adopted exclusion criteria were the absence of intratendinous or intramuscular calcific deposits and evidence of muscle strain injury, infection, tumor, or denervation myopathy. We reviewed the available medical records, radiology reports, and surgical notes of every patient.
A total of 11 subjects met imaging inclusion criteria for intramuscular migration of rotator cuff hydroxyapatite deposits after a thorough review of our medical records. The patient group was composed of seven men (64 %; 7/11) and four women (36 %; 4/11) with ages that ranged from 51 to 79 years (mean age = 63 years).
MR images were available in all 11 patients with the majority performed at 1.5 T (91 %; 10/11), although a 3 T system was used for one patient (9 %; 1/11). All patients had a combination of T1-weighted and fluid-sensitive sequences performed in the oblique coronal, transverse and oblique sagittal planes, although exact parameters varied depending on particular institution protocol. Acquired sequences included a combination of fast spin-echo (FSE) T1-weighted (TR/TE, 367–700 ms/9–14 ms; 10/11 patients), FSE T2-weighted (2,132–4,000/96–131; 5/11 patients), FSE PD-weighted (2,000–2,800/20–51; 5/11 patients), FSE T2-weighted with fat-suppression (2,500–6,050/62–84; 6/11 patients), FSE PD-weighted with fat-suppression (1,400-3,010/22-51; 9/11 patients). In two patients each, additional gradient-echo T2*-weighted (908–910/24), and short tau inversion-recovery (4,466–4,666/31–38) sequences were utilized. In two patients, (18 %; 2/11), MR arthrography was performed utilizing an intra-articular injection of a solution containing gadolinium DTPA, followed by three plane acquisition of FSE T1-weighted with fat-suppression (563–676/22–23). One patient (9 %; 1/11) underwent intravenous gadolinium DTPA administration.
Conventional radiographs were acquired in four patients (36 %; 4/11), and CT was obtained in one patient (9 %; 1/11). Radiographs included routine and true anteroposterior (AP) views of the shoulder in both neutral and internal rotation, axillary views, and scapular outlet views. The CT examinations were obtained in a multi-slice detector with a slice thickness of 2.5 mm.
Two fellowship-trained musculoskeletal radiologists with 4 years (E.Y.C.) and 29 years (M.P.) of experience evaluated the examinations and established a consensus opinion regarding the imaging findings. All examinations were assessed for the side of involvement. The specific tendon affected, the anatomic extent of involvement of the tendon, myotendinous unit and muscle, as well as the size and configuration of the calcific deposit within the tendon and muscle were evaluated. Periarticular and intramuscular reactive edema was graded as mild (limited to the muscle fibers that were immediately adjacent to the deposits), moderate (involving less than 50 % of the muscle substance) or severe (involving more than 50 % of the muscle substance). Identification of any additional sites of calcific migration was noted. All of the imaging studies in each patient were assessed at the same time.
Results
The calcific deposition was right-sided in eight subjects (73 %; 8/11) and left-sided in the remaining three (27 %; 3/11). In subjects for whom upper limb dominance information was available (27 %; 3/11), the non-dominant limbs (67 %; 2/3) were more commonly affected than the dominant limbs (33 %; 1/3). There were no documented cases of bilateral involvement, although imaging examinations of both shoulders were available in only two patients (18 %; 2/11).
The most commonly affected muscle was the supraspinatus, which was affected in nine patients (82 %; 9/11). Two patients (18 %; 2/11) showed isolated infraspinatus muscle involvement, and two patients (18 %; 2/11) showed involvement of both the supraspinatus and the infraspinatus muscles. There were no cases involving the subscapularis or the teres minor muscle.
All 11 patients presented with acute shoulder pain. Three patients (27 %; 3/11) also reported night pain. Seven patients (64 %; 7/11) reported the spontaneous onset of the acute symptoms, three patients (27 %; 3/11) reported weight lifting as the cause of their symptoms, and one subject (9 %; 1/11) reported the acute onset of pain following a fall on the arm. Seven patients (64 %; 7/11) had a history of chronic shoulder pain and experienced a superimposed acute exacerbation that led to the imaging examinations. The remaining four patients (36 %; 4/11) reported no prior symptoms and presented with acute symptoms. In subjects for whom information regarding the range of motion of the affected joint was available (82 %; 9/11), all patients (100 %; 9/9) reported limited range of motion or such limitation was noted during the initial clinical examination.
Rotator cuff calcifications were identified in all initial radiographs (100 %; 4/4). Radiographs in three persons (75 %; 3/4) demonstrated amorphous calcifications that were medially located within the muscle substance and had no relationship with the greater tuberosity of the humerus (Fig. 1). In the remaining subject (25 %; 1/4), radiographs showed linear, slightly irregular “comet-tail” calcifications that extended medially from the greater tuberosity towards the myotendinous junction of the rotator cuff muscle (Fig. 2).
A 62-year old man with acute exacerbation of chronic right shoulder pain. a The scapular Y-view radiograph shows amorphous calcifications within the infraspinatus muscle (dashed arrow), as well as smaller calcifications overlying the supraspinatus tendon (arrowhead). Axial (b) and coronal (c) fat suppressed PD-weighted fast spin-echo MR images illustrate several large intramuscular hydroxyapatite deposits (arrows) within the infraspinatus accompanied by prominent muscle edema
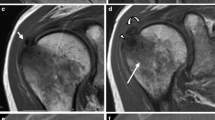
A 75-year old man with acute exacerbation of chronic right shoulder pain. Coronal PD-weighted fat-suppressed (a) and sagittal T1-weighted (b) fast spin-echo MR images show intramuscular migration of hydroxyapatite deposits (arrows) within the supraspinatus. Sagittal (c) and axial (d) PD-weighted fat-suppressed fast spin-echo MR images show bursal extrusion (asterisk) as well as intraosseous extension (dashed arrow) of hydroxyapatite deposits. An AP radiograph of the shoulder (e) shows calcifications extending medially to the myotendinous junction of the supraspinatus in a “comet-tail” configuration (arrowheads)
The majority of the MR examinations (91 %; 10/11) demonstrated intramuscular hydroxyapatite deposits that were associated with edema of the adjacent muscle fibers. The deposits were hypointense on all imaging sequences, had either a linear (45 %; 5/11) or globular (45 %; 5/11) morphology, and were located at or about the myotendinous junction of the rotator cuff muscles. In the linear form, the width of the calcification was relatively uniform, whereas the globular form had an enlarged rounded or oval area of calcification, typically located at the medial edge of the calcification. The surrounding muscle edema was striated in appearance, did not involve the entirety of the muscle, and was usually limited to the fibers that were adjacent to the calcific deposits. The amount of edema was categorized as mild (45 %; 5/11), moderate (36 %; 4/11), and severe (18 %; 2/11), based on its size and signal increase on fluid sensitive sequences. Two patients (18 %; 2/11) showed coexistence of the intramuscular migration pattern with other migration patterns, namely bursal extrusion (50 %; 1/2) and intraosseous extension (100 %; 2/2; Fig. 2). Enhancement of the soft tissues that surrounded the deposits after intravenous gadolinium DTPA administration was seen in one examination (9 %; 1/11; Fig. 3). One patient (9 %; 1/11) presented with an intramuscular cyst that contained layered calcific material and surrounding muscle edema (Fig. 4).
Sequential imaging in a 51-year old woman presenting with acute right shoulder pain. a A coronal PD-weighted fast spin-echo MR image obtained 4 years prior to presentation shows discrete hydroxyapatite deposits in the supraspinatus tendon (arrow) and a large intermuscular lipoma (asterisk) between the trapezius and supraspinatus muscles. b Coronal PD-weighted fast spin-echo MR image obtained 1 year prior to presentation (following lipoma excision) shows intratendinous growth of the calcific deposits (arrow). Coronal (c) and axial (d) T1-weighted fat-suppressed fast spin-echo MR images after intravenous gadolinium administration acquired at the time of presentation show migration of the calcific deposits (arrows) into the supraspinatus muscle with enhancement of the adjacent soft tissues
A 60-year old man with acute left shoulder pain. Axial CT acquired with the patient in a prone position (a) and axial T1-weighted fat-suppressed fast spin-echo MR arthrographic image obtained with the patient in a supine position (b) show an intramuscular cyst (arrows) within the supraspinatus containing calcific material that layers in a variable pattern depending on the position of the patient (arrowheads). Coronal T2-weighted fat-suppressed fast spin-echo MR image (c) delineates the cyst (arrow) and edema of the adjacent muscle fibers
Imaging follow-up was available in two patients (18 %; 2/11). One subject (50 %; 1/2) showed resolution of the deposits after surgical treatment, and the other (50 %; 1/2) showed persistence of the deposits on a radiograph that was performed 7 days after the first examination.
Follow-up information regarding management was available in five subjects (45 %; 5/11). All of these patients (100 %; 5/5) underwent subacromial injection of anesthetics and steroids. Three subjects (60 %; 3/5) underwent physical therapy sessions, and two (40 %; 2/5) underwent arthroscopic surgery with bursectomy and cannulation of the calcium deposits after failure of conservative treatment. All patients (100 %; 5/5) experienced pain relief during follow-up examinations after treatment.
Discussion
Many authors have proposed different theories regarding the pathogenesis of HADD such as repeated microtrauma, decreased local vascularity, failed healing response, and erroneous differentiation of tendon-derived stem cells; however, the exact pathogenesis of this disorder remains controversial [1, 7, 8].
Hydroxyapatite deposits usually change over time and undergo three distinct stages: pre-calcific, calcific, and post-calcific [4]. The presence of fibrocartilaginous metaplasia without visible calcifications characterizes the pre-calcific stage. The calcific stage is characterized by the presence of macroscopic calcifications, and it is further divided into three phases: formative, resting, and resorptive. Homogeneous, well-defined deposits that are contained within the tendon and cause minimal symptoms are observed in the formative and resting phases. In the resorptive phase, however, the deposits grow in size, become ill-defined, and often migrate to the adjacent tissues [1, 9], where they may extend beneath or within the overlying bursa, erode into the underlying bone [10, 11] or, as described in this report, migrate medially within the tendon substance to the myotendinous junction of the corresponding rotator cuff muscle. The dispersion of hydroxyapatite crystals into neighboring tissues triggers an acute inflammatory response with severe pain and tenderness that is frequently seen with MRI as bursal fluid, soft tissue edema, or marrow edema about intraosseous deposits.
The disappearance of the calcifications shortly after the resorptive phase is not uncommon and is frequently accompanied by improvement of the inflammatory symptoms. This characterizes the post-calcific stage, when granulation tissue replaces the space left behind by the hydroxyapatite crystals and eventually matures into a fibrotic scar [3].
In our series, we observed a pattern of reactive edema involving the rotator cuff muscles that, in part, surrounded the hydroxyapatite deposits that had migrated medially along the tendon fibers. The deposits were located at or near the myotendinous junction and were likely associated with coexisting intrasubstance delaminating-type tendon tears that served as a migration pathway for the calcifications, although such tears were not always clearly visualized. The fact that one of our patients presented with an intramuscular cyst that contained layered calcific material supports this hypothesis, taking into account the strong association between this variety of cyst and delaminating rotator cuff tears [12]. It is unclear, however, if the crystals migrate toward the myotendinous junction through pre-existing tears or if they create intrasubstance tears during the migration process. Many authors have reported the association between intratendinous hydroxyapatite deposits and tendon rupture [13, 14], and some even suggest that HADD may be a predisposing factor for rotator cuff tendon tears [15].
CT remains the best method to evaluate the precise location and extent of the calcific deposits, but MR is the modality of choice to assess muscle involvement in HADD [6, 16]. The presence of hydroxyapatite crystals within the muscle creates an inflammatory response that is easily identified with MRI. The high signal intensity of the muscle edema on fluid-sensitive sequences creates a background that makes the hypointense calcium deposits even more conspicuous.
Given its relatively low frequency and unusual appearance, intramuscular migration of hydroxyapatite deposits may be easily confused with other entities. Injury to the myotendinous junction of rotator cuff muscles is the most important differential diagnosis. The striated appearance of the edema seen in rotator cuff strains is very similar to the edema pattern that is visible about intramuscular hydroxyapatite crystals. Furthermore, when there are associated partial tears of the tendon following injury, the retracted torn fibers may assume a globular appearance that resembles that of hydroxyapatite deposits, making the distinction between the two entities even more difficult with MRI [17]. The slightly younger age group of the patients, the history of acute trauma, and the lack of radiographic evidence of calcific tendinitis are helpful in differentiating between these two entities. It is noteworthy, however, that some authors have reported a high incidence of calcific tendinitis amongst patients with infraspinatus myotendinous disruption [18, 19], so it remains unclear if the two entities are related or if there is any overlap between them.
Isolated edema involving rotator cuff muscles may also raise concern for subacute denervation injuries or pyogenic myositis. The most common denervation injuries involving the muscles of the rotator cuff are cervical radiculopathy, brachial neuritis (Parsonage-Turner syndrome), and suprascapular nerve compression at the spinoglenoid notch [6, 20–23]. Denervation edema, unlike intramuscular HADD-related edema, results in a mild increase in signal intensity that uniformly involves the entirety of the affected muscles and is not associated with perimuscular fluid accumulation [20, 23, 24]. Diffuse muscle edema may also be seen in the context of myositis, either pyogenic or idiopathic. The clinical history of infection is key to differentiate it from intramuscular HADD [24]. The most reliable way to distinguish these entities from intramuscular HADD through imaging, however, is achieved through the documentation of rotator cuff calcifications on radiographs or CT.
Management of patients with intramuscular HADD deposition was very similar to that of patients with acute HADD-related symptoms in general. Physical therapy sessions helped recovering the lost range of motion and pain relief was frequently accomplished following subacromial injection of anesthetics and steroids. In 40 % of the patients where complete management information was available, arthroscopic removal of the hydroxyapatite deposits was necessary due to failure of conservative therapy, but our study is too small to determine if conservative treatment is more likely to fail in patients with intramuscular extension of HADD.
We acknowledge that our study has limitations, mainly because of its retrospective design. Follow-up examinations were not frequently performed, compromising the assessment of progression or resolution of the imaging findings. Furthermore, detailed clinical notes were not available for all subjects, and information such as symptom resolution, the side of arm dominance, and chosen therapeutic regimen was lacking.
In conclusion, the accurate diagnosis of intramuscular migration of hydroxyapatite deposits is most dependent on the identification of focal edema involving portions of the muscle that contain hypointense calcific foci on MR fluid-sensitive sequences. Correlation with clinical data and results of other imaging studies is also essential to avoid misinterpretation of the MR findings. We hope that our study encourages future investigations on the clinical outcomes and imaging features of intramuscular HADD not only within the rotator cuff but also at other locations throughout the body.
References
Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031–48.
Hayes CW, Rosenthal DI, Plata MJ, Hudson TM. Calcific tendinitis in unusual sites associated with cortical bone erosion. Am J Roentgenol. 1987;149:967–70.
Kachewar SG, Kulkarni DS. Calcific tendinitis of the rotator cuff: a review. J Clin Diagnostic Res. 2013;7:1482–5.
Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183–91. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10797220.
Bosworth BM. Examination of the shoulder for calcium deposits. J Bone Jt Surg. 1941;23:567–77.
Mileto A, Gaeta M. Calcific tendonitis of supraspinatus simulating acute brachial neuritis (Parsonage-Turner syndrome). Clin Radiol. 2011;66:578–81. Available at: http://dx.doi.org/10.1016/j.crad.2011.01.001.
Rui YF, Lui PPY, Chan LS, Chan KM, Fu SC, Li G. Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J. 2011;124:606–10.
Oliva F, Via A, Maffulli N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012. p. 95. Available at: http://www.biomedcentral.com/1741-7015/10/95
Cho NS, Lee BG, Rhee YG. Radiologic course of the calcific deposits in calcific tendinitis of the shoulder: does the initial radiologic aspect affect the final results? J Shoulder Elb Surg. 2010;19:267–72.
Sola WC, Drake GN, Ramos CH, Gomes A, Gartsman GM. Calcific tendinitis of the rotator cuff associated with intraosseous loculation: two case reports. J Shoulder Elb Surg. 2009;18:e6–8.
Flemming DJ, Murphey MD, Shekitka KM, Temple HT, Jelinek JJ, Kransdorf MJ. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. Am J Roentgenol. 2003;181:965–72.
Kassarjian A, Torriani M, Ouellette H, Palmer WE. Intramuscular rotator cuff cysts: association with tendon tears on MRI and arthroscopy. Am J Roentgenol. 2005;185:160–5.
Jim YF, Hsu HC, Chang CY, Wu JJ, Chang T. Coexistence of calcific tendinitis and rotator cuff tear: an arthrographic study. Skeletal Radiol. 1993;22:183–5.
Hsu H-C, Wu J-J, Jim Y-F, Chang C-Y, Lo W-H, Yang D-J. Calcific tendinitis and rotator cuff tearing: a clinical and radiographic study. J Shoulder Elb Surg. 1994;3:159–64. Available at: http://dx.doi.org/10.1016/S1058-2746(09)80095-5.
Gotoh M, Higuchi F, Suzuki R, Yamanaka K. Progression from calcifying tendinitis to rotator cuff tear. Skelet Radiol. 2003;32:86–9.
Ramon FA, Degryse HR, De Schepper AM, Van Marck EA. Calcific tendinitis of the vastus lateralis muscle. Skelet Radiol. 1991;20:21–3.
Taneja AK, Kattapuram SV, Chang CY, Simeone FJ, Bredella MA, Torriani M. MRI findings of rotator cuff myotendinous junction injury. Am J Roentgenol. 2014;203:406–11. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25055277.
Lunn JV, Castellanos-Rosas J, Tavernier T, Barthélémy R, Walch G. A novel lesion of the infraspinatus characterized by musculotendinous disruption, edema, and late fatty infiltration. J Shoulder Elb Surg. 2008;17:546–53.
Walch G, Nové-Josserand L, Liotard JP, Noël E. Musculotendinous infraspinatus ruptures: an overview. Orthop Traumatol Surg Res. 2009;95:463–70.
Helms CA, Martinez S, Speer KP. Acute brachial neuritis (Parsonage-Turner syndrome): MR imaging appearance—report of three cases. Radiology. 1998;207:255–9.
Fritz RC, Helms CA, Steinbach LS, Genant HK. Suprascapular nerve entrapment: evaluation with MR imaging. Radiology. 1992;182:437–44.
Beltran J, Rosenberg ZS. Diagnosis of compressive and entrapment neuropathies of the upper extremity: value of MR imaging. Am J Roentgenol. 1994;163:525–31.
Yanny S, Toms AP. MR patterns of denervation around the shoulder. Am J Roentgenol. 2010;195:W157–63.
May DA, Disler DG, Jones EA, Balkissoon AA, Manaster BJ. Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics. 2000;20(1):S295–315.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, B.P.G., Chang, E.Y., Resnick, D.L. et al. Intramuscular migration of calcium hydroxyapatite crystal deposits involving the rotator cuff tendons of the shoulder: report of 11 patients. Skeletal Radiol 45, 97–103 (2016). https://doi.org/10.1007/s00256-015-2255-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2255-9