Abstract
Calcium hydroxyapatite crystal deposition is a common disorder, which sometimes causes acute pain as calcifications dissolve and migrate into adjacent soft tissue. Intraosseous calcium penetration has also been described. We illustrate the appearance of these lesions using a series of 35 cases compiled by members of the French Society of Musculoskeletal Imaging (Société d’Imagerie Musculo-Squelettique, SIMS). The first group in our series (7 cases) involved calcification-related cortical erosions of the humeral and femoral diaphyses, in particular at the pectoralis major and gluteus maximus insertions. A second group (28 cases) involved the presence of calcium material in subcortical areas. The most common site was the greater tubercle of the humerus, accompanying a calcifying tendinopathy of the supraspinatus. In addition, an extensive intramedullary diffusion of calcium deposits was observed in four of these cases, associated with cortical erosion in one case and subcortical lesions in three cases. Cortical erosions and intraosseous migration of calcifications associated with calcific tendinitis may be confused with neoplasm or infection. It is important to recognize atypical presentations of hydroxyapatite deposition to avoid unnecessary investigation or surgery
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Calcific tendinitis is a very common disorder found in 3 % of adults [1]. It is most common between the ages of 40 and 70 years, affecting women slightly more often. In order of frequency, calcium deposits mainly involve the shoulders, hips, elbows, wrists, and knees [1–3].
These deposits are usually asymptomatic but may cause pain of varying intensity, including occasionally acute painful attacks, accompanied by swelling, erythema, and even fever [1, 4]. Laboratory tests usually are normal, but in some cases of acute crisis, tests can show moderate signs of inflammation, including mild leucocytosis, and erythrocyte sedimentation rate or C-reactive protein elevations [4].
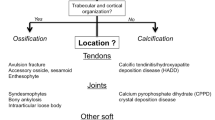
Many names have been given to this disorder, such as calcareous peritendinitis, calcifying tendinitis, calcific tendinosis, calcific periarthritis, etc. Since this type of calcification can also be involved in destructive arthropathy, it may be more appropriate to call this disorder “calcium hydroxyapatite crystal deposition disease” [5–7].
Pathogenesis
The pathogenesis of calcific tendinitis is not completely understood. Theories regarding its cause include soft tissue degeneration, soft tissue necrosis, trauma, and hypoxia-induced metaplasia of less perfused tendon insertions with resultant calcification [2, 8]. Histological examinations have not uncovered inflammatory polymorphonuclear cells but have shown the calcium deposits to be situated within zones of finely vascularized fibrocartilage accompanied by macrophages [9].
The theory that is most widely cited [2, 3, 7] is the one by Uhthoff [8], who described calcific tendinitis in several stages:
-
1.
The initial stage is caused by the transformation of tendinous tissue into fibrocartilage, followed by the appearance of calcification within this fibrocartilage. Persistent hypoxia causes parts of the tendon to transform into fibrocartilage, where chondrocytes mediate deposition of calcium. This fibrocartilaginous metaplasia may be provoked by mechanical and vascular factors. In the shoulder, for instance, tendinous calcifications generally occur in critical zones, which are situated near tendon insertions and are thought to be poorly vascularized areas.
-
2.
Next, the calcific deposits are the site of phagocytosis and increased local vascularization, which may then lead to the synthesis of a new tendinous matrix that is qualitatively normal.
Both phases may coexist in the same area, and the histological appearance may differ depending on the clinical phase. Indeed, very painful tendinopathy exhibits much more resorption activity histologically than clinically silent tendinopathy [8].
Natural history of calcific tendinitis
Stable deposits appear radiographically as homogeneous, amorphous zones of increased density that do not possess a cortical or trabecular structure, unlike heterotopic ossification and accessory ossicles [3]. During the dissolution phase, their density diminishes while their volume increases, and their contours become ill-defined, presenting a “fluffy” or “snowflake” appearance [6, 7] (Fig. 1).
Based on more than 300 surgically treated shoulder tendinopathies, Moseley [9] described the natural history of intratendinous calcium deposits in several phases (Fig. 2):
Natural history of intratendinous calcium deposits of the shoulder (adapted from Moseley [9]). a In the silent phase, the compact intratendinous calcium deposit does not have any modifying effect on its environment. b At the beginning of the “mechanical” phase, the deposit increases in volume, creating a focal bulge that may lead to impingement with the acromion. c Calcific material disperses and partially migrates between the tendon and the adjacent synovial bursa. d The deposit is evacuated into the subdeltoid bursa (generally during an episode of acute pain). e The tendinous calcification may extrude into adjacent bone
-
Silent phase (usually asymptomatic): compact deposits in the tendons have a “dry powder” appearance.
-
Mechanical phase: the deposit’s volume increases, provoking focal bulging of the tendon’s surface under the coracoacromial ligament. This creates an impingement that can lead to local bursitis.
-
Subbursal rupture: during a hyperemic phase, a portion of the calcium deposit may dissolve at the tendon’s surface, beneath the adjacent synovial bursa. After several recurrences, the calcium deposit may be totally eliminated.
-
Intrabursal rupture: during a sudden dissolution of a larger quantity of calcific material, the calcium may disseminate into the adjacent synovial bursa, provoking an intense inflammatory reaction.
This dispersion may occur into sites other than the synovial bursa: for example, between or into muscles (Fig. 3). Note that in his illustration (Fig. 2e), Moseley shows the migration of the tendon calcium deposit not only into adjacent soft tissues, but also in the adjacent bone at the tendon insertion.
Intramuscular dissemination. a A CT scan shows compact para-acetabular calcification (arrow) exhibiting a lower density extension (arrowheads) into a portion of the iliacus muscle. b and c T1-weighted and FS T2-weighted MRI scans show the central intramuscular calcium migration with a low-signal intensity, surrounded by an infiltration of the muscle with high signal intensity on FS T2
Osseous involvement
Although intraosseous penetration of calcium from calcific tendinitis of the supraspinatus has been previously described by Moseley based on observations made during surgical procedures [9], the first case reported at imaging was that of Hayes et al. in 1987 [3]. Since then, many other cases have been reported. Most of them were isolated cases or small series, except that of Flemming et al., which brought together 50 cases from several teams, including that of the Armed Forces Institute of Pathology in Washington, DC [1].
The osseous manifestations described in this paper come from 35 cases compiled by members of the French Society of musculoskeletal imaging (Société d’Imagerie Musculo-Squelettique, SIMS) from their data banks. This series was presented at the SIMS Congress of June 2013 and published in the monograph of the SIMS Congress [10]. Our series includes diaphyseal cortical erosions and calcifications situated in a subcortical location, similar to previous reported cases in the literature. It also includes four less typical cases of more extensive calcium diffusion into the bone marrow.
The epidemiology of these lesions is similar to that of calcific tendinitis without osseous involvement: mean age was 50 years, with 58 % of cases involving women and 42 % involving men in Flemming et al.’s series [1]; mean age was 54 years, with 63 % of cases involving women and 37 % involving men in our series [10].
Cortical erosions
The initial description of osseous involvement in calcific tendinitis was that of a diaphyseal location, affecting the humerus in two cases (pectoralis major insertion) and the femur in three cases (gluteus maximus insertion and adductor magnus insertion) [3]. The predilection for these sites remained unchanged in later reports of isolated cases and small series, which included 7 humeral locations (pectoralis major insertion) and 14 femoral locations (gluteus maximus insertion) across 8 publications [11–18], and in other examples illustrated in review articles and book chapters [6, 7, 19, 20]. It largely predominated in Flemming’s series too (9 humeral locations at the pectoralis major insertion and 19 femoral locations in the proximal femoral linea aspera) [1] and was also present in our series, which included 2 humeral diaphyses at the pectoralis major insertion (Fig. 4) and 3 femoral diaphyses at the gluteus maximus insertion (Fig. 5). In our series, we also observed calcium erosion in the cervical spine (n = 1) and in the calcaneus (calcaneal tendon insertion) (n = 1) (Fig. 6).
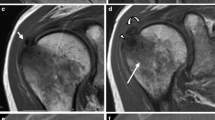
Superficial femoral cortical erosion opposite calcific tendinitis at the gluteus maximus insertion. a A radiograph of the upper femur imprecisely shows low density calcification (arrowheads) partly obscured by the femur. b An additional radiograph with internal rotation of the hip shows the large, heterogeneous calcium deposit at the upper part of the linea aspera at the gluteus maximus insertion. c The corresponding CT scan shows the penetration of calcium material into a small cortical erosion
Erosive calcific tendinitis of the calcaneal tendon. a A radiograph shows a small, amorphous, partially compact calcification in the lower end of the calcaneal tendon. b A CT scan shows that this calcification penetrates into a small osseous erosion. c, d T1- and FS T2-weighted MR images, respectively, confirm this erosion, which is accompanied by edema-like high signal intensity of the bone marrow on FS T2-weighted sequences
Erosion is, of course, more apparent on computed tomography (CT) scans than it is on standard radiographs [1]. Bone scans, when performed, have shown intense focal tracer uptake [1]. Magnetic resonance imaging (MRI) in the acute phase of calcific tendinitis has shown pronounced edema in neighboring soft tissue and in bone marrow. The signal intensity at the site of the cortical erosion is low on T1-weighted and heterogeneous on T2-weighted MRI, with low and high signal intensity especially on fat-suppressed (FS) T2-weighted sequences [1]. Edema in adjacent bone marrow was visible in all cases of cortical erosion explored using MRI in our series.
The erosive lesions associated with soft tissue and bone marrow edema, which are occasionally associated with severe pain, can be easily mistaken for osteitis or aggressive neoplastic lesions [19]. This mistake is especially common on MRI, as the calcifications themselves are poorly visible [16]. In such situations, examining radiographs and, above all, CT scans can be critical for establishing a positive diagnosis by revealing tendinous calcifications. CT can be particularly useful when calcifications are no longer dense enough to be visible on radiographs.
If performed, biopsy reveals the characteristics of calcific tendinitis in the acute phase: scattered calcification in the fibrovascular tissue with histiocytes and macrophages [1, 8, 9]. The resultant inflammatory response may produce focal hypervascularity, leading to local bone resorption. The erosions may also be due to the mechanical pulling effect at the tendon insertion [1, 3, 14]. It is essential to note that the remodeling may include zones of chondroid metaplasia. These lesions can therefore easily be mistaken for chondrosarcoma [1], hence the need for very good collaboration between pathologists and radiologists.
Subcortical calcium migration
Intraosseous migration of calcific material of tendinous origin was already mentioned by Moseley in 1969 as part of the spectrum of the possible evolution of calcific tendinitis. He based his theory on surgical observations of calcific shoulder tendon deposits extending into cavities measuring up to 1 by 0.5 cm in the adjacent greater tuberosity (so-called “intraosseous loculation”), usually not visible on radiographs [9] (Fig. 2). However, there have been few cases reported in the literature. The first case illustrated at imaging was described by Chagnaud et al. in 1998 [21]. The authors reported the spontaneous disappearance of a large calcification (15 mm) documented on radiography and MRI in the greater tuberosity of the humerus. Later, a few other isolated cases were reported affecting the greater tuberosity of the humerus [2, 22–24] or the anterior inferior iliac spine [25]. Several examples affecting the greater tuberosity of the humerus were also illustrated in review articles and book chapters [5, 7, 20, 26]. In their series of 50 calcific tendinitis-related osseous lesions, Flemming et al. [1] reported 11 cases of calcium penetration into the subcortical bone marrow [at the greater tuberosity of the humerus (n = 8), medial femoral condyle (n = 1), cervical spine at C2 (n = 1), and acetabulum (n = 1)]. Furthermore, cases of calcium deposits in erosions of the greater tuberosity of the humerus were reported in articles describing the arthroscopic treatment of calcific tendinitis of the shoulder (5 cases of osseous erosion out of 30 shoulders treated in one paper [27] and 43 out of 126 patients in another [28]).
In our series, 28 cases of more or less deep subcortical calcium migration were observed, mostly affecting the shoulder (19 greater tuberosity and 1 lesser tuberosity of the humerus) (Figs. 7, 8, 9, and 10), but also one wrist (lunate) (Fig. 11), one greater femoral trochanter, one ischium, one anterior femoral intertrochanteric region, one patella (quadriceps insertion) (Fig. 12), two medial femoral condyles of the knee, and one cervical spine (longus colli tendon insertion at C2) (Fig. 13). These lesions appeared as dense calcified or heterogeneous geode-like areas, with a diameter that was usually around a centimeter, located beneath cortical erosions of varying size.
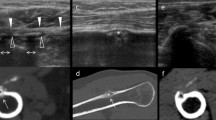
Subcortical calcium deposit demonstrated by means of ultrasound (courtesey of S. Bianchi). a A radiograph shows an ill-defined calcium deposit, probably in dissolution, in the region of the supraspinatus tendon. b Ultrasonography shows that the heterogeneous echogenic focus in the tendon (arrow) extends into an osseous lacuna of the superior facet of the greater tuberosity of the humerus (arrowhead)
Similar lesion demonstrated by ultrasound and CT (courtesy of G. Morvan). a Ultrasound shows a heterogeneous echogenic focus in the tendon (arrow) that extends into a large lesion of the superior facet of the greater tuberosity of the humerus (arrowheads). b A CT scan confirms that the large tendinous calcification (arrow) extended into the subcortical bone (arrowheads)
Calcific subcortical lesion at the greater tuberosity of the humerus, with large neighboring medullary edema on MRI (courtesy of V. Vuillemin). a A CT scan shows an area of amorphous subcortical calcification opposite a calcific tendinitis of the supraspinatus tendon. b An FS T2-weighted MRI scan shows this large low-signal area of calcium, with a large high-signal edematous infiltration in neighboring bone marrow
Suspicious subcortical lesion accompanying calcific tendinitis of the shoulder (courtesy of O. Fantino). a A CT scan of the upper end of humerus shows small calcifications in the tendinous area (arrows) and within a large lytic lesion of the greater tuberosity of the humerus (arrowhead). b A T1-weighted MRI scan confirms the presence of a large focus of bone marrow replacement. Biopsy/curettage yielded heterogeneous tissue (areas of calcified necrosis with granulomatous response) whose culture was negative. The clinical course was favorable, without any specific treatment (c: nearly 1 year later)
Calcific intraosseous extension into the patella (courtesy of O. Hauger). a A radiograph of the knee shows an amorphous calcium deposit in the lower end of the quadriceps tendon. b A few weeks later, during an attack of acute pain, a CT scan shows that the tendinous calcification has practically disappeared and that a large calcification is occupying a subcortical lacuna in the patella. c An FS T2-weighted MRI scan shows the lesion’s heterogeneous content, with edematous infiltration of high signal intensity in adjacent bone marrow and soft tissues
Calcific tendinitis and subcortical lesion in the cervical spine (courtesy of A. Cotten). a A sagittal CT view shows heterogeneous calcification of relatively low density in the region of the longus colli tendon with an adjacent subcortical lesion. b An FS T2-weighted MRI scan shows high signal intensity in the subcortical lesion and well-defined edematous infiltration in the neighboring bone marrow
The intraosseous calcium deposits may be dense and appear as bone islands on radiographs [21]. However, the deposits may be less compact and poorly visualized at radiography (Fig. 7). CT is more sensitive than radiographs for the detection, particularly when the calcium deposits are sparse within cyst-like cavities. CT is the optimum imaging modality for the detection of the osseous lesions but also for depicting the continuity between the subcortical and tendinous calcifications (Fig. 8). Ultrasound is also highly sensitive for identifying intratendinous calcification and can also depict the continuity between the calcified tendon and the heterogeneous subcortical lytic area (Figs. 7 and 8). However, the role of ultrasound in the analysis of the intraosseous calcification is generally limited. This continuity is important to demonstrate to differentiate these lesions from others such as in neoplasms. When they are dense, the calcium deposits may appear as areas of very low signal intensity on both T1- and T2-weighted MR images (Fig. 9) [1, 2]. However, when the deposits are less dense and sparse within cyst-like lesions, the lesions appear as heterogeneous areas on T2-weighted images [1]. The lesions are accompanied by an edematous reaction in the bone marrow and neighboring soft tissues, with high signal intensity on T2- and mainly FS T2-weighted MRI. In our series, the edema-like reaction was intense in 12 cases, moderate in 3, and absent in 1 case that was practically asymptomatic.
Histologic findings in cases described in the literature and in two of our patients who underwent biopsy (Fig. 10) included findings seen with ordinary calcific tendinitis, that is, a polymorphic collection of calcific deposits intermingled with a proliferation of histiocytic cells and giant cells, as well as an occasional cystic appearance like that of mucous cysts [1].
The mechanism that causes these cystic cavities to form is, of course, unclear. Focal osseous resorption may result from a reparative response caused by calcium penetration into cancellous bone, or the cystic formation may be linked to a communication with the articulation that precedes intraosseous calcification migration [1].
The clinical course probably does not differ much from that of other symptomatic forms of calcific tendinitis, generally improving after a few weeks or months [1, 2, 23, 25]. In an arthroscopic series, the presence of osseous lesions seems to correlate with more severe symptoms and with less improvement after treatment [28].
Intramedullary diffusion
Besides erosive cortical lesions and subcortical calcium migration, our series included four cases of extensive intramedullary calcium migration. The lesions originated from a subcortical lesion in the greater tuberosity of the humerus in two cases, from an erosion of the pectoralis major humeral insertion in one case, and from a subcortical lesion of the medial aspect of the medial femoral condyle of the knee in one case. To our knowledge, such extensive intramedullary calcium diffusion had never before been described.
In one case that originated from the greater tuberosity of the humerus, intramedullary calcifications were suspected on standard radiographs and confirmed by CT (Fig. 14). On MRI, edematous infiltration of the bone marrow extended as far as the metaphyseal region and included multiple small foci of hypointense signal in all sequences matching the calcium deposits visible on CT. On T2* (gradient echo), the zones of hypointense signal were larger than on T2. This may have resulted from a magnetic susceptibility artifact caused by the calcium. The abnormalities had faded on check-ups conducted a few weeks later and had disappeared on check-ups 3 years later.
Extensive intramedullary calcium diffusion in tendinopathy of the shoulder. a, b Radiographs taken after an attack of acute pain show the presence of amorphous heterogeneous calcium deposits in the tendinous region (arrows) along with other deposits that appear to be intraosseous (arrowheads). c A CT scan confirms the presence of calcium deposits in a subcortical area and disseminated in the bone marrow through to the metaphyseal region (arrowhead). d, e, f The same day, T1-, FS T2-, and T2*-weighted coronal views show a large low-signal deposit in the subcortical region and multiple smaller low-signal deposits in the epiphyseal/metaphyseal region, with a global edematous high-signal area on FS T2. Note the massive low-signal intensity of the greater tuberosity on T2*-weighted images (probably as a result of magnetic susceptibility effect). g, h, i Similar scans taken 1 month later show disappearance of the presumably calcic focal subcortical low-signal intensity area on T1- and T2-weighted images, and a persistent widespread hyperintense signal intensity on FS T2-, and decreased hypointense signal on T2*-weighted images, indicating diminished calcifications. j, k, l The same scans taken 3 years later (patient asymptomatic) show that the entire region had almost completely returned to normal
In the second case, extensive humeral involvement was revealed by bone scan conducted as part of an assessment of breast cancer (Fig. 15). High tracer uptake in the upper third of the humerus was correlated with edematous infiltration on MRI extending from the abnormalities of the greater tuberosity of the humerus to the upper third of the humeral diaphysis. A further CT examination revealed ill-defined tendinous calcification opposite the greater tuberosity, along with small calcified areas in the upper third of the diaphyseal cavity. One year later, the bone scan was nomalized .
Bone scan in the follow-up of a breast cancer shows a hot spot at the greater tuberosity of the humerus (arrow) and high signal intensity at the proximal part of the humeral diaphysis (arrowheads in a). A previous scan, performed 10 months earlier, was normal (not shown). Sagittal T2-w (b) and FS T2-weighted (c) images show heterogeneous high signal intensity from the humeral epiphysis to the upper diaphysis. Note the high signal intensity around a small focus of low signal intensity at the posterior aspect of the humeral head (arrow in c). The focal abnormality is confirmed on axial FS T2-weighted (d) as well as axial CT images (e). The latter shows a small tendon calcification as well as small cortical erosions (arrows). Sagittal (f) and coronal (g) CT reformats show the tendon calcification (arrowhead in f) as well as a high-density area in the humeral diaphysis (arrows). A bone scan performed a year later showed no abnormality (image not shown)
A third case involved a humeral diaphysis that was the site of cortical erosion opposite a calcification of the pectoralis major tendon insertion (Fig. 16). Further CT scans showed that calcium had penetrated through the cortical erosion and migrated into the center of the diaphyseal medullary cavity up to several centimeters from the cortical erosion.
Extensive intramedullary diffusion of a calcification of the pectoralis major tendon (courtesy of JD. Laredo). a A radiograph shows a large amorphous calcium deposit next to the humeral diaphysis (arrowhead). b, c CT scans performed 2 weeks later show the partial disappearance of this calcification and the presence of calcium deposits not only within a cortical lesion, but also extending several centimeters into the medullary cavity
The fourth case accompanied a calcification next to the medial femoral condyle (Fig. 17). MRI showed a small subcortical “cystic” cavity with extensive medullary edema that encompassed multiple small foci of hypointense signal in all sequences. These foci of low signal intensity corresponded to multiple small high-density areas on CT within the trabecular bone matrix.
Intramedullary diffusion of calcium deposits in a femoral condyle. a A radiograph shows amorphous, heterogeneous calcifications (arrow) of the superficial medial femoral condyle along with other possibly intraosseous calcifications (arrowheads). b Seven days later, extraosseous calcification had almost disappeared and calcifications that were possibly intraosseous had a more ill-defined appearance (arrowheads). c A CT scan taken on the same day shows a subcortical lesion and the presence of small calcifications that were disseminated within the cancellous bone (arrows). d An FST2-weighted MRI scan of the same region showing multiple small areas of hypointense signal corresponding to the disseminated calcifications (arrows) within a large medullary edematous infiltration of high signal intensity
In all cases, the clinical setting was similar to that of the other acute calcific tendinitis: more or less intense painful attacks (leading to imaging examinations), with spontaneous improvement over a few weeks. As the clinical course does not seem to differ much from all acute calcific tendinitis, additional specific treatment probably is unnecessary. Acute crises are generally treated symptomatically, usually with non-steroidal anti-inflammarory drugs.
The pathogenesis of the intramedullary dissemination of tendinous calcium material is speculative. One hypothesis is that this involves intramedullary migration of more fluid dissolved calcium material; however, this is not scientifically substantiated.
Conclusion
Osseous involvement in calcific tendinitis is far from uncommon. The most frequent form of osseous involvement reported in the literature is diaphyseal cortical erosion, but focal subcortical migration of calcifications is also relatively frequent, particularly in the greater tuberosity of the humerus. In fact, these subcortical lesions were the most common form of lesion reported in our series.
However, extensive dissemination of calcium material into adjacent bone marrow is rare, as seen in the series presented here, but not previously reported.
Cortical erosions and intraosseous migration of calcifications can mislead the radiologist by suggesting an infectious or tumorous lesion. It is important to recognize the continuity between the osseous lesion and calcific tendinitis to avoid unnecessary investigation or surgery.
References
Flemming DJ, Murphey MD, Shekitka KM, Temple HT, Jelinek JJ, Kransdorf MJ. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol. 2003;181:965–72.
Chan R, Kim DH, Millett PJ, Weissman BN. Calcifying tendinitis of the rotator cuff with cortical bone erosion. Skeletal Radiol. 2004;33:596–9.
Hayes CW, Rosenthal DI, Plata MJ, Hudson TM. Calcific tendinitis in unusual sites associated with cortical bone erosion. AJR Am J Roentgenol. 1987;149:967–70.
Paik NC. Acute calcific tendinitis of the gluteus medius: an uncommon source for back, buttock, and thigh pain. Semin Arthritis Rheum. 2014;43:824–9.
Bonavita JA, Dalinka MK, Schumacher Jr HR. Hydroxyapatite deposition disease. Radiology. 1980;134:621–5.
Resnick D. Calcium hydroxyapatite crystal deposition disease. In: D. Resnick. Diagnosis of bone and joint disorders. 4th edition. Philadelphia. WB Saunders Company. 2002; 1619–1657.
Deries B, Demondion X, Delfaut E, Paul C, Chastanet P, Cotten A. Maladie des dépôts d’hydroxyapatite. Encycl Med Chir (Editions Scientifiques et Médicales Elsevier SAS, Paris), Radiodiagnostic-Squelette normal-Neuroradiologie-Appareil locomoteur, 31-316-B-10, 2002,12p.
Uhthoff HK, Sarkar K, Maynard JA. Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res. 1976;118:164–8.
Moseley HF. Shoulder lesions. 3rd ed. Linvingstone: Edinburgh and London; 1969. 318 p.
Malghem J, Lecouvet F, Omoumi P, Larbi A, Maldague B, Vande Berg B, Bianchi S, Cotten A, Fantino O, Hauger O, Laredo JD, Morvan G, Vuillemin V. Migration intra-osseuse de calcifications tendineuses. In: Bard H, Bianchi S, Brasseur JL, Djian P. Le tendon et son environnement. Sauramps Médical, Montpellier, 2013.
Chadwick CJ. Tendinitis of the pectoralis major insertion with humeral lesions. A report of two cases. J Bone Joint Surg (Br). 1989;71-B:816–8.
Fritz P, Bardin T, Laredo JD, Ziza JM, D’Anglejan G, Lansaman J, et al. Paradiaphyseal calcific tendinitis with cortical bone erosion. Arthritis Rheum. 1994;37:718–23.
Mizutani H, Ohba S, Mizutani M, Otake S, Otsuka T, Nakamura T. Calcific tendinitis of the gluteus maximus tendon with cortical bone erosion: CT findings. J Comput Assist Tomogr. 1994;18:310–2.
Dürr HR, Lienemann A, Silbernagl H, Nerlich A, Refior HJ. Acute calcific tendinitis of the pectoralis major insertion associated with cortical bone erosion. Eur Radiol. 1997;7:1215–7.
Thornton MJ, Harries SR, Hughes PM, Whitehouse R, Carradine S. Calcific tendinitis of the gluteus maximus tendon with abnormalities of cortical bone. Clin Radiol. 1998;53:296–301.
Kraemer EJ, El-Khoury GY. A typical calcific tendinitis with cortical erosions. Skeletal Radiol. 2000;29:690–6.
Siegal DS, Wu JS, Newman JS, Del Cura JL, Hochman MG. Calcific tendinitis: a pictorial review. Can Assoc Radiol J. 2009;60:263–72.
El-Essawy MT, Vanhoenacker FM. Calcific tendinopathy of the pectoralis major insertion with intracorticalprotrusion of calcification. JBR-BTR. 2012;95:374.
Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031–48. Review.
Cotten A, Boutry N, Demondion X, Flipo RM. Affections microcristallines. In: A. Cotten. Imagerie musculosquelettique. Pathologies générales. Paris. Masson. 2005; p.95.
Chagnaud C, Gaubert JY, Champsaur P, Marciano S, Petit P, Moulin G. Vanishing osteosclerotic lesion of the humeral head. Skeletal Radiol. 1998;27:50–2.
Sola Jr WC, Drake GN, Ramos CH, Gomes A, Gartsman GM. Calcific tendinitis of the rotator cuff associated with intraosseousloculation: two case reports. J Shoulder Elbow Surg. 2009;18:e6–8.
Martin S, Rapariz JM. Intraosseous calcium migration in calcifying tendinitis: a rare cause of single sclerotic injury in the humeral head. Eur Radiol. 2010;20:1284–6.
Sola Jr WC, Drake GN, Ramos CH, Gomes A, Gartsman GM. Calcific tendinitis of the rotator cuff associated with intraosseous loculation: two case reports. J Shoulder Elbow Surg. 2009;18:e6–8.
Kim YS, Lee HM, Kim JP. Acute calcific tendinitis of the rectus femoris associated with intraosseous involvement: a case report with serial CT and MRI findings. Eur J Orthop Surg Traumatol. 2013;23 Suppl 2:S233–9.
Chen L, Chung CB. Miscellaneous conditions of the shoulder. In: CB Chung and LS Steinbach. MRI of the upper extremity. Philadelphia. Lippincot Williams & Wilkins. 2010; 346–366.
Seyahi A, Demirhan M. Arthroscopic removal of intraosseous and intratendinous deposits in calcifying tendinitis of the rotator cuff. Arthroscopy. 2009;25:590–6.
Porcellini G, Paladini P, Campi F, Pegreffi F. Osteolytic lesion of greater tuberosity in calcific tendinitis of the shoulder. J Shoulder Elbow Surg. 2009;18:210–5.
Acknowledgments
The presented series was collected thanks to the participation of several members of the French-speaking Society of Musculo-skeletal Imaging (Société d’Imagerie Musculosquelettique, SIMS): S. Bianchi, A. Cotten, O. Fantino, O. Hauger, JD. Laredo, G. Morvan, and V. Vuillemin, allowing us to gather the second most important series of intraosseous migrations of tendinous calcifications in the literature.
Conflicts of interest
The authors declare that they have no conflicts of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malghem, J., Omoumi, P., Lecouvet, F. et al. Intraosseous migration of tendinous calcifications: cortical erosions, subcortical migration and extensive intramedullary diffusion, a SIMS series. Skeletal Radiol 44, 1403–1412 (2015). https://doi.org/10.1007/s00256-015-2165-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2165-x