Abstract
Objective
To assess if diffusion-weighted MRI (DWI) can differentiate between central enchondromas and chondrosarcomas (CS) and if DWI can distinguish low-grade chondroid lesions from high-grade CS.
Materials and methods
Fifty-two patients with central cartilage tumors were included. Patients underwent conventional MRI and DWI with ADC mapping. The slice on MRI with the most aggressive imaging feature was identified. The corresponding mean and minimum ADC maps of the tumor at this position were measured.
Results
There were 24 enchondromas, five atypical cartilaginous lesions, 15 grade 1, three grade 2, two grade 3, and three dedifferentiated CS. Mean ADC values (×10−6 mm2/s) for enchondromas, atypical cartilaginous tumors, grade 1 CS, grade 2, CS, grade 3 CS and dedifferentiated CS were 1,896, 2,048, 2,152, 2,170, 2,076, and 1,261, respectively. Minimum ADC values (×10−6 mm2/s) for enchondromas, atypical cartilaginous tumors, grade 1 CS, grade 2, CS, grade 3 CS and dedifferentiated CS were 1,820, 1,752, 2,010, 1,829, 1,752, and 767, respectively. ANOVA test demonstrated a statistically significant difference in mean and minimum ADC values in all groups. Post hoc analysis revealed this was due to difference in mean and minimum ADC values in dedifferentiated CS. The mean ADC value in low-grade chondroid lesions was 2,001, while the ADC value for high-grade CS were 2,132. The minimum ADC value for low-grade chondroid lesions was 1,896, while the minimum ADC for high-grade CS was 1,837. The difference in minimum and mean ADC values was not statistically significant.
Conclusions
DWI cannot differentiate between enchondromas and CS and DWI does not aid in the distinction of low-grade chondroid tumors from high-grade CS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central chondroid tumors are among the most common primary bone tumors [1]. The spectrum of central chondral tumors ranges from a benign and indolent lesion (an enchondroma) to the highly aggressive tumor entity, a high-grade chondrosarcoma (CS).
Both enchondroma and CS are common primary bone tumors. Enchondroma is the second most common chondral tumor after osteochondroma and is observed in approximately 3 % of all routine MRI examinations of the knee [2] and in approx. 2 % of all routine MRI studies of the shoulder [3]. Similarly, the malignant spectrum, CS is the second most common primary bone tumor after osteosarcoma [4]. Although the differentiation of an enchondroma from a high-grade CS does not represent a diagnostic challenge, the diagnostic conundrum for both radiologist and pathologist alike lies in the distinction of enchondromas from low-grade CS [5].
In recent years, there has been increased interest in functional MR imaging in oncological imaging for the characterization of benign from malignant lesions. In particular, diffusion-weighted imaging (DWI) has emerged as a promising tool in oncological MR imaging for the characterization of lesions and the assessment of treatment response. DWI exploits the random, Brownian motion of water molecules in the body. Water molecules outside the human body are in constant, random motion. In contrast, the movement of water molecules within the human body is impeded by intracellular organelles, macromolecules, and cell membranes. DWI is therefore a non-invasive imaging tool which has the potential to assess cellularity within biologic tissues [6, 7]. The apparent diffusion coefficient (ADC) is a quantitative measurement of Brownian movement with highly cellular tissues demonstrating lower ADC values then less cellular tissues which return higher ADC values [6]. Therefore, DWI has the potential to be a non-invasive tool in the assessment of cellularity in bone tumors. Particularly, as cellularity is one of the differentiating histopathological features in the differentiation of enchondromas from low-grade CS [1], we hypothesized that DWI may potentially be able to differentiate enchondromas from low-grade CS. However, at present, there remains a paucity of evidence supporting or refuting the role of DWI in the characterization of primary bone tumors [8, 9]. In particular, the role of DWI in the differentiation of enchondromas from low-grade CS remains elusive [10]. The aim of this study was therefore to evaluate the role of DWI in the distinction of enchondromas from CS.
Materials and methods
Since October 2012, we have introduced DWI into our routine bone tumor protocol. This is therefore a retrospective study of all patients who underwent conventional MRI with DWI at our institution from October 2012 to October 2013. Institutional Review Board approval was obtained in the form of a service evaluation. Informed consent was not required in view of the retrospective nature of the study. The electronic bone tumor database was accessed and all patients with central chondral tumors who underwent conventional MRI with DWI were identified.
MRI protocol
All MRI examinations were performed on a 3.0-T scanner (Skyra; Siemens Medical Systems, Erlangen, Germany). The conventional imaging included T1-weighted sequences in the short and long axis of the tumor, fat-suppressed T2-weighted sequences in the short axis, STIR sequences in the long axis of the tumor plane, and chemical-shift imaging. Subsequently, DWI was performed in the axial plane using a spin-echo, single-shot echo-planar imaging (EPI) sequence. The technical parameters for DWI are described in Table 1. The b-values used were 50 s/mm2 and 1,200 s/mm2.
The image quality of DWI in the spine, ribs, pelvis, femora, tibia, and the humerus was largely good with a few cases where the image quality was deemed acceptable but still diagnostic. Image quality of DWI in the hands was acceptable and remained diagnostic using the dual-source parallel radiofrequency (RF) excitation technique on the Skyra system. Ghosting and air-interface artefacts were occasionally observed in the extremities (particularly in the hands) however this did not impede the interpretation of the images.
Apparent diffusion coefficient maps using a mono-exponential fitting algorithm as provided by Siemens were automatically generated on the operating console using the above stated b-values. Noise levels for the calculation of the ADC maps were kept constant at 15.
Image analysis
One musculoskeletal radiologist with 4 years of experience in bone tumor imaging (HD) retrospectively reviewed the conventional MRI examinations. The MR images were analyzed on a dedicated workstation (Leonardo Workstation, Siemens). On the axial T1-weighted images, the slice with the most aggressive imaging feature was selected to identify the corresponding ADC map on the DWI. Aggressive features on the conventional MR images included one of the following imaging features: cortical destruction or soft tissue mass. In the absence of these imaging findings, the slice with the most endosteal scalloping was selected as this area was thought to represent the most aggressive and biologically most active part of the tumor. Subsequently, the corresponding slice on the ADC map of the DWI was identified. First, the mean ADC value of the lesion at this slice position was measured drawing a region of interest (ROI) of the entirety of the chondral lesion at this slide. Then, minimum ADC values were measured by drawing a ROI on three areas within the tumor at the same slice position. Circular ROIs were drawn in areas of the tumor which on the corresponding anatomical imaging (T1WI) appeared most aggressive. The size of each circular ROI within the same lesion was kept constant. The ADC map was correlated with the conventional axial T1WI sequences to avoid drawing ROIs in areas of matrix calcification as this would have led to falsely low results in the quantification of ADC values because the aim of the study was to identify areas of most increased cellularity within the chondroid tumors. The lowest ADC value of the three ROIs was used for analysis of the minimum ADC value.
Diagnosis and grading of chondroid tumors
The electronic histopathology database was accessed, the histopathology reports for all patients who underwent histopathological confirmation were retrospectively reviewed, and the diagnosis and grading of the chondroid tumor were documented. In patients who underwent biopsy only (which occurred in 14 enchondromas and five atypical cartilaginous tumor), the histopathology report from the biopsy was taken as the final diagnosis. All biopsied enchondromas and atypical cartilaginous tumors were biopsied under CT guidance by one musculoskeletal radiologist (HD) targeting the most aggressive area on CT. In contrast, patients who underwent biopsy and subsequent curettage or resection, the final histopathology report, which was based on the curettage or resection specimen, was taken as the final diagnosis. All patients with biopsy-proven CS either underwent curettage or resection. The term “atypical cartilaginous tumor” or “CLUMP” (cartilaginous lesion of unknown malignant potential) is used at our institution for low-grade cartilaginous tumors that may be modestly cellular, show myxoid change, mild nuclear atypia, and have readily identifiable binucleate forms but however do not demonstrate permeative growth [11]. This term is used in a similar fashion in other institutions and has recently been included in the WHO classification of chondroid tumor highlighting the difficulty in differentiating between enchondromas and CS [1, 12]. At our institution, all chondroid lesions are reported by two musculoskeletal pathologists. Furthermore, all chondroid lesions at our institution are discussed in a multidisciplinary meeting and the diagnosis of enchondroma, atypical cartilaginous tumor and CS is made in conjunction with clinicians, radiologists, and histopathologists. Histological grading of enchondromas, atypical cartilaginous tumor, grade 1, grade 2, grade 3, and dedifferentiated CS was determined according to widely accepted definitions [1, 13]. In the absence of histological confirmation in enchondromas, the diagnosis of an enchondroma was based on widely accepted imaging features [14].
Statistical analysis
Data was analyzed using SPSS version 19.0 (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA with post hoc Tukey analysis) was used to compare the minimum and mean ADC values of enchondromas, atypical cartilaginous lesions, grade 1 CS, grade 2 CS, grade 3 CS, and dedifferentiated CS. Subsequently, independent samples t test was used to compare the minimum and mean ADC values of low-grade chondroid lesions and high-grade chondroid lesions. p values < 0.05 were considered significant.
Results
Fifty-two patients with chondroid tumor were identified. There were 25 male (48 %) and 27 female (52 %) patients. The median age was 49.5 years and the mean age was 49.8 years (age range, 11–80 years). Nineteen chondral lesions were located within the humerus (36.5 %), 17 in the femur (32.7 %), five in the tibia (9.6 %), five in the pelvis (9.6 %), three in the hand (5.8 %), two in the ribs (3.8 %), and one in the spine (1.9 %). In the CS group, the final histological diagnosis and grading was made on curettage, resection or amputation specimens, considering the highest grade of tumor in the specimen as the final diagnosis. Similarly, all atypical chondroid tumors were histologically confirmed using CT-guided biopsy. In contrast, in the enchondroma group, 58 % (14) of lesions were histologically confirmed based on biopsy or curettage while 42 % (10) of all enchondromas were diagnosed based on typical MR-imaging findings.
Twenty-four patients (46.2 %) had an enchondroma, five patients (9.6 %) had an atypical cartilaginous tumor, 15 patients (28.8 %) had a grade 1 CS, three patients (5.8 %) had a grade 2 CS, two patients (3.8 %) had a grade 3 CS, and three patients (5.8 %) had a dedifferentiated CS.
For the subsequent subgroup analysis in the differentiation of low-grade CS from high-grade CS, dedifferentiated CS were excluded. Enchondromas, atypical cartilaginous tumor, and grade 1 CS were defined as low-grade CS while grade 2 and grade 3 CS were defined as high-grade CS. Thus, there were 44 (89.8 %) low-grade CS and five (10.2 %) high-grade CS.
The mean ADC values (×10−6 mm2/s) for enchondromas was 1,896 (range, 1,281–2,370), for atypical cartilaginous tumor: 2,048 (range, 1,816–2,427), for grade 1 CS: 2,152 (range, 1,804–2,951), for grade 2 CS: 2,170 (range 1,762–2,430), for grade 3 CS: 2,076 (range, 1,774–2,378) and for dedifferentiated CS: 1,261 (range, 1,086–1,627). ANOVA test showed that there was a statistically significant difference in the mean ADC values in all groups (p = 0.001). Post hoc analysis, however, revealed that this difference was due to the lower mean ADC values of dedifferentiated CS.
In contrast, the minimum ADC values (×10−6 mm2/s) for enchondromas was 1,820 (range, 1,183–2,370) (Fig. 1), for atypical cartilaginous tumor: 1,752 (range, 1,650–1,999), for grade 1 CS: 2,010 (range, 1,691–2,350) (Fig. 2), for grade 2 CS: 1,829 (range, 1,543–2,171) (Fig. 3), for grade 3 CS: 1,752 (range, 1,645–1,859) (Fig. 4) and for dedifferentiated CS group: 767 (range, 512–1,224) (Fig. 5). ANOVA test demonstrated a statistically significant difference in the minimum ADC values in all groups (p < 0.001). Post hoc analysis, however, showed that this difference was due to the lower minimum ADC values in the dedifferentiated CS group.
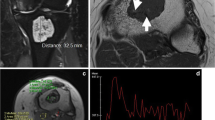
A 31-year-old female patient with an enchondroma of the right proximal humerus. a Coronal STIR sequence demonstrates a 4.2 cm in the maximum craniocaudal dimension measuring chondroid lesion. b Axial T1W SE MR image shows no significant endosteal scalloping. c Corresponding ADC map reveals a mean ADC value of 2,405 and a minimum ADC value of 2,184 ×10−6 mm2/s and a mean ADC of 2,405 ×10−6 mm2/s
A 67-year-old male patient with a grade 1 CS of the right femur. a Coronal STIR sequence shows a 21.8 cm in the maximum craniocaudal dimension measuring chondroid lesion. b Axial fat-suppressed T2W FSE MR image shows more than two-thirds endosteal scalloping (arrow) and cortical thickening. c ADC map demonstrates a mean ADC value of 2,444 ×10−6 mm2/s and a minimum ADC value of 2,403 ×10−6 mm2/s
A 69-year-old male patient with a grade 2 CS of the right pelvis. a Axial fat-suppressed T2W FSE MR image shows a diffusely infiltrative lesion within the right acetabulum (arrow) which demonstrates chondroid matrix calcification within it and surrounding soft issue edema. b Corresponding ADC map (arrow) shows a mean ADC value of 2,318 ×10−6 mm2/s and a minimum ADC value of 2,104 ×10−6 mm2/s
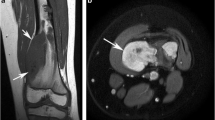
A 77-year-old male patient with a grade 3 CS of the left pelvis. a Axial fat-suppressed T2W FSE MR image shows a destructive lesion of the left ilium with a large soft tissue mass extending into the left buttock and invading the left sacral ala (arrow). c Corresponding ADC map demonstrates a mean ADC value of 1,774 ×10−6 mm2/s and a minimum ADC value of 1,645 ×10−6 mm2/s
A 70-year-old female patient with a dedifferentiated CS of the right humeral head. a Axial fat-suppressed T2W FSE MR image shows a destructive lesion of the right humeral head with a surrounding soft tissue mass (arrow) and areas of necrosis. Corresponding DWI (b 1,200s/mm2) (c) and ADC map (d) demonstrates marked restricted diffusion within areas of the lesion with a mean ADC value of 1,086 ×10−6 mm2/s and a minimum ADC value of 564 ×10−6 mm2/s
The mean ADC value (×10−6 mm2/s) for all low-grade chondroid lesions was 2,001 while the mean ADC of all high-grade chondroid lesions was 2,132. Using Student’s t test, this difference in mean ADC values between the two groups was not statistically significant (p = 0.37). The minimum ADC value (×10−6 mm2/s) for all low-grade chondroid lesions was 1,896, while the minimum ADC of all high-grade chondroid lesions was 1,837. Using Student’s t test, this difference in minimum ADC values was also not statistically significant (p value = 0.71).
Discussion
The differentiation of enchondromas from low-grade CS is one of the most challenging diagnoses for the musculoskeletal radiologist and musculoskeletal pathologist alike [5, 15]. Therefore, the radiological and histopathological criteria used to differentiate the two disease entities may significantly vary from institution to institution. There is therefore significant diagnostic uncertainty in the distinction of the two disease entities. This observation may thus explain the differences in surgical outcome of low-grade chondroid lesions and the different treatment approaches for low-grade CS published in the literature. While some centers perform intralesional curettage with or without local adjuvants [16], other institutions treat low-grade CS with en bloc resection and reconstruction [17]. In contrast, asymptomatic enchondromas that are frequently depicted incidentally on routine MRI examinations of the knee [2] or shoulder [3] do not require surgical intervention. Although symptomatic enchondromas may be treated with intralesional curettage, a more aggressive treatment approach for this benign disease entity is not warranted. Furthermore, the diagnosis of a CS, be it low-grade or high-grade, usually instigates a wide range of investigations such as staging CT of the chest and skeletal scintigraphy, which is not required in an enchondroma. Moreover, the diagnosis of sarcoma may result in emotional distress to patients and may negatively affect other aspects of life such as insurance policies and mortgage applications. It is therefore vital to derive an accurate diagnosis in the distinction of enchondromas from low-grade CS. There have therefore been attempts to utilize advanced non-anatomical MR techniques such as diffusion-weighted MRI to aid in the distinction of the two disease entities. However, at present, there remains a paucity of evidence supporting or refuting the role of DWI in the differentiating of enchondromas from low-grade CS [18].
To the best of our knowledge, there has been only one publication that evaluated the role of DWI in the distinction of enchondromas from CS [10]. This retrospective study suggested that there was a statistically significant difference in the mean ADC values of enchondromas and CS. The authors, however, only included 14 chondroid lesions and therefore stated that a larger sample size was required to investigate the role of DWI in differentiating enchondromas from low-grade CS [10]. In contrast, the current study included 52 patients with chondroid lesions of which 24 lesions were enchondromas and 15 tumors were grade 1 CS.
Our study did not corroborate the previously stated findings. On the contrary, the current study demonstrates no statistically significant difference in mean or minimum ADC values between enchondromas, grade 1 CS, grade 2 CS, or grade 3 CS. This difference in findings is therefore most likely due to the small sample size in the previously reported study. Although in our study, ANOVA test demonstrated a statistically significant difference in the minimum and mean ADC values in all groups, the post hoc analysis revealed that this difference was due to the lower minimum and mean ADC values in the dedifferentiated CS group. This finding is however due to the dedifferentiated component within the lesions, which usually represents a high-grade undifferentiated sarcoma or an osteosarcoma while the cartilaginous component of the tumor can range from an enchondroma-like appearance to a grade 1 or grade 2 CS [13]. This has also been observed in our three cases of dedifferentiated CS where in two lesions the dedifferentiated component represented a high-grade undifferentiated sarcoma while in one case the dedifferentiated component of the tumor was an osteosarcoma. In contrast, the chondroid components of the three dedifferentiated CSs in our study were low-grade CSs.
Furthermore, we evaluated if DWI can differentiate between low-grade chondroid lesions (enchondromas, atypical chondroid tumor, grade 1 CS) and high-grade CS (grade 2 CS, grade 3 CS). The rationale for this distinction between low-grade chondroid lesions and high-grade CS is that in some institutions, low-grade chondroid lesions are treated with intralesional curettage while high-grade CSs are widely treated with limb-salvage surgery and endoprosthetic reconstruction or amputation [16, 19, 20]. Our study, however, demonstrates that there is no statistically significant difference in minimum or mean ADC values of low-grade chondroid lesions from high-grade CS. In contrast, it has previously been demonstrated that conventional MRI can reliably differentiate low-grade CS from high-grade CS [12, 21].
The choice of b-values for quantitative evaluation of ADC values remains uncertain. In our study, we have chosen a low b-value of 50 s/mm2 and a high b-value of 1,200 s/mm2 for calculation of ADC values. While most radiologists would agree with our choice of low b-value (50 s/mm2) in order to suppress the high-signal flow from vessels, which results in so-called “black blood images” [6], our choice of a high b-value of 1,200 s/mm2 may prove slightly more controversial, as most publications quote a high b-value of 800–1,000 s/mm2. The reason for this high b-value is based on two considerations. Firstly, chondroid lesions demonstrate high water content trapped within the cells. The aim was therefore to suppress the signal derived from free water molecules within the chondroid cells thereby allowing evaluation of cellularity within the tumor. Secondly, evidence in the literature suggests that the optimum b-value for bone marrow imaging is 1,400 s/mm2 [22].
There are several limitations to our study. Firstly, the retrospective nature of the study may have resulted in a selection bias. Secondly, 42 % (10) of enchondromas did not undergo histological confirmation. However, we applied stringent criteria for designating a lesion as an enchondroma based on widely accepted imaging features [14]. Lastly, minimum and mean ADC values were only measured in one slice. This slice was, however, carefully chosen to correspond with the slice on the conventional MR imaging, which demonstrated the most aggressive features.
In conclusion, our study demonstrates that DWI cannot differentiate enchondromas from low-grade CS and that DWI cannot distinguish low-grade chondroid lesions from high-grade CS. Although DWI can aid in the distinction of dedifferentiated CS from conventional CS, this differentiation usually does not represent a diagnostic challenge based on conventional MRI. Future research may therefore focus on the question if other advanced MR techniques such as MR spectroscopy (MRS) could potentially differentiate enchondromas from low-grade CS.
Despite the introduction of advanced MR imaging techniques in musculoskeletal oncology, the distinction of enchondromas from low-grade CS currently continues to be based on the close collaboration between radiologists, pathologists, and orthopedic oncologists. Therefore, the multidisciplinary team approach cannot be overemphasized in the distinction of the two disease entities.
References
Hogendoorn PCW, Bovee JVMG, Nielsen GP. Chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organization classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. p. 264–8.
Walden MJ, Murphey MD, Vidal JA. Incidental enchondromas of the knee. AJR Am J Roentgenol. 2008;190:1611–5.
Hong ED, Carrino JA, Weber KL, Fayad LM. Prevalence of shoulder enchondromas on routine MR imaging. Clin Imaging. 2011;35:378–84.
Douis H, Saifuddin A. The imaging of cartilaginous bone tumours. II. Chondrosarcoma. Skeletal Radiol. 2013;42:611–26.
Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. J Bone Joint Surg Am 2007; 89: 2113–2123.
Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35.
Khoo MM, Tyler PA, Saifuddin A, Padhani AR. Diffusion-weighted imaging (DWI) in musculoskeletal MRI: a critical review. Skeletal Radiol. 2011;40:665–81.
Hayashida Y, Hirai T, Yakushiji T, Katahira K, Shimomura O, Imuta M, et al. Evaluation of diffusion-weighted imaging for the differential diagnosis of poorly contrast-enhanced and T2-prolonged bone masses: initial experience. J Magn Reson Imaging. 2006;23:377–82.
Yakushiji T, Oka K, Sato H, Yorimitsu S, Fujimoto T, Yamashita Y, et al. Characterization of chondroblastic osteosarcoma: gadolinium-enhanced versus diffusion-weighted MR imaging. J Magn Reson Imaging. 2009;29:895–900.
Stratta M, Robba T, Clementi V, Regis G, Gallo A, Piana R, et al. DWI in the differential diagnosis of enchondroma and central chondrosarcoma. Skeletal Radiol. 2012;41:1179–87.
Grimer RJ, Carter SR, Tillman RM, Mangham DC, Abudu A, Fiorenza F. Chondrosarcoma of bone. J Bone Joint Surg Am. 2000;82-A:1203–4.
Douis H, Singh L, Saifuddin A. MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur Radiol. 2014;24:232–40.
Inwards C, Hogendoorn PCW. Dedifferentiated chondrosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organization classification of tumours of soft tissue and bone. Lyon: IARCP; 2013. p. 269–70.
Murphey MD, Flemming DJ, Boyea SR, Bojescul JA, Sweet DE, Temple HT. Enchondroma versus chondrosarcoma in the appendicular skeleton: differentiating features. Radiographics. 1998;18:1213–37. quiz 1244–1215.
Eefting D, Schrage YM, Geirnaerdt MJ, Le Cessie S, Taminiau AH, Bovee JV, et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33:50–7.
Verdegaal SH, Brouwers HF, van Zwet EW, Hogendoorn PC, Taminiau AH. Low-grade chondrosarcoma of long bones treated with intralesional curettage followed by application of phenol, ethanol, and bone-grafting. J Bone Joint Surg Am. 2012;94:1201–7.
Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;391:224–33.
Subhawong TK, Jacobs MA, Fayad LM. Diffusion-weighted MR, imaging for characterizing musculoskeletal lesions. Radiographics. 2014;34:1163–77.
Hanna SA, Whittingham-Jones P, Sewell MD, Pollock RC, Skinner JA, Saifuddin A, et al. Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol. 2009;35:1343–7.
Donati D, Colangeli S, Colangeli M, Di Bella C, Bertoni F. Surgical treatment of grade I central chondrosarcoma. Clin Orthop Relat Res. 2010;468:581–9.
Yoo HJ, Hong SH, Choi JY, Moon KC, Kim HS, Choi JA, et al. Differentiating high-grade from low-grade chondrosarcoma with MR imaging. Eur Radiol. 2009;19:3008–14.
Messiou C, Collins DJ, Morgan VA, Desouza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol. 2011;21:1713–8.
Conflict of interest
There is no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douis, H., Jeys, L., Grimer, R. et al. Is there a role for diffusion-weighted MRI (DWI) in the diagnosis of central cartilage tumors?. Skeletal Radiol 44, 963–969 (2015). https://doi.org/10.1007/s00256-015-2123-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2123-7