Abstract
Objective
To review imaging features of fibrous hamartoma of infancy (FHI), focusing on ultrasonography (US) findings.
Materials and methods
We retrospectively reviewed pediatric patients who were diagnosed with pathologically confirmed FHI in two children’s hospitals from 2004 to 2013. Imaging features of US, Doppler US, and magnetic resonance imaging (MRI) were evaluated.
Results
Thirteen pediatric patients (M:F = 7:6; age 5–22 months, mean 11.3 months) were included. Mean lesion size was 3.2 cm (range, 0.7–8.0 cm). The tumors were located in the back (n = 4), scrotum (n = 2), scalp, shoulder, axilla, forearm, intergluteal cleft, inguinal area, and thigh. US was performed in 11 patients. With the exception of two scrotal masses, all masses were located in the dermal and subcutaneous layer. All masses demonstrated heterogeneous hyperechogenicity with a “serpentine pattern” of intervening hypoechoic portions in the hyperechoic mass. The margins were ill-defined (n = 9) or lobulated (n = 2). Doppler US was performed in nine patients and showed no (n = 6) or minimal (n = 3) vascularity. MRI was performed in five patients and the masses showed heterogeneous signal intensity with the presence of fat on T1- and T2-weighted images.
Conclusions
FHI is a tumor that is typically located in the dermal and subcutaneous layer in young children less than 2 years old and presents as a heterogeneously hyperechoic mass with a “serpentine pattern” and ill-defined or lobulated margin on US and no remarkable vascularity on Doppler US.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fibrous hamartoma of infancy (FHI) was first described as a subdermal fibrous tumor of infancy by Reye in 1956 [1], and Enzinger introduced the current nomenclature of FHI in 1965 [2]. It is a rare benign soft tissue tumor that usually occurs in the first 2 years of life and is reported to be known congenital in 23 % of cases [3]. There is a predilection for male gender. FHI presents as a single lesion in the subcutaneous layer in most cases and may show rapid growth. The tumor is most commonly found in the truck, especially the shoulder region including axilla, and upper extremities [3, 4]. However, it has also been reported in the foot, scalp, perineal region, gluteal region, and scrotum [4–7].
For the treatment of FHI, conservative excision with positive margin status is often curative [4]. The recurrence rate after incomplete excision is approximately 16 % [2]. Delayed surgery is not associated with an increased risk of operative complications [8]. It is therefore important to limit surgical extension in this tumor.
There are several case reports describing MRI findings of FHI since the first report by Loyer et al. in 1992 [9]. MRI features of FHI reveal the histologic character of this tumor as a mass with poorly defined margin and a reticular pattern with an interposing fat component that shows a reduced signal on fat-suppression inversion recovery image [9–11].
Ultrasonography (US) is a fundamental imaging modality for the evaluation of palpable lesions in young children. US is capable of distinguishing solid and cystic masses, evaluating the internal vascularity, and defining the extent of the lesions and the relationship of the lesions to adjacent structures [12]. Even though US findings of solid masses are usually nonspecific, they can help to narrow down the differential diagnosis in certain cases. However, US findings of FHI have not been well established. Therefore, the purpose of this study was to evaluate the imaging features of FHI, focusing on US findings.
Materials and methods
This retrospective study involving two institutions was approved by the institutional review boards and the requirement for informed consent from patients and parents was waived. We reviewed imaging features of pathologically proven FHI diagnosed in two children’s hospitals from 2004 to 2013, and 14 cases of FHI were confirmed by histologic evaluation after excisional biopsy. Among them, 13 patients underwent preoperative imaging studies including US (n = 11), Doppler US (n = 9), and MRI (n = 5). The other one patient who had palpable mass on axilla underwent operation without preoperative imaging study and showed no remained mass on postoperative imaging study.
The study population included seven boys and six girls with a mean age of 11.3 months (range, 5–22 months). All patients had a solitary mass. The majority of tumors were located in the trunk or upper arm, including back (n = 4), shoulder (n = 1), axilla (n = 1), and forearm (n = 1). The remaining cases were located in the scrotum (n = 2), inguinal area (n = 1), intergluteal cleft (n = 1), scalp (n = 1), and thigh (n = 1).
On gray-scale US, the depth of lesion, echotexture (homogeneous or heterogeneous), echogenicity compared with subcutaneous fat (hyperechoic, isoechoic, or hypoechoic), and margin (well circumscribed, lobulated, or ill-defined) were evaluated. On Doppler US, presence or absence of internal vascular flow was evaluated. In addition, the signal intensity on T1- and T2-weighted images and the pattern of contrast enhancement after gadolinium injection on MRI were also evaluated if available.
Results
Tumor size was 0.7-8.0 cm, with a mean size of 3.2 cm. On US (n = 11), the lesions were found to be located in the dermal and subcutaneous layer (n = 8), only in the subcutaneous layer (n = 1), or in the scrotal sac separated from testis (n = 2). The echotexture and echogenicity was heterogeneously hyperechoic in all cases. All of the masses showed characteristic heterogeneity that we called a “serpentine pattern” formed by intervening hypoechoic portions in the hyperechoic mass (Figs. 1 and 2). The hyperechoic portions can represent fat component and the hypoechoic portions can be the fibrous component on pathology (Fig. 1c). The margins were ill-defined (n = 9) or lobulated (n = 2). Doppler US was performed in nine patients and the masses showed either no definite internal vascularity (n = 6) or minimal internal vascularity (n = 3).
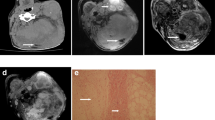
A 7-month-old boy who presented with a palpable mass on the axilla. a Ultrasonography reveals a 4.5-cm heterogeneously hyperechoic mass in the subcutaneous layer of the axilla. The mass shows a “serpentine pattern” as intervening hypoechoic portions in the hyperechoic mass and a lobulated margin. b The mass shows minimal internal vascularity on Doppler image. c Histopathology reveals the three essential components of fibrous hamartoma of infancy: fibrocollagenous tissue (long solid arrow), immature appearing cells (short solid arrow), and interposing mature adipose tissue (dashed arrow). And these histologic components are located with a intervening or serpentine pattern as shown on US
A 22-month-old girl who presented with a palpable mass on the back. a Ultrasonography demonstrates a 3.5-cm, ill-defined, heterogeneously hyperechoic mass with intervening hypoechoic portions in a “serpentine pattern” in the subcutaneous layer of the back. b The mass shows no vascularity on Doppler ultrasonography
MRI was performed in five patients. The masses showed low signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images with an intervening high signal intensity fat component on both T1- and T2-weighted images. These findings are also correlated with the “serpentine pattern” on US (Fig. 3). Gadolinium enhancement study was performed in two patients and the masses showed a heterogeneous enhancement pattern.
A 6-month-old girl who presented with a palpable mass on the back. a Ultrasonography shows a 4.2-cm, ill-defined, heterogeneously hyperechoic mass with a “serpentine pattern” in the subcutaneous layer of the back. b–d On MRI, the heterogeneous mass shows intervening low signal intensity portions on T2-weighted image (b) with a fat component that shows a signal reduction on fat suppression T2-weighted image (c). These findings are comparable with the “serpentine pattern” on US. After gadolinium enhancement, the mass shows heterogeneous enhancement on fat-suppression T1-weighted image (d)
Discussion
In this study we present the US appearance of FHI. On US, FHI showed the characteristic feature of heterogeneous hyperechogenicity with a “serpentine pattern”. Almost all masses showed an ill-defined or lobulated margin without remarkable vascularity on Doppler US.
With respect to diagnosis, histologic analysis of FHI tumors reveals three distinctive kinds of tissue: (1) fibrocollagenous tissue forming trabeculae or bundles; (2) immature appearing cells that represent primitive mesenchyme; and (3) interposing mature adipose tissue [3]. Mitotic figures and necrosis are absent or rare in all components [5, 13]. Encapsulation is a rare feature of the tumor and margins are poorly circumscribed with common microscopic spread [3, 4]. The previously reported MRI appearance of FHI largely reflects the relative proportion of the different tissue components present within the lesion [9, 14, 15]. The fibrous component appears as areas of low signal intensity and the fatty component shows characteristic high signal intensity on both T1- and T2-weighted images [16, 17]. In our study, five cases underwent MRI and the masses showed similar characteristics to those described above. However, when we encounter a superficial mass with a fat component in young children other differential diagnoses should include lipoma, lipoblastoma, and involuting hemangioma.
Compared with MRI reports, there are limited studies on the US findings of FHI. The few case reports that do include US features describe nonspecific findings such as homogeneous or heterogeneous hyperechogenicity with ill-defined margins [14, 18, 19]. In 1998, Eich et al. [18] reported fibrous tumors in children including one case of FHI that was described as a hyperechoic mass with poorly defined margin and areas of acoustic shadowing on US. In 2006, Arioni et al. [14] reported one case of FHI of the knee and proposed that the hyperechogenicity was probably due to the large number of interfaces. In 2009, Rho et al. [20] reported US findings of FHI in five children as showing purely solid, heterogeneously hyperechoic and hypovascular masses. In three cases they described an internal architecture with a “layering” appearance, which is comparable to our “serpentine pattern”, and lastly, in 2009 Kang et al. [19] reported nine cases of FHI including US findings of four cases. They described US findings as one isoechoic and three heterogeneously hyperechoic masses without available images. Ours is the largest study to evaluate imaging findings of FHI. All 11 masses that underwent US showed heterogeneous hyperechogenicity with a “serpentine pattern” of intervening hypoechoic portions in the hyperechoic mass. This characteristic pattern may be caused by hyperechogenicity from the fat component and intervening hypoechogenicity from the fibrous component of the tumor, although we could not directly match the US and pathologic findings. Still other soft tissue masses with different components intervening pathologically can show the similar findings of the serpentine pattern on US. Additional study including other common soft tissue masses in young children is needed to evaluate the specificity of this finding.
The masses also showed ill-defined or lobulated margins and no remarkable vascularity on Doppler US. When we consider superficial masses with these findings in young children, there are many differential diagnoses including other fibrous tumors such as myofibroma, calcifying aponeurotic fibroma, infantile digital fibromatosis, infantile fibrosarcoma, and embryonal rhabdomyosarcoma. In 2013, Wu et al. [21] reported the unsatisfactory concordance rate of US diagnosis for soft tissue lesions of the limbs except Baker cyst and neurofibroma. However, they included not only pediatric patients and there was no case of FHI in that study. Being aware of the specific US findings of the FHI might allow promising diagnostic accuracy for evaluating superficial lesions in infants. If diagnosis of the FHI on US is uncertain, additional MRI would be helpful to depict internal fat component.
In conclusion, a mass associated with FHI demonstrates typical US features of ill-defined and heterogeneous hyperechogenicity with a “serpentine pattern” and poor vascularity on Doppler US and is usually located in the dermal and subcutaneous layer. When we see these characteristic imaging findings in young children less than 2 years old, it is reasonable to consider FHI as the first differential diagnosis.
References
Reye RD. A consideration of certain subdermal fibromatous tumours of infancy. J Pathol Bacteriol. 1956;72(1):149–54.
Enzinger FM. Fibrous hamartoma of infancy. Cancer. 1965;18:241–8.
Dickey GE, Sotelo-Avila C. Fibrous hamartoma of infancy: current review. Pediatr Dev Pathol. 1999;2(3):236–43.
Carretto E, Dall'Igna P, Alaggio R, Siracusa F, Granata C, Ferrari A, et al. Fibrous hamartoma of infancy: an Italian multi-institutional experience. J Am Acad Dermatol. 2006;54(5):800–3.
Groisman G, Lichtig C. Fibrous hamartoma of infancy: an immunohistochemical and ultrastructural study. Hum Pathol. 1991;22(9):914–8.
Sotelo-Avila C, Bale PM. Subdermal fibrous hamartoma of infancy: pathology of 40 cases and differential diagnosis. Pediatr Pathol. 1994;14(1):39–52.
Vinayak RS, Kumar S, Chandana S, Trivedi P. Fibrous hamartoma of infancy. Indian Dermatol Online J. 2011;2(1):25–7.
Efem SE, Ekpo MD. Clinicopathological features of untreated fibrous hamartoma of infancy. J Clin Pathol. 1993;46(6):522–4.
Loyer EM, Shabb NS, Mahon TG, Eftekhari F. Fibrous hamartoma of infancy: MR-pathologic correlation. J Comput Assist Tomogr. 1992;16(2):311–3.
Ashwood N, Witt JD, Hall-Craggs MA. Fibrous hamartoma of infancy at the wrist and the use of MRI in preoperative planning. Pediatr Radiol. 2001;31(6):450–2.
Song Y, Lee I, Kim H, Choi K-U, Song J. Fibrous hamartoma of infancy in the hand: unusual location and MR imaging findings. Skeletal Radiol. 2010;39(10):1035–8.
Lin J, Jacobson JA, Fessell DP, Weadock WJ, Hayes CW. An illustrated tutorial of musculoskeletal sonography: part 4, musculoskeletal masses, sonographically guided interventions, and miscellaneous topics. AJR Am J Roentgenol. 2000;175(6):1711–9.
Scott DM, Pena JR, Omura EF. Fibrous hamartoma of infancy. J Am Acad Dermatol. 1999;41(5 Pt 2):857–9.
Arioni C, Bellini C, Oddone M, Risso FM, Scopesi F, Nozza P, et al. Congenital fibrous hamartoma of the knee. Pediatr Radiol. 2006;36(5):453–5.
Chang WC, Huang GS, Lee HS, Lee CH. Fibrous hamartoma of infancy at the wrist. Pediatr Int. 2010;52(2):317–8.
Laffan EE, Ngan BY, Navarro OM. Pediatric soft-tissue tumors and pseudotumors: MR imaging features with pathologic correlation: part 2. Tumors of fibroblastic/myofibroblastic, so-called fibrohistiocytic, muscular, lymphomatous, neurogenic, hair matrix, and uncertain origin. Radiographics. 2009;29(4):e36.
Song YS, Lee IS, Kim HT, Choi KU, Song JW. Fibrous hamartoma of infancy in the hand: unusual location and MR imaging findings. Skeletal Radiol. 2010;39(10):1035–8.
Eich GF, Hoeffel JC, Tschappeler H, Gassner I, Willi UV. Fibrous tumours in children: imaging features of a heterogeneous group of disorders. Pediatr Radiol. 1998;28(7):500–9.
Kang G, Suh YL, Han J, Kwon GY, Lee SK, Seo JM. Fibrous hamartoma of infancy: an experience of a single institute. J Korean Surg Soc. 2011;81(1):61–5.
Rho BH, Lee HJ, Kwon SY. Imaging findings of fibrous hamartoma of infancy. J Korean Soc Radiol. 2009;61(3):189–92.
Wu S, Tu R, Liu G, Shi Y. Role of ultrasound in the diagnosis of common soft tissue lesions of the limbs. Ultrasound Q. 2013;29(1):67–71.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Choi, YH., Cheon, JE. et al. Ultrasonographic features of fibrous hamartoma of infancy. Skeletal Radiol 43, 649–653 (2014). https://doi.org/10.1007/s00256-014-1838-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-014-1838-1