Abstract
The classification of vascular bone tumors remains challenging, with considerable morphological overlap spanning across benign to malignant categories. The vast majority of both benign and malignant vascular tumors are readily diagnosed based on their characteristic histological features, such as the formation of vascular spaces and the expression of endothelial markers. However, some vascular tumors have atypical histological features, such as a solid growth pattern, epithelioid change, or spindle cell morphology, which complicates their diagnosis. Pathologically, these tumors are remarkably similar, which makes differentiating them from each other very difficult. For this rare subset of vascular bone tumors, there remains considerable controversy with regard to the terminology and the classification that should be used. Moreover, one of the most confusing issues related to vascular bone tumors is the myriad of names that are used to describe them. Because the clinical behavior and, consequently, treatment and prognosis of vascular bone tumors can vary significantly, it is important to effectively and accurately distinguish them from each other. Upon review of the nomenclature and the characteristic clinicopathological, radiographic and genetic features of vascular bone tumors, we propose a classification scheme that includes hemangioma, hemangioendothelioma, angiosarcoma, and their epithelioid variants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vast majority of both benign and malignant vascular bone tumors are readily diagnosed based on their characteristic histological features, such as the formation of vascular spaces and the expression of endothelial markers. However, some vascular tumors have atypical histological features, such as a solid growth pattern, epithelioid change, or spindle cell morphology, which complicates their diagnosis [1]. For this rare subset of vascular tumors, there remains considerable controversy with regard to the terminology and the classification that should be used [2, 3].
For example, Evans et al. [3] argued that epithelioid hemangioma is not a distinct tumor entity, but rather a misdiagnosed hemangioendothelioma, a tumor that, unlike hemangioma, has metastatic potential. Furthermore, hemangioendothelioma of the bone is not listed as a distinct diagnostic entity in current classification systems [4].
Although the genetic hallmark of vascular tumors is still under investigation, we think that a better understanding of the molecular signature of vascular bone tumors may help to refine the present classification system based on immunophenotype alone. To date, only epithelioid hemangioendothelioma has been characterized by a specific chromosomal translocation involving chromosomes 1 and 3 [t(1;3)(p36.3:q25)], which represents a characteristic rearrangement for this histopathological entity [5].
In the most recent World Health Organization classification, vascular bone tumors are categorized as hemangioma or angiosarcoma, with hemangioendothelioma being subsumed by the latter [6]. This review focuses on the clinicopathological, radiographic, and genetic features of vascular bone tumors with the intent to clarify the classification of these rare entities.
Hemangioma
Skeletal hemangiomas are benign lesions consisting of newly formed and anomalous blood vessels. They may occur in patients at any age, but prevalently in adolescents and young adults. Lesions have often been present for many years and it is therefore likely that many examples are congenital [4, 7]. Bone hemangiomas are relatively common in the spine and the skull, occurring in the vertebrae of approximately 10% of the adult population [8]. By contrast, they are rare in other axial bones and in the appendicular skeleton and account for less than 1% of all primary bone tumors [8]. In the spine, the hemangioma is usually located in the thoracic and lumbar regions, rarely involving more than one vertebra. It involves primarily the vertebral body, occasionally extending to the posterior arch. Hemangiomas are more common in female subjects by a ratio of 3:2 [9].
The majority of vertebral and skull hemangiomas are small and are discovered as incidental findings on imaging studies, obtained for unrelated reasons [7]. Rarely, the vertebral hemangioma causes severe pain and may be associated with a pathological fracture and with signs of neurological impingement. Unlike vertebral or skull hemangiomas, the majority of extremity lesions are symptomatic and occur predominantly in long bones, the tibia and the femur being the most common sites. Although most hemangiomas are medullary and considered to be metaphyseal lesions, they can also be periosteal or intracortical, and occur in the diaphysis or metadiaphysis [9].
Radiographically, a hemangioma presents as a well-demarcated lucent mass that frequently contains coarse trabeculations or striations [4]. The classic imaging findings of vertebral hemangioma is that of parallel coarse and vertical trabeculations without enlargement of the vertebral body. On conventional radiographs (X-ray), the normal trabecular pattern is replaced by coarsened vertical trabeculae with relative demineralization of the vertebra, resulting in a characteristic corduroy pattern [8].
Indolent lesions frequently contain fat and sclerotic trabeculae on computed tomography (CT) and magnetic resonance imaging (MRI). CT imaging mirrors the radiographic appearance, demonstrating well-defined lytic lesions with high-density round foci corresponding to thickened vertical trabeculae mixed with low attenuation fat. The cross-sectional images may demonstrate this as a group of dots, resulting in the characteristic “polka dot” pattern. When present, this pattern is pathognomonic [7, 9]. More rarely, the imaging pattern of vertebral hemangioma is “honeycomb-like”, or finely reticulated, or even purely osteolytic. This imaging is found in symptomatic hemangiomas [8] (Fig. 1).
Hemangioma of the bone. a Lateral radiograph of a thoracic vertebra shows coarsened vertical trabeculae involving the vertebral body with a characteristic corduroy pattern. b Axial CT image demonstrates the characteristic polka dot pattern of a vertebral hemangioma created by thickened vertical trabeculae
On MRI, the majority of asymptomatic hemangiomas have characteristic imaging features and exhibit high signal intensity on T1- and T2-weighted images owing to the presence of fat within the tumor [7]. MRI provides the clearest depiction of the extent of skeletal hemangiomas, which are clearly defined by their contrast with normal marrow fat and cortical bone [9].
On the other hand, extra-spinal hemangiomas are less characteristic. In fact, the classic corduroy and sunburst patterns of vertebral and skull hemangiomas are uncommon in extremity sites. In the latter, one may see an non specific, well-defined osteolytic lesion with or without a radiating, lattice-like or web-like coarse trabecular pattern, which is highly suggestive of the diagnosis. Symptomatic tumors usually show loss of fat and reveal a low signal on T1-weighted images and high signal on T2-weighted images. In such cases, correlation with the patient’s clinical presentation is necessary to obtain a correct diagnosis [4, 7, 9].
Macroscopically, hemangiomas manifest as a soft, well-demarcated, dark red mass. On the transection surface, large blood-filled cavities with delicate and well-defined walls are seen. Moreover, thick bony trabeculae may be seen, imparting the honeycomb pattern [8].
Hemangiomas have variable histological features. The variant lesions may be of cavernous, capillary, or venous types. Histologically, hemangiomas are composed of thin-walled, blood-filled vessels lined by a single layer of fat. Cytologically, they are characterized by banal endothelial cells (Fig. 2). When hemangiomas spread throughout the skeleton, this is known as angiomatosis. Disappearing bone disease or Gorham’s disease display hypervascular bone, which can be indistinguishable from hemangioma [7].
The genetic hallmark of hemangioma is still under investigation. The Notch pathway plays a crucial role in vascular development and tumor angiogenesis [10]. There are several lines of evidence of an aberrant activation of the Notch pathway in benign vascular tumors, such as hemangiomas [11–13]. A switch in Notch gene expression seems to mirror the progression from immature cells to endothelial differentiation, which characterizes the growth and involution of infantile hemangioma [12]. Moreover, after an analysis of eight members of the HES/HEY family, Adepoju et al. demonstrated that Notch signaling is active in infantile hemangioma cells [13].
Interestingly, Mihm and Nelson [14] proposed that the metastatic niche theory can elucidate infantile hemangioma development. They reported that infantile hemangiomas may be metastases from the fetal component of placenta. In fact, certain aspects of the biology of infantile hemangioma cells suggest a relationship to the placenta as a possible site of origin for hemangioma precursor cells. First, distinct immunohistochemical markers are uniquely co-expressed by fetal microvessels of the human placenta and juvenile hemangiomas [15]. Second, the genome-wide gene expression profiles of the placenta and hemangiomas exhibit a higher degree of global similarity relative to other tissues [16]. Finally, the natural progression of infantile hemangiomas is similar to that of the placenta (rapid proliferation followed by subsequent stabilization). Therefore, Mihm and Nelson hypothesized that the site where a hemangioma is formed is prepared by humoral factors that determine the site of infantile hemangioma development, in the same way that malignant tumor cells prepare a site for tumor metastases [14]. Taken together, these findings suggest that hemangioma precursor cells arise from the placenta as a “benign metastasis.”
Many investigations have endorsed this metastatic theory, by defining the importance of a “metastatic niche” and its cellular preconditioning by bone marrow cells contributing to the organ-specific metastasis of individual types of tumors [17]. Kaplan et al. [18] demonstrated that bone marrow-derived hematopoietic progenitor cells that express vascular endothelial growth factor receptor 1 (VEGFR1) home to tumor-specific pre-metastatic sites and form cellular clusters before the arrival of tumor cells. These findings introduce the concept that tumor metastasis is initiated by a sequence of events dependent on cellular “bookmarking” through the site-specific delivery of VEGFR1-positive cells to form permissive niches within target organs. These data suggest that differences in tumor-secreted humoral factors promote metastatic spread in specific distant organs [18].
Indolent hemangiomas should be considered “leave me alone lesions” and do not require any treatment. However, when the lesion is active, repeated arterial embolization or curettage are different options of treatment, according to the anatomical and clinical presentation. Only a minority of cases complicated by a local recurrence [7]. Conversely, symptomatic hemangioma involving the vertebral column may pose a therapeutic challenge, often requiring the active involvement of several disciplines. The coupling of preoperative transarterial embolization followed by vertebroplasty, with or without surgical decompression depending on the patients’ presenting symptoms, is a relatively safe treatment and may offer long-term symptomatic relief in these patients [19].
In summary, hemangiomas of the bone are usually not life-threatening lesions. The distinctive trabecular pattern and the presence of fat are the most helpful diagnostic imaging features for making diagnosis in any location.
Epithelioid hemangioma
There is much debate over the existence of epithelioid hemangioma as a distinct entity, because of its overlapping features with other malignant vascular neoplasms and aggressive clinical characteristics, including multifocal or lymph node involvement. This has resulted in frequent misdiagnosis and inappropriate treatment of epithelioid hemangioma, particularly in skeletal locations [20].
Epithelioid hemangiomas have a wide skeletal distribution and usually manifest as painful masses. They occur in male and female subjects with equal frequency and, at the time of diagnosis, the patients are in their second to eighth decades of life and have a mean age of 34 years [21]. The vast majority of bone epithelioid hemangiomas are solitary. However, up to 25% of bone epithelioid hemangiomas can affect the skeleton in a multifocal fashion [2, 18]. Moreover, Floris et al. [22] reported a case of epithelioid hemangioma of the 2nd toe with secondary involvement of the ipsilateral inguinal, iliac, and paraortic lymph nodes. The groin lymph nodes were excised, and the presence of epithelioid hemangioma was subsequently confirmed.
Pain has been the most common complaint for patients with epithelioid hemangiomas, but the lesion may also be identified as an incidental radiographic abnormality [7]. Radiographically, epithelioid hemangiomas are well-defined lytic lesions involving the metaphysis or diaphysis of the affected bone. They do not commonly cause cortical destruction. However, when the cortex is involved there is usually focal destruction with associated thick reactive periosteal new bone formation [7].
Epithelioid hemangioma continues to be confused with hemangioendothelioma [2]. In a series of 13 patients with so-called hemangioendothelioma reported by Evans et al. [3], 3 of the patients were treated with chemotherapy, and another 3 underwent amputation. Remarkably, none of the patients in their series died. However, in a “Letter to the Editor” in the International Journal of Surgical Pathology, Rosenberg argued that Evans et al.’s illustrations of the tumors showed characteristics of epithelioid hemangioma, a benign neoplasm [22].
This example not only illustrates the current confusion surrounding the classification of this rare subset of vascular tumors, but also indicates the danger inherent in using poorly defined and inappropriate terminology to classify them.
Although imaging is extremely helpful in the diagnosis of hemangioma and usually excludes the need for biopsy, it cannot be used effectively in the diagnosis of epithelioid hemangioma and other vascular tumors because these entities lack characteristic radiological features [23, 24]. In fact, the presence of multifocal lesions may be the only clue indicating a diagnosis of a vascular tumor [7]. On conventional X-rays, the bone lesions are usually lucent with well-defined margins. However, the bone can sometimes be expanded and focally destroyed with tumor extending into adjacent soft tissue (Fig. 3).
Morphological and immunohistochemical features thus remain the cornerstone of the diagnosis of vascular tumors and their epithelioid variants. The differential diagnosis of epithelioid hemangioma includes epithelioid hemangioendothelioma and epithelioid angiosarcoma. Features that distinguish epithelioid hemangioma from epithelioid angiosarcoma include the absence of significant cytological atypia, brisk mitotic activity, and necrosis and the presence of well-formed vessels [6] (Fig. 4). The more difficult distinction between epithelioid hemangioma and epithelioid hemangioendothelioma could be made on the basis of our recent discovery of a novel genetic rearrangement that is specific to epithelioid hemangioendothelioma, [t(1;3)(1p36.23;3q25.1)], which is not present in epithelioid hemangioma [5].
The correct differential diagnosis of these two entities is critical because epithelioid hemangioendothelioma exhibits a more aggressive clinical course than epithelioid hemangioma. It is also more frequently multifocal when occurring in bone [25].
The article by Floris et al. sparked a controversy reflected in an exchange of opinions in the form of “Letters to the Editor” in the International Journal of Surgical Pathology [22]. In his letter, Evans reiterated his opinion that epithelioid hemangioma is not a distinct clinicopathological entity, but rather a misdiagnosed hemangioendothelioma, a tumor with malignant potential. However, in his own letter, Rosenberg argued that these neoplasms are histologically and biologically different from one another. Clearly, the classification of epithelioid vascular tumors remains a topic of considerable controversy as epithelioid hemangioma continues to be confused with epithelioid hemangioendothelioma or some other type of vascular sarcoma.
Crucial to the significance of this controversy is what effect, if any, the classification of these vascular tumors has on their treatment and prognosis [2]. In a recent study, Nielsen et al. [20] analyzed 50 cases of epithelioid hemangioma of the bone. In their series, most patients had a single lesion, but 9 patients (18%) had lesions involving more than one bone. Two of the patients with multifocal epithelioid hemangiomas had discontinuous lesions of the bone, skin, artery, and lymph node, but none of these patients with an unusual multifocal presentation of epithelioid hemangioma experienced an adverse outcome. Therefore, the non-aggressive behavior of epithelioid hemangioma reported in the literature [3, 20, 22] supports the hypothesis that this tumor is indeed benign.
Our findings confirm that epithelioid hemangioma does not behave aggressively and is thus a benign tumor. In fact, although most patients received conservative treatment, including only a biopsy in one case, their long-term prognosis was excellent, and none of them died of the disease [26]. By contrast, as we previously reported, epithelioid hemangioendothelioma is also associated with good prognosis, but it can metastasize in some cases and produce a fatal outcome [5].
Management of epithelioid hemangioma is still controversial given the limited experience reported in the literature [7]. Treatment varies widely, ranging from biopsy to segmental resection. Most patients can be effectively treated with intralesional curettage. Like other vascular tumors, however, epithelioid hemangioma may be present with multifocal involvement and rare loco-regional lymph node metastasis. In such cases, the treatment remains surgical as the hypothesis suggests a benign metastases [26]. However, careful radiographic follow-up may be considered [7].
The possible existence of benign metastasis is further supported by the behavior of giant cell tumors, another type of benign bone tumor that can metastasize without producing a fatal outcome. At the Rizzoli Institute, the overall metastatic rate of 349 giant cell tumors of the extremity was 4%, and all tumors were associated with a good long-term prognosis [27]. Similarly, Klenke et al. [28] found the same rate of pulmonary metastases in 118 patients with giant cell tumors, and none of the patients died of the disease. However, we ultimately agree with Rosenberg, who pointed out that “Currently, it seems we are limited to our subjective interpretations and we must wait for a molecular analysis of vascular tumors before a more definitive and objective answer becomes apparent” [22].
In summary, epithelioid hemangioma does not behave aggressively and therefore supports the contention that it is a benign tumor. Like other vascular tumors, however, epithelioid hemangioma may be present with multifocal involvement. Therefore, epithelioid hemangioma seems to be a benign tumor with metastatic potential. It is important to distinguish epithelioid hemangioma from other epithelioid vascular tumors because of the significant differences in their management and clinical outcome.
Hemangioendothelioma
Hemangioendothelioma is described as a rare low-grade malignant endothelial neoplasm. It can occur at any age, but is more commonly seen in adults. The male to female ratio is 2:1. The most frequent sites are in long bones: the femur, tibia, and humerus, in that order, but these tumors can also occur in any location. Hemangioendothelioma can be metaphyseal, with possible epiphyseal extension, but the tumor may also affect the diaphysis [8].
Localized pain or, less commonly, soft tissue swelling, are the two most common clinical manifestations. Hemangioendothelioma is multifocal in approximately 25% of patients and the multiple lesions can develop in the same or in contiguous bones. Rarely, pathological fractures can also occur in advanced cases [8].
Although the majority of hemangioendotheliomas are purely or predominantly osteolytic, they may be present as mixed lytic and sclerotic lesions. These tumors are associated with variable degrees of osseous expansion, endosteal erosion, and cortical thinning. CT and MRI provide useful information regarding the extent and pattern of bone destruction and soft tissue extension of the tumor (Fig. 5). However, whereas imaging is extremely helpful in the diagnosis of hemangioma and usually obviates the need for biopsy, there are no characteristic CT or MRI features in other vascular neoplasms, including hemangioendothelioma [23]. A bone scan is indicated for the staging of the disease, since the optimal treatment plan is determined by the presence or absence of a multicentric disease [29].
The tumor is soft, dark red or brownish. It can be grey and firmer, in areas of solid cellular tumor, or of prominent collagenization. The histological appearance of this tumor is variable. The tumor generally has a vasoformative nature with numerous spaces lined by plump endothelial cells. The endothelial cells have abundant eosinophilic cytoplasm [29]. However, hemangioendothelioma could also be less vasoformative with more primitive vascular spaces lined with plump endothelial cells (Fig. 6). Because of this variable histological appearance, the term hemangioendothelioma of the bone remains a nebulous, most likely “waste basket” diagnosis and should be avoided as much as possible [5].
The terminology and classification applied to the intra-osseous vascular tumors at the low end of the spectrum has proven particularly controversial [2, 3]. This illustrates the lack of objective diagnostic criteria and the confusion surrounding the classification of this rare subset of vascular tumors. The differential diagnosis of these tumors can be very difficult because of their remarkably similar histopathological and morphological features. Although morphological and immunohistochemical features remain the cornerstone of diagnosis, tumor-specific genetic alterations can be very helpful in making a diagnosis [30]. The recent identification of WWTR1-CAMTA1 fusion, as the genetic hallmark of an epithelioid hemangioendothelioma irrespective of its anatomical location, provides an objective and powerful diagnostic tool that can be used to distinguish an epithelioid hemangioendothelioma from a hemangioendothelioma, with limited biopsy material or a challenging diagnosis [5]. However, the genetic hallmarks of hemangioendothelioma are still under investigation.
The treatment of patients with hemangioendothelioma of the bone is variable depending upon whether the tumor is unifocal or multifocal. Patients with unifocal tumors are preferably treated by a wide en bloc resection or intralesional surgery followed by radiation therapy. Multifocal or challenge sites can be treated by curettage and radiation therapy or only by radiotherapy, especially in view of the fact that a wide to radical surgery would imply amputation [29]. However, Nielsen et al. [20] have demonstrated that over the years many authors have reported vascular tumors of the bone labeled as hemangioendothelioma, causing confusion about the proper treatment of this rare entity.
The prognosis of patients with a hemangioendothelioma of the bone is variable depending on whether the tumor is solitary or multifocal. However, local recurrence and metastasis occurred in a relatively small proportion of cases. It seems that the major prognostic factor is the degree of differentiation and cytological atypia of neoplastic endothelial cells [29].
Epithelioid hemangioendothelioma
An epithelioid hemangioendothelioma is a recently identified entity with intermediate malignancy that may arise in a variety of sites, including the bone. It occurs over a broad age range, but predominantly in middle age [5, 31]. There is a slight male predominance [2]. Patients show non-specific signs and symptoms, with pain being the most common complain [29].
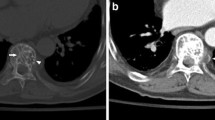
The radiographic appearance of epithelioid hemangioendothelioma of the bone can show an expansive, osteolytic, and poorly demarcated lesion. CT and MRI are not entirely specific in this setting either. Matrix mineralization is uncommon and periosteal reaction is rare. Epithelioid hemangioendotheliomas are usually centred in the medullary cavity and grow in an infiltrative and destructive fashion. The lesions are usually confined to the bone, but a small soft tissue mass can be seen in approximately 40% of the cases (Fig. 7) [29].
Pathologically, the gross appearance of an epithelioid hemangioendothelioma is a lobulated hemorrhagic lesion. The histological appearance is that of a lesion composed of a hyalinized stroma and cords or strands of plump cells with abundant eosinophilic cytoplasm [29]. The lobular growth pattern that characterizes epithelioid hemangioma is absent. The most distinguishing morphological feature of this tumor is the extracellular stroma that surrounds the endothelial cells (Fig. 8). Characteristically, it has a hyalinized or basophilic appearance and lacks inflammatory cells [2]. Owing to the epithelioid morphological features, an epithelioid hemangioendothelioma may be mistaken for epithelial tumors, including carcinoma, melanoma, mesothelioma, epithelioid hemangioendothelioma, and epithelioid sarcoma [31].
Epithelioid hemangioendothelioma of the bone. Proliferation of plump epithelioid endothelial cells arranged in a short strands, cords, solid nests, or single cells among the host bone trabeculae. Neoplastic cells show slightly atypical vesicular nuclei with small nucleoli (hematoxylin-eosin staining, ×20)
Epithelioid hemangioendothelioma, similar to other vascular tumors, shows multiple non-contiguous tumors in approximately 50% of cases, and it is unclear whether the separate lesions represent multicentric disease or metastases [2, 6]. Multicentricity in mesenchymal neoplasms is defined as the presence of a tumor at two or more anatomically separate sites before the manifestation of the disease in sites where sarcomas most commonly metastasize, such as the lungs [32]. Because the clinical course of an epithelioid hemangioendothelioma is frequently indolent, the concept that different lesions are independent primary tumors often prevails [2, 23].
In our recent study, an in-depth molecular analysis of 17 cases of epithelioid hemangioendothelioma arising in different anatomical locations revealed an identical genetic translocation [t(1;3)(1p36.23;3q25.1)] involving the CAMTA1 and WWTR1 genes on chromosomes 1 and 3 respectively. As a result of the translocation, two protein-coding regions were fused in-frame, producing a chimeric protein. To our knowledge, this is the first time that a CAMTA1–WWTR1 fusion has been reported. This is especially important because the CAMTA1 and WWTR1 genes have been shown to play an important role in oncogenesis [33–37].
In addition, we tested two multifocal epithelioid hemangioendotheliomas with different rearrangements of WWTR1 and CAMTA1 genes. An identical monoclonal rearrangement was found in each lesion from each patient, but not in tumors from different patients. The identical WWTR1–CAMTA1 rearrangement suggests that a multifocal epithelioid hemangioendothelioma resulted from a metastasis of the same neoplastic clone rather than a simultaneous neoplastic formation of multiple epithelioid hemangioendothelioma cell clones [17].
Our conclusions are supported by the results of a recent study that reported a series of patients with liver epithelioid hemangioendothelioma. Sixteen patients received a liver transplant and 5 of them (31%) had a recurrence of disease in the new liver [38]. This finding follows the “seed and soil” theory that Paget proposed in 1889, namely, “When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil” [39].
Recently, many investigators have validated this metastatic theory [40–42]. They defined the metastatic niche (soil) as a friendly site to which the tumor cell (seed) will attach and grow. In addition, Norton and Massagué [42] proposed that cancer was a self-seeding disease and that the appearance of multifocality was conveyed by self-seeds returning to the primary tumor’s organ of origin, but not attaching to the primary tumor mass. Following these hypotheses, we can speculate that in both of our cases the epithelioid hemangioendothelioma cells were able to attach and grow only in the tissue of origin. Therefore, it seems that a multifocal epithelioid hemangioendothelioma is more likely a metastatic disease rather than a manifestation of multicentricity.
These data could have therapeutic implications. In fact, metastatic disease suggests an aggressive tumor that warrants further treatment; in contrast, tumors arising independently may simply reflect the propensity of an organ to develop occult tumors, which may or may not progress to a clinically significant disease.
Although most studies recommend a wide surgical resection, curettage is also an option [43]. Multicentric tumors in contiguous bone can sometimes be controlled with amputation, but otherwise, radiation therapy with or without surgery, is the mainstay of management. The role of chemotherapy is not yet clear, but generally it is not a standard approach [44]. However, a small number of patients may show multiple tumors involving the skeleton, soft tissue, liver and lung, concurrently. In these cases, chemotherapy has been employed [29]. Therefore, because of the variable clinical course, treatment plans should be tailored to the individual patient in correlation with the extension and location of the disease.
Angiosarcoma
Angiosarcoma is a high-grade malignant tumor that is composed of tumor cells that show endothelial differentiation [4]. It is a very rare neoplasm, occurring in less than 1% of malignant bone tumors. Patients may show osseous angiosarcoma at any age, with a peak incidence in adulthood. Males and females are affected more or less equally. Angiosarcomas show a wide skeletal distribution; however, they tend to affect the long bones of the extremity and the axial skeleton, mainly the spine. About a third of these lesions are multifocal [4]. Patients with a multifocal bone disease may have the involvement of one or more anatomical regions [29]. Pain is the most common presenting symptom. A soft-tissue swelling may be seen, but in general there are no characteristic clinical findings specific to an angiosarcoma of the bone.
The radiological appearance of an angiosarcoma of the bone is also non-specific. An angiosarcoma usually develops poorly marginated lytic bone lesions. They can erode the cortex and extend into the soft tissue (Fig. 9) [4]. As with the other vascular tumors, the feature that most commonly allows one to suggest the diagnosis is the presence of multifocal disease. Although multicentric lesions may involve a single anatomical area, some cases involve multiple anatomical regions [29].
Angiosarcoma of the bone. a Conventional X-ray shows a pathological fracture of the proximal humerus secondary to an ill-defined lytic destructive lesion with a suggestion of a soft-tissue mass. Axial b T1-weighted and c T2-weighted MR images demonstrate a large heterogeneous mass with predominantly high signal intensity on the T2-weighted image. d A skip lesion is visible on the coronally injected MR image
Pathologically, angiosarcomas usually show as red, bloody lesions with irregular margins. They are usually composed of atypical endothelial cells that exhibit nuclear atypia and an increased number of mitoses with atypical mitotic figures. The essential feature of this neoplasm is its vasoformative appearance. Areas with necrosis may be present (Fig. 10) [4, 29].
The differential diagnosis for angiosarcoma of the bone includes other primary sarcomas as well as metastatic carcinoma. In this regard, immunohistochemical stains for factor VIII, CD31, CD34 or Fli1 may be helpful in identifying those tumors that are endothelial in origin [29].
An angiosarcoma represents a heterogeneous group of malignant vascular tumors, occurring not only in different anatomical locations, but also in distinct clinical settings, such as after radiation therapy or in association with chronic lymphedema [10]. This clinical heterogeneity mirrors the genetic heterogeneity of an angiosarcoma. Because of that, the genetic and molecular aberrations involved in angiosarcoma oncogenesis remains poorly understood. However, Italiano et al. [10] reported that a genomic amplification of MYC may not only occur in a radiation-induced angiosarcoma, but also in a subset of primary angiosarcoma. Several studies have shown how MYC is not only involved in the control of cell proliferation, apoptosis, and differentiation, but also in non-cell autonomous cancer processes such as angiogenesis. Interestingly, a significant up-regulation of the miR-17-92 cluster was observed in MYC-amplified angiosarcoma compared with an angiosarcoma lacking MYC amplification. Moreover, MYC amplified angiosarcomas were associated with a significantly low expression of thrombospodin-I, a potent endogenous inhibitor of angiogenesis. Altogether, these data confirm that non-cell autonomous cancer processes such as angiogenesis represent the main functional consequences of MYC amplification in angiosarcoma [10].
The treatment of patients with angiosarcoma of the bone depends on several factors: the age of patient, size and location of the tumor, solitary or multifocal disease. Due to their aggressive nature, the treatment of angiosarcoma of the bone consists of a multidisciplinary approach based on surgery, radiotherapy, and chemotherapy [45]. The surgical approach represents the cornerstone of treatment for patients with a localized disease. Surgery has to be very aggressive with wide surgical margins and it can be associated with adjuvant radiotherapy. Reconstruction of the skeleton following resection can be performed utilizing a variety of techniques that are dependent upon the location of the lesion [29]. Patients with a multicentric angiosarcoma involving a single anatomical location may also be candidates for radical resection. Radiotherapy alone should also be considered for those patients with multifocal disease in a different anatomical location or with unresectable tumors. Although chemotherapy may be used, the efficacy has not yet been determined [29]. In fact, there is a paucity of clear information or meta-analyses on the management of these tumors in the literature. Anti-angiogenic monoclonal antibodies may offer angiosarcoma-specific treatment in the future, although their use is explicitly limited to trials at the moment [43]. Local and distant recurrence is common, with the lungs noted as a prime site for a metastasis in 50% of cases [45].
The histological degree of differentiation is the most important factor in the prognosis of patients affected by angiosarcoma of the bone. However, despite adequate locoregional treatment, up to 50–80% of patients will develop metastatic relapse and will die of the disease [10, 29].
Epithelioid angiosarcoma
An epithelioid angiosarcoma is an extremely rare tumor of the bone that is characterized by large cells with an epithelioid morphology. This tumor seems to have a predilection for long bones, a striking male predominance and occurs in the adult population. Accordingly, literature is limited to only several case reports and a series of ten cases [46]. In the latter series, Desphande et al. [46] reported that 8 patients were male, the age range was 26–83 years old, and that 6 patients had multifocal involvement. Three of the solitary lesions involved the femur and one involved the calcaneus. Of the 6 patients with multicentric lesions, 2 of them involved the same bones (femur and tibia), and others involved contiguous bones.
A variety of clinical presentations may be encountered, ranging from painful and enlarging soft tissue mass to long bone fractures. The non-specific initial presentation depends on the size of the tumor, the tissue involved, and its resultant dysfunction [47]. Once again, as with other vascular tumors, the only diagnostical clue is the possible multifocal presentation of an epithelioid angiosarcoma.
The radiographic appearance of these tumors is that of a destructive, predominantly, or exclusively lytic tumor that originates in the medullary cavity and invades the cortex and neighboring soft tissues [2] (Fig. 11).
Epithelioid angiosarcoma of the bone. Multiple, not sharply demarcated lytic lesions of the spine on a axial T2, b sagittal T1, and c T2 fat-saturated MR images involving the sacrum, the first, second, and fifth lumbar vertebra. Lesions are heterogeneous, some with a high signal on T1 and T2 sequences, indicating blood, some with a lower signal compatible with fibrous tissue or bone sclerosis. The sacral periosteum is expanded, with some destruction of the right posterior iliac spine
An epithelioid angiosarcoma is usually a poorly differentiated and biologically aggressive neoplasm. Macroscopically, this tumor is friable, red, and hemorrhagic, with often an extra-osseous extension. Microscopically, it is composed of epithelioid cells that usually demonstrate marked pleomorphism and cytological atypia with irregular nuclear membranes and prominent nucleoli. Necrosis and mitotic figures, including atypical forms, are abundant. By contrast, hyalinized or basophilic stroma are absent [2, 6]. Well-formed vascular channels are frequently numerous (Fig. 12). However, because of their epithelioid appearance, an epithelioid angiosarcoma may be misdiagnosed as a metastatic carcinoma. Therefore, antibodies against certain vascular and endothelial antigens have been shown to be helpful in differentiating vascular tumors from metastatic carcinomas [48]. A panel of vascular markers such as CD31, CD34, and Fli-1 should be used, as not all tumors are positive for all markers [6].
Epithelioid angiosarcoma of the bone. Proliferation of solid sheets of large atypical endothelial cells with an epithelioid morphology among the host bone trabeculae. Tumor cells show abundant eosinophilic cytoplasm and large nuclei. Nuclear atypia is prominent and mitoses are numerous (hematoxylin–eosin staining, ×10)
The genetic studies on epithelioid angiosarcoma are extremely limited. Cao et al. [49] examined and compared the cytogenetic characteristics of epithelioid hemangioendothelioma and epithelioid angiosarcoma utilizing the Array-Comparative Genomic Hybridization (Array-CGH) method. Considerable differences in the cytogenetic characteristics were observed between the two types of tumors. Small fragment gains (<10 MB) were dominant in an epithelioid hemangioendothelioma, whereas large fragment gains and deletions (>10 MB) were dominant in an epithelioid angiosarcoma. Some large fragment alterations, such as gains in chromosomes 19q and 19p, and deletions in chromosomes 9p and 13q, involved over half of a chromosome arm.Therefore, an epithelioid hemangioendothelioma and an epithelioid angiosarcoma showed great cytogenetic differences. However, further genetic studies on epithelioid angiosarcomas are warranted.
Treatment modalities vary among individual cases. It is important to ascertain whether the disease is solitary or multicentric. The therapeutic alternatives in the management of epithelioid angiosarcoma of the bone are similar to the treatment approaches to patients with bone sarcomas of other types [29]. Wide surgical resection and adjuvant radiation therapy are usually used. If the lesion is solitary and surgically accessible, surgery is the treatment of choice. Radiation therapy should be considered for those patients with multicentric lesions or patients with surgically inaccessible tumors [29]. Chemotherapeutic regimens are still under investigation. However, despite wide surgical resection with or without adjuvant radiation and chemotherapy, an epithelioid angiosarcoma frequently metastasizes, and this usually results in the patient’s death after a short clinical course [2, 46].
Summary
One of the most confusing issues related to vascular tumors is the myriad of names that are used to describe them. Pathologically, these tumors are remarkably similar, which makes differentiating them from each other very difficult [29]. This issue is compounded by the fact that current surgical pathology textbooks inadequately describe and classify these tumors. Moreover, most of these textbooks do not even acknowledge the existence of the three subtypes of epithelioid vascular neoplasms.
For this rare subset of vascular tumors, there remains considerable controversy regarding the terminology and the classification that should be used. For instance, epithelioid hemangioma continues to be confused with hemangioendothelioma [2, 3].
Because clinical behavior, treatment, and prognosis of vascular bone tumors can vary significantly, it is important to effectively and accurately distinguish them from each other.
Upon a review of the English language literature, we propose a classification scheme of vascular bone tumors that includes hemangioma, hemangioendothelioma, angiosarcoma, and their epithelioid variants (Table 1).
References
Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25(8):1061–6.
O’Connell JX, Nielsen GP, Rosenberg AE. Epithelioid vascular tumors of bone: a review and proposal of a classification scheme. Adv Anat Pathol. 2001;8(2):74–82.
Evans HL, Raymond AK, Ayala AG. Vascular tumors of bone: a study of 17 cases other than ordinary hemangioma, with an evaluation of the relationship of hemangioendothelioma of bone to epithelioid hemangioma, epithelioid hemangioendothelioma, and high-grade angiosarcoma. Hum Pathol. 2003;34(7):680–9.
World Health Organization Classification of Tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002.
Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is consistent abnormality in epithelioid hemangioendotelioma of different anatomic sites. Gene Chromosome Cancer. 2011;50(8):644–53. doi:10.1002/gcc.20886
Deyrup AT, Montag AG. Epithelioid and epithelial neoplasm of bone. Arch Pathol Lab Med. 2007;131(2):205–16.
Wenger DE, Wold LE. Benign vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29(2):63–74.
Campanacci M. Bone and soft tissue tumors. 2nd ed. New York: Springer; 1999.
Kaleem Z, Kyriakos M, Totty WG. Solitary skeletal hemangioma of the extremities. Skeletal Radiol. 2000;29(9):502–13.
Italiano A, Thomas R, Breen M, Zhang L, Crago AM, Singer S, Khanin R, Maki RG, Mihailovic A, Hafner M, Tuschl T, Antonescu CR. The miR-17-92 cluster and its target THBS1 are differentially expressed in angiosarcomas dependent on MYC amplification. Genes Chromosomes Cancer. 2012;51(6):569–78.
Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174(5):1638–49.
Wu JK, Adepoju O, De Silva D, Baribault K, Boscolo E, Bischoff J, Kitajewski J. A switch in Notch gene expression parallels stem cell to endothelial transition in infantile hemangioma. Angiogenesis. 2010;13(1):15–23.
Adepoju O, Wong A, Kitajewski A, Tong K, Boscolo E, Bischoff J, Kitajewski J, Wu JK. Expression of HES and HEY genes in infantile hemangiomas. Vasc Cell. 2011;3:19.
Mihm Jr MC, Nelson JS. Hypothesis: the metastatic niche theory can elucidate infantile hemangioma development. J Cutan Pathol. 2010;37 [Suppl 1]:83–7.
North PE, Waner M, Mizeracki A, Mrak RE, Nicholas R, Kincannon J, Suen JY, Mihm Jr MC. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol. 2001;137(5):559–70.
Barnés CM, Huang S, Kaipainen A, Sanoudou D, Chen EJ, Eichler GS, Guo Y, Yu Y, Ingber DE, Mulliken JB, Beggs AH, Folkman J, Fishman SJ. Evidence by molecular profiling for a placental origin of infantile hemangioma. Proc Natl Acad Sci U S A. 2005;102(52):19097–102.
Errani C, Sung YS, Zhang L, Healey JH, Antonescu CR. Monoclonality of multifocal epithelioid hemangioendothelioma of the liver by analysis of WWTR1-CAMTA1 breakpoints. Cancer Genet. 2012;205(1–2):12–7.
Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7.
Blecher R, Smorgick Y, Anekstein Y, Peer A, Mirovsky Y. Management of symptomatic vertebral hemangioma: follow-up of 6 patients. J Spinal Disord Tech. 2001;24(3):196–201.
Nielsen GP, Srivastava A, Kattapuram S, Deshpande V, O’Connell JX, Mangham CD, Rosenberg AE. Epithelioid hemangioma of bone revisited: a study of 50 cases. Am J Surg Pathol. 2009;33(2):270–7.
Sung MS, Kim YS, Resnick D. Epithelioid hemangioma of bone. Skeletal Radiol. 2000;29(9):530–4.
Floris G, Deraedt K, Samson I, Brys P, Sciot R. Epithelioid hemangioma of bone: a potentially metastasizing tumor? Int J Surg Pathol. 2006;14(1):9–15. discussion 16–20.
Gupta A, Saifuddin A, Briggs TW, Flanagan AM. Subperiosteal hemangioendothelioma of the femur. Skeletal Radiol. 2006;35(10):793–6.
Shah ZK, Peh WC, Shek TW, Wong JW, Chien EP. Hemangioendothelioma with an epithelioid phenotype arising in hemangioma of the fibula. Skeletal Radiol. 2005;34(11):750–4.
O’Connell JX, Kattapuram SV, Mankin HJ, Bhan AK, Rosenberg AE. Epithelioid hemangioma of bone. A tumor often mistaken for low-grade angiosarcoma or malignant hemangioendothelioma. Am J Surg Pathol. 1993;17(6):610–7.
Errani C, Zhang L, Panicek DM, Healey JH, Antonescu CR. Epithelioid hemangioma of bone and soft tissue: a reappraisal of a controversial entity. Clin Orthop Relat Res. 2012;470(5):1498–506.
Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, Rossi G, Longhi A, Mercuri M. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1–7.
Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591–9.
Wenger DE, Wold LE. Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29(11):619–31.
Bovée JV, Hogendoorn PC. Molecular pathology of sarcomas: concepts and clinical implications. Virchows Arch. 2010;456(2):193–9.
Murali R, Zarka MA, Ocal IT, Tazelaar HD. Cytologic features of epithelioid hemangioendothelioma. Am J Clin Pathol. 2011;136(5):739–46.
Antonescu CR, Elahi A, Healey JH, Brennan MF, Lui MY, Lewis J, Jhanwar SC, Woodruff JM, Ladanyi M. Monoclonality of multifocal myxoid liposarcoma: confirmation by analysis of TLS-CHOP or EWS-CHOP rearrangements. Clin Cancer Res. 2000;6(7):2788–93.
Barbashina V, Salazar P, Holland EC, Rosenblum MK, Ladanyi M. Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene. Clin Cancer Res. 2005;11(3):1119–28.
Henrich KO, Fischer M, Mertens D, Benner A, Wiedemeyer R, Brors B, Oberthuer A, Berthold F, Wei JS, Khan J, Schwab M, Westermann F. Reduced expression of CAMTA1 correlates with adverse outcome in neuroblastoma patients. Clin Cancer Res. 2006;12(1):131–8.
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial–mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–36.
Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284(21):14347–58.
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial–mesenchymal transition. J Biol Chem. 2009;284(20):13355–62.
Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, Edelman G, Yeh J, Prasad S, Weinberg G. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140(5):1312–8.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101.
Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66(23):11089–93.
Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95.
Norton L, Massagué J. Is cancer a disease of self-seeding? Nat Med. 2006;12(8):875–8.
Larochelle O, Périgny M, Lagacé R, Dion N, Giguère C. Best cases from the AFIP: epithelioid hemangioendothelioma of bone. Radiographics. 2006;26(1):265–70.
Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133(6):967–72.
Lewis CJ, Gerrand C, Barnes DE, Murray S, Milner RH, Ragbir M. Experience of angiosarcoma in the North of England Bone and Soft Tissue Tumour Service. J Plast Reconstr Aesthet Surg. 2011;64(7):884–91.
Deshpande V, Rosenberg AE, O’Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27(6):709–16.
Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135(2):268–72.
Kleer CG, Unni KK, McLeod RA. Epithelioid hemangioendothelioma of bone. Am J Surg Pathol. 1996;20(11):1301–11.
Cao Y, Zou SM, Zhang KT, Lu N, Liu Y, Feng L, Wen P, Han NJ, Lin DM. Genetic alterations in pulmonary epithelioid hemangioendothelioma and epithelioid angiosarcoma. Histol Histopathol. 2011;26(4):491–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Errani, C., Vanel, D., Gambarotti, M. et al. Vascular bone tumors: a proposal of a classification based on clinicopathological, radiographic and genetic features. Skeletal Radiol 41, 1495–1507 (2012). https://doi.org/10.1007/s00256-012-1510-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-012-1510-6