Abstract
Rotator cuff calcific tendinitis is a very common disease and may result in a very painful shoulder. Aetiology of this disease is still poorly understood. When symptoms are mild, this disease may be treated conservatively. Several treatment options have been proposed. Among them, ultrasound-guided procedures have been recently described. All procedures use one or two needles to inject a fluid, to dissolve calcium and to aspirate it. In the present article, we review some tips and tricks that may be useful to improve performance of an ultrasound-guided double-needle procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Detection of calcifications within the tendons of the rotator cuff is a common occurrence, being found in up to 7.5 % of asymptomatic adults and up to 20 % of painful shoulders. [1–4]. Tiny scattered calcifications may be found on the insertional portion of the tendons as a result of a degenerative process (i.e., calcific enthesopathy). Conversely, rotator cuff calcific tendinitis (RCCT) is a completely different disease, which usually occurs in healthy tendon, as a result of fibrocartilaginous metaplasia of collagen fibroblasts associated with calcium deposition, predominantly hydroxyapatite. This event is probably triggered by oxygen tension decrease and pressure increase within the tendon [2, 3], although etiopathogenesis is still not completely understood. The term tendinitis is not really appropriate, as inflammatory cells are rarely encountered [4]. Thus, calcific tendinopathy seems to be the most appropriate name for this disease [3]. Two stages of the disease have been recognized: precalcific and calcific. In turn, calcific stage consists of formative, resorptive, and postcalcific phase. Resorptive phase is characterized by vascular invasion, oedema, migration of phagocytic cells, and increased intratendinous pressure. At this time, patients may report the occurrence of highly disabling sharp acute pain, usually scarcely responding to common painkillers. Occasionally, calcium may drain within the subacromial-subdeltoid bursa (SASD), thus promoting the occurrence of calcium-related bursitis [2, 5].
RCCT is more frequently seen in women in their fourth and fifth decades, and it seems not to be related to physical activity. Theoretically, all tendons of the body may be affected by RCCT, although rotator cuff is by far the most common site. There, the supraspinatus tendon is involved in about two thirds of cases [2, 6].
Notably, RCCT is considered a self-healing condition, with complete restitutio ad integrum of tendon matrix and pain regression [3]. However, the temporal self-response of RCCT has been demonstrated to be extremely variable [2, 3]. Being RCCT a self-limiting condition, the chosen treatment should be effective, complication free, and minimally invasive. As far as the treating options are concerned, there are different possibilities, but no standard of care has been established. Nevertheless, no treatment is required for asymptomatic patients, while mild symptoms can benefit from conservative treatments, such as physical therapy and oral non-steroidal anti-inflammatory drugs [5]. Different treatment options have been reported. Iontophoresis has proven not to be more effective than physiotherapy or placebo on long-term follow-up [7]. Shockwave therapy resolves calcifications in 57–60 % of the shoulders and achieves substantial or complete clinical improvement [8]. However, shockwaves are extremely painful when performed during the hyperalgesic crisis [9]; at least three applications are usually needed , and they are relatively expensive [8, 9]. Arthroscopy [10, 11] achieves substantial or complete clinical improvements in 79–100 % of the shoulders, but long rehabilitation is required and even major complications may occasionally occur. Nowadays, it is considered the last option when other methods have failed.
Imaging-guided procedures are considered alternative and effective treatments. Slightly different approaches have been described, performed under fluoroscopic [12] or ultrasound (US) guidance, all implying the use of a fluid (local anesthetic or saline solution) to dissolve calcific deposits. Percutaneous treatment is currently most often performed under US guidance [2, 13–22], thanks to absence of ionizing radiation, three-dimensional capabilities in localizing the calcification, real-time monitoring during needle placement, decreased risk of injury to the neighboring structures, and real time confirmation of procedure success [2]. These procedures are also very quick, having an average duration of 10 to 20 minutes [21]. US-guided percutaneous procedures can be effectively used as first line treatment for RCCT, especially in the acute phase.
US-guided procedures can be performed using one [13, 15, 17, 19] or two needles [2, 14, 16, 18, 20–22] to inject fluid within calcifications. Some authors used one needle, believing that double needle insertion could be harmful to the tendon [25], but safety and efficacy of two-needle technique has been proven up to one and ten years, respectively, and it actually is an effective, quick, and low-cost therapy [2, 21]. Purposes of using two needles are to create a continuous inflow and outflow of saline solution to remove calcium and to control saline pressure inside the calcification during injection [18]. This also provides escape for fluid, avoiding disruption of the peripheral calcific rim that causes calcium to spread throughout the periarticular soft tissues, possibly promoting the occurrence of post-procedural calcific bursitis [2, 21].
The purpose of the present work is to review some tips and tricks based on a daily routine experience that can improve the practical performance of a double-needle procedure for treating RCCT.
Procedure details
US probe disinfection
Preliminary disinfection of instruments is necessary before starting the procedure [6], as US machines are ideal vectors for cross infections [23]. In terms of potential infections, US-guided procedures are argued to be safe for patients and operators with ordinary antisepsis [24]. Although most operators use either sterile gels or liquids in combination with sterile sheaths, condoms or gloves, or non-sterile gel-filled condoms or gloves, this may be an unnecessary procedural effort. Caturelli et al. [25] reported no infection when US transducer was cleaned with a 70 % alcoholic solution prior to each procedure of abdominal fine-needle-puncture of solid organs. To our knowledge, no study is available investigating the infection rate in US-guided joint procedures. When dealing with US-guided percutaneous treatment of RCCT, no infective complications were reported in studies in which the US probe was simply cleaned with antiseptic sterile solution [2, 21]. However, a sterile probe cover can be also used, but it represents an additional cost and may be uncomfortable in a procedure that lasts more than seconds.
Use of a two-step skin cleaning procedure (coloured/uncoloured antiseptics)
We currently use a two-step antiseptic procedure for skin cleaning. It consists of cleaning with a coloured disinfectant (i.e., 7.5–10 % iodopovidone solution) for at least two minutes, immediately followed by a second cleaning with an uncoloured disinfectant (e.g., benzalkonium chloride solution) (Fig. 1). This approach has three practical advantages. First, a two-step procedure improves antisepsis of shoulder skin. Second, using no sterile covers, the US probe is not stained by coloured antiseptic solution. Third, keeping a peripheral ring of coloured antiseptic solution allows for a better control of the cleaned area during the entire procedure. Thus, not only is the area optimally cleaned, but we are also aware of the external borders of the cleaned area. In case of reported allergy to iodine, we perform a double cleaning with the same uncoloured antiseptic solution reported above [2, 21].
Use of sterile equipment and sterile contact gel
Although this procedure is not usually performed in a surgical theatre, sterility levels should be kept at maximum. Thus, the use of sterile drapes and gloves is advisable [2, 21].
Some authors do not use any sterile contact gels during US-guided procedures [23–25]. This is agreeable, as for short procedures (e.g., injections, biopsies) it is enough to keep both the skin and the probe wet with the antiseptic solution that was used to clean the skin. However, in longer procedures, such as US-guided percutaneous treatment of RCCT, this approach is suboptimal, as the antiseptic solution gets dry quite quickly and it needs to be reapplied frequently. For these reasons, the use of a small amount of sterile lubricant contact gel (e.g., Glissen 12.5 g) may be advisable [2, 21].
How to inject local anaesthesia
Different anaesthetic solutions can be used (e.g., 2 % lidocaine chloridrate). Whatever solution is used, the maximum dose recommended for adults is 400 mg, to be administered during the entire procedure [26]. Anaesthetic solution must be injected mostly (almost two thirds) into the SASD bursa, thus allowing for its complete distension. The remaining solution should be injected in the subcutaneous tissues and around calcification. In order to preserve the peripheral calcific rim, no anesthetic solution should be injected directly within the calcification. No anaesthetic injection is required directly in the deltoid muscle (Fig. 2).
How to position the needles
In musculoskeletal procedures, selection of type and size of the needles depends on the joint and on the procedure that is being performed. Drug injection normally needs thin needles of 22–27 gauge (G) [27], while fluid aspiration normally needs thicker needles of 18–21 G. When dealing with RCCT, needle size must be chosen in order to maximize calcium retrieval and avoid obstruction. Aina et al. [15] indicated that 22 G needles are adequate for performing calcification fragmentation, aspiration, and reduction, without the need of larger and more traumatic needle sizes. The caliber of the needles used in other published studies for RCCT treatment varies between 16 and 22 G [13–22]. In our experience [2, 21], using two 16 G needles has been demonstrated to be safe, without risk of tendon tears and a good compromise between calcium retrieval and avoiding obstruction that may occur when thinner needles are used.
Needles are inserted into calcification under continuous US monitoring with a free-hand technique. No needle guidance kit is required, as freehand technique allows for a faster and more flexible approach (i.e., with no fixed angles) [28].
Correct needle positioning and an adequate visualization of needle tips are crucial [2]. Two aspects must be taken into account: needle positioning with respect to the US probe, and positioning of the two needles with respect to each other and to calcifications.
As far as the position of the needle with respect to the US probe is concerned, methods used to insert the two needles vary according to location, accessibility, and calcification size. Anyhow, both needles must be inserted as parallel as possible to the US probe so they will be as perpendicular as possible to US beam. In this way, anisotropy artefacts are drastically reduced and needles can be seen thoroughly (Fig. 3).
Positioning of the needles in respect to the probe. The best needle visualization is obtained when (a) the needle is as parallel as possible to the US probe, which means as perpendicular as possible to the ultrasound beam. (b) As the angle between the beam and the needle decreases, the needle becomes less visible. C calcification
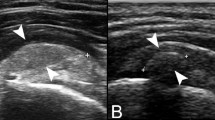
With respect to each other and to calcifications, the deeper needle is first inserted. This prevents the first needle from being covered by the second, which is inserted superficially (Fig. 4). Needles should be placed on a plane so that the US beam can demonstrate both of them and the calcification simultaneously. Angulation between the needles should be 25–30°, with tips very close to each other (maximum distance 2–3 mm). The bevel of the first needle must be rotated upward, while the bevel of the second needle should be rotated downward, so that both bevels are facing each other (Fig. 5). This would allow for facilitating a continuous flow of water that is injected by one needle and drained by the other.
Needles should be inserted in the same coronal plane, such that they form a maximum angle of 25–30° and their tips are very close to each other (maximum distance 2–3 mm). Deep needle (arrowheads) bevel (thick arrow) must be rotated upward and superficial needle (thin arrows) bevel (thick arrow) should be rotated downward so that they face each other. C calcification
Use of warm saline solution
As mentioned before, all percutaneous treatments for RCCT imply the use of a fluid (saline or anesthetic) to dissolve calcium. Most authors reported the use of room temperature fluid [2, 14–20, 22]. However, we recently demonstrated that use of warm saline solution improves treatment of RCCT [21]. This hypothesis has been tested, starting from the well-known concept that most salts dissolve better in high temperature liquids [29]. Using warm saline solution when treating RCCT allows for significant reduction in procedure duration, compared to the same procedure performed using room temperature saline solution. Using warm saline also improves calcium dissolution and helps in reducing the occurrence of post-procedural bursitis, maintaining a similar efficacy of the treatment.
Saline solution injection
Saline solution is normally injected using a 20-mL syringe. It is connected to one of the needles, and the plunger is repeatedly pushed. Once calcification starts to dissolve, water and calcium debris are drained from the free needle (Fig. 6a). Another option is to build a homemade lavage closed circuit. A saline bottle is hung on a phleboclysis rod and is connected directly to one of the needles using a long tube. A three-way stopcock connected to a syringe is inserted in the line, thus allowing a continuous aspiration and injection of saline within the calcification (Fig. 6b). Although slightly more complicated, this system ensures complete sterility of the procedure [14].
How to increase calcium removal
It is not clear whether there is a correlation between removal of a large amount of calcium and a good outcome, as some studies report satisfactory results, with minimal calcium retrieval [12, 15, 17]. However, removing a large amount of calcium may reduce the occurrence of post-procedural bursitis induced by calcium debris that spreads around the cuff. Although the double-needle technique allows for optimal calcium removal in most cases, hard calcifications are occasionally difficult to melt. In these cases, needles can be gently rotated and laterally displaced (Fig. 7) to increase calcium disaggregation and fragmentation. This usually results in easier and more effective calcium washing (Fig. 8). These manoeuvres are performed carefully, trying not to disrupt the peripheral calcific rim.
Conclusion
Double-needle US-guided percutaneous treatment of RCCT has been demonstrated to be an effective, quick, minimally invasive and low-cost therapy. It facilitates shoulder function improvement and prompt pain relief, and post-procedural complications are almost absent. The use of the tips and tricks discussed in the present article may further improve the execution of this procedure in the daily practice.
References
Speed CA, Hazelman BL. Calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1582–4.
Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment–nonrandomized controlled trial. Radiology. 2009;252:157–64.
Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin Rheumatol. 1989;3:567–81.
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202.
Bianchi S, Martinoli C, Shoulder in Bianchi S, Martinoli C, eds. Ultrasound of the musculoskeletal system, Berlin, Germany: Springer-Verlag, 2007;190–331
Hamada J, Ono W, Tamai K, Saotome K, Hoshino T. Analysis of calcium deposits in calcific periarthritis. J Rheumatol. 2001;28:809–13.
Leduc BE, Caya J, Tremblay S, Bureau NJ, Dumont M. Treatment of calcifying tendinitis of the shoulder by acetic acid iontophoresis: a double-blind randomized controlled trial. Phys Med Rehabil. 2003;84:1523–7.
Ebenbichler G. Ultrasound therapy for calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1582–4.
Mouzopoulos G, Stamatakos M, Mouzopoulos D, Tzurbakis M. Extracorporeal shock wave treatment for shoulder calcific tendonitis: a systematic review. Skeletal Radiol. 2007;36:803–11.
Wittenberg RH. Surgical or conservative treatment for chronic rotator cuff calcifying tendinitis—a matched-pair analysis of 100 patients. Arch Orthop Trauma Surg. 2001;121:56–9.
Rubenthaler F. Prospective randomized surgical treatments for calcifying tendinopathy. Clin Orthop Relat Res. 2003;410:278–84.
Comfort TH, Arafiles RP. Barbotage of the shoulder with image-intensified fluoroscopic control of needle placement for calcific tendinitis. Clin Orthop Relat Res. 1978;135:171–8.
Bradley M, Bhamra MS, Robson MJ. Ultrasound guided aspiration of symptomatic supraspinatus calcific deposits. Br J Radiol. 1995;68:716–9.
Galletti S, Magnani M, Rotini R, et al. The echo-guided treatment of calcific tendinitis of the shoulder. Chir Organi Mov. 2004;89:319–23.
Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fine-needle method. Radiology. 2001;221:455–61.
Farin PU, Rasanen H, Jaroma H, Harju A. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 1996;25:551–4.
del Cura JL, Torre I, Zabala R. Legórburu A Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am J Roentgenol. 2007;189:W128–34.
Sconfienza LM, Serafini G, Sardanelli F. Treatment of calcific tendinitis of the rotator cuff by ultrasound-guided single-needle lavage technique. AJR Am J Roentgenol. 2011;197:W366.
Giacomoni P, Siliotto R. Echo-guided percutaneous treatment of chronic calcific tendinitis of the shoulder. Radiol Med (Torino). 1999;98:386–90.
Farin PU, Jaroma HJ. Sonographic findings of rotator cuff calcifications. J Ultrasound Med. 1995;14:7–14.
Sconfienza LM, Bandirali M, Serafini G, et al. Rotator Cuff Calcific Tendinitis: Does Warm Saline Solution Improve the Short-term Outcome of Double-Needle US-guided Treatment? Radiology. 2012;262:560–6.
De Zordo T, Ahmad N, Ødegaard F, et al. US-guided therapy of calcific tendinopathy: clinical and radiological outcome assessment in shoulder and non-shoulder tendons. Ultraschall Med. 2011;32:S117–23.
Fowler C, McCracken D. US probes—Risk of cross infection and ways to reduce it—Comparison of cleaning methods. Radiology. 1999;213:299–300.
Weidner S, Kellner W, Kellner H. Interventional radiology and the musculoskeletal system. Best Pract Res Clin Rheumatol. 2004;18:945–56.
Caturelli E, Giacobbe A, Facciorusso D, et al. Free-hand technique with ordinary antisepsis in abdominal US-guided fine-needle punctures: three-year experience. Radiology. 1996;199:721–3.
Niesel HC. Local anesthetics: maximum recommended doses. Anaesthesiol Reanim. 1997;22:60–2.
De Smet AA. Ultrasound-guided injections and aspirations of the extremities. Semin Roentgenol. 2004;39:145–54.
del Cura JL. Ultrasound-guided therapeutic procedures in the musculoskeletal system. Curr Probl Diagn Radiol. 2008;37:203–18.
Wiberg E, Wiberg N, Holleman AF. Inorganic chemistry. London, England: Academic Press; 2001. p. 810–1.
Conflict of Interest
Authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sconfienza, L.M., Viganò, S., Martini, C. et al. Double-needle ultrasound-guided percutaneous treatment of rotator cuff calcific tendinitis: tips & tricks. Skeletal Radiol 42, 19–24 (2013). https://doi.org/10.1007/s00256-012-1462-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-012-1462-x