Abstract
Minimally-invasive treatments for chronic Achilles tendinopathy may prevent the need for surgery when conservative methods have failed. Whilst injections have traditionally been used to manage symptoms, recently described therapies may also have disease-modifying potential. Ultrasound provides the ability to guide therapeutic interventions, ensuring that treatment is delivered to the exact site of pathology. Treatments can be broadly categorised according to their intended therapeutic targets, although some may act through several possible mechanisms. In this article, we review the ultrasound-guided techniques currently used to treat chronic Achilles tendinopathy, with reference to the available literature. There is strong pilot-level evidence supporting the use of many of these techniques, although large definitive trials are lacking. An approach towards the management of chronic Achilles tendinopathy is suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic Achilles tendinopathy is an overuse injury that is common in athletes, but is also seen in the non-athletic population. The term Achilles tendinopathy encompasses a spectrum of painful conditions at or around the Achilles tendon, including the histopathological entities tendinosis and paratendinitis [1]. Clinical differentiation between these disorders can be difficult, and lesions may co-exist. Chronic Achilles tendinopathy most commonly affects the mid-portion of the tendon, but also occurs at the bone–tendon junction. There are no specific temporal criteria to classify overuse injuries as acute or chronic, although some investigators have defined chronic tendinopathy as symptoms present for more than 6 weeks [2].
Histopathology
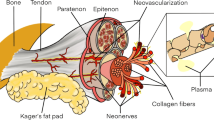
The pathophysiological hallmark of chronic tendinopathy is the presence of “degenerative” changes, including disorganised collagen fibres, increase in ground substance and neovascularity [3]. The precise cause of degeneration and pain in patients with tendinopathy is not clear. Mechanical, vascular, neural and “failure of healing” models have been proposed [4]. There is no cellular evidence of inflammation in chronic Achilles tendinopathy, although inflammation may play a role in the early phases of the condition [5].
Diagnosis
Achilles tendinopathy is clinically characterised by pain, swelling and impaired performance. A correct diagnosis can often be established by clinical examination alone. However, differentiating between Achilles tendon disorders can be difficult, and Achilles tendon ruptures are reportedly missed by up to 20% of primary care physicians [6]. If a diagnosis is not clear, ultrasound or magnetic resonance imaging (MRI) may reveal the pathology within the tendon [7]. Ultrasound provides a readily available, quick, safe and inexpensive method of verifying the existence and location of intra- and peritendinous lesions. Ultrasound carries a high positive predictive value for Achilles tendinopathy, although a negative examination can occur in patients with clinically proven tendinopathy [8]. MRI is also a very sensitive method of depicting pathological changes in the Achilles tendon. A study comparing ultrasound, MRI and surgical findings in 27 histologically verified cases of chronic Achilles tendinopathy showed good correlation between the two imaging modalities [9].
The healthy Achilles tendon exhibits an echogenic pattern of parallel fibrillar lines in the longitudinal plane, reflecting the acoustic borders between collagen fibrils and loose connective tissues between fascicles, and an echogenic round-to-ovoid shape in the transverse plane. Normal tendon thickness ranges between 4 and 7 mm (mean 5.2 mm) [10]. Typical ultrasound findings in patients with Achilles tendinopathy may be graded according to characteristic grey-scale ultrasound appearances: Grade 1, normal tendon; Grade 2, swollen tendon demonstrating spindle shape/fusiform thickening; Grade 3, intratendinous hypoechoic areas, which may be localised or generalised [11]. Studies have shown that hypoechoic areas seen on ultrasound correspond to histological evidence of collagen degeneration [12]. There may also be associated peritendinous adhesions, seen as thickening of the hyperechoic paratenon. Simultaneous use of colour or power Doppler can add information about blood flow. In the Achilles tendon, blood flow is not detected in normal tendons, but colour Doppler often reveals blood flow in tendinopathic tendons (Fig. 1) [13]. This vascular ingrowth, termed neovascularisation, has been linked to greater pain, poorer function and longer duration of symptoms in Achilles tendinopathy [7]. When present, neovessels predominate on the ventral side of the Achilles tendon. There is some evidence that nerve structures related to neovessels may be the source of pain in tendinopathy [14].
Treatment
Conservative methods of treatment for chronic Achilles tendinopathy are directed towards correcting possible aetiological factors and controlling symptoms. Such strategies include physiotherapy, eccentric stretching exercises, cryotherapy, shockwave lithotripsy and glyceryl trinitrate patches [15–19]. However, conservative management is unsuccessful in 24–45% of patients with Achilles tendinopathy and surgery may be recommended if other methods have been tried for at least 6 months [20–22]. The aim of surgery is to excise fibrotic adhesions, remove degenerate tendon and make longitudinal tenotomies to stimulate cell matrix response and healing. Most authors report good results from open surgery in up to 85% of patients, but this may be lower in non-specialised clinical practice [23, 24]. It is therefore usual to exhaust all non-operative measures before considering surgery.
Local injection therapies for the management of chronic soft-tissue disorders have been in use for many years. Historically, injections were used primarily to manage symptoms. Recently, several novel techniques and injectates have been used with the aim of promoting healing [25]. Blind administration, even by experienced operators, can be inaccurate and there is some evidence that injection accuracy correlates with efficacy [26, 27]. As a method of providing guidance for therapeutic injections, ultrasound provides the important ability to visualise the needle passage and make adjustments in real time, ensuring that treatment is delivered to the appropriate location (Figs. 2, 3). Ultrasound-guided injection is now increasingly considered to be best practice [1, 28].
Transverse ultrasound image in a patient with chronic mid-portion Achilles tendinopathy obtained during a brisement procedure. The tip of the needle (arrow) is within the peritendinous space. Dilute local anaesthetic injectate is seen as a hypoechoic rim (between arrowheads) around the Achilles tendon
Standard patient preparation for ultrasound-guided Achilles tendon interventions
For most ultrasound-guided Achilles tendon interventions, the patient is positioned prone on the examination couch, with a pillow under the distal tibia and the foot hanging freely at approximately 90°. The skin is disinfected with chlorhexidine and draped with a sterile cover to expose only the affected part of the Achilles tendon. The skin and subcutaneous tissues may be anaesthetised locally with 3–5 ml of lidocaine (1%) or bupivacaine (0.5%) [29].
A linear or small footprint high frequency (7–12 MHz) ultrasound probe is used. The probe is held on the dorsal aspect of the Achilles tendon, parallel to the fibres. Injections are generally approached from the medial side of the tendon to minimise the risk of injuring the sural nerve. Interventions are performed dynamically, with the aid of real-time grey-scale and colour Doppler imaging. For injection therapies, the finest needle that will reach the targeted area should be used—typically 21-gauge or smaller [29].
Ultrasound-guided treatments
Local anti-inflammatory injections
Local corticosteroid injections are one of the most commonly used treatments for chronic tendon lesions. However, the role of inflammation in chronic tendinopathy is unclear, and the rationale for the use of anti-inflammatory injections in Achilles tendinopathy is controversial [1, 30]. Indeed, inflammation, if present, may be an essential part of the healing response. Whilst corticosteroids may have effects upon other non-inflammatory sources of pain, these are also poorly understood. Furthermore, many clinicians advise against the injection of corticosteroid near heavily loaded tendons because of the potential risk of tendon rupture [1]. However, although there are case reports associating glucocorticoids with Achilles tendon ruptures, the majority of studies dealing with peritendinous corticosteroid injections have not shown any direct adverse effects to the tendon [1, 31].
Corticosteroid injections have traditionally been administered blindly, although inadvertent intratendinous injection may be difficult to avoid in a thickened and inflamed tendon. Most practitioners recommend needle placement deep to the paratenon for mid-portion Achilles tendinopathy or directly into the retrocalcaneal bursa for insertional tendinopathy [32]. Ultrasound-guided peritendinous injection enables steroid to be injected as close as possible to the tendon, without being intratendinous. Fredberg et al. [33] performed a double-blind, placebo-controlled study of peritendinous steroid injection in 24 athletes with chronic Achilles tendonitis, in which ultrasound was used in the diagnosis and guidance of injection. The steroid injection contained 3.5 ml of 10 mg/ml lidocaine and 0.6 ml of kenalog containing 20 mg of triamcinolone in a 5-ml syringe. The placebo injection contained 3.5 ml of 1% lidocaine and 0.5 ml of 20% intralip (in order to achieve a milky appearance) in a 5-ml syringe. Injections were placed peritendinously about the thickest point on both sides of the tendon. After 6 months, the steroid injections had a significant beneficial effect in reducing pain and thickening of tendons, compared with no change in the placebo-treated group. However, when combined with aggressive rehabilitation, many patients suffered a relapse of symptoms within 6 months. Furthermore, reversible tissue atrophy was seen in 11 out of 24 steroid-treated patients, although in nearly half the athletes the changes resolved within 6 months and in none did the atrophy give any problems.
In an attempt to address the high frequency of relapse after local corticosteroid injections, the same group tested the effect of two new anti-inflammatory treatments [33]. Ultrasound-guided peritendinous injections of adalimumab (a tumour necrosis factor-alpha blocker) and anakinra (an interleukin-1 receptor antagonist) were evaluated with regard to reducing pain, tendon thickness and blood flow in patients with chronic Achilles tendinopathy. Adalimumab had a significant effect on pain sensation at rest and showed a significant tendency to reduce the blood flow in the tendon over 12 weeks, although the drug had no effect on tendon thickness. Anakinra had no effect on the blood flow and, surprisingly, the tendon thickness increased significantly after 12 weeks.
Koenig et al. [34] hypothesised that the intratendinous hyperaemia seen in chronic Achilles tendinopathy represented active inflammation. They performed colour Doppler-guided intratendinous injections of 1 ml of 40 mg/ml methylprednisolone acetate and 0.5 ml of 1% lidocaine directly into areas of hyperaemia in 6 tendons in 5 patients. Pain and colour Doppler activity decreased during a mean follow-up of 182 days. Although one tendon relapsed because of continuation of the provoking activity, no ruptures were encountered. The authors concluded that intratendinous hyperaemia represents an ongoing inflammatory reaction rather than a reparative process.
Obliteration of neovessels
Tendon neovascularisation is seen as a prominent feature on colour Doppler ultrasound of painful Achilles tendons, but not in those that are pain free. This finding led to the hypothesis that obliterating the neovasculature may reduce refractory Achilles tendon pain.
Sclerotherapy
Öhberg and Alfredson performed a series of studies targeting neovessels in chronic tendinopathies, using polidocanol as a sclerosing agent. Polidocanol was first developed as a local anaesthetic, and is now used as a sclerosing agent with a good safety profile. In pilot studies, polidocanol (5 mg/ml) was injected in small volumes (2–4 ml) against the neovessels outside the tendon under colour Doppler control until the vessels were no longer visible. In 8 out of 10 patients with chronic mid-portion Achilles tendinopathy and in 8 out of 11 patients with chronic insertional Achilles tendinopathy, this treatment cured the pain and allowed full Achilles tendon-loading activity [35, 36]. The researchers then performed a double-blind randomised controlled trial comparing injection treatment with either the polidocanol (5 mg/ml) or lidocaine hydrochloride (5 mg/ml) + adrenaline (5 μg/ml) in 20 patients with chronic painful mid-portion Achilles tendinopathy [37]. The combination of lidocaine hydrochloride + adrenaline ensured that the control treatment also had immediate local anaesthetic and vasoconstrictive effects, although unlike polidocanol, it did not have a sclerosing effect. The short-term results (mean follow-up 3 months) after a maximum of two injections showed significantly reduced tendon pain during activity following treatment with polidocanol, but not with the non-sclerosing combination. Neovascularisation was absent after treatment in the pain-free tendons, but not in the painful tendons. At 2-year follow-up, polidocanol injections showed continuing good clinical results and absence or decrease in neovascularity in the successfully treated tendons [38].
A potential criticism of sclerosing therapy is that multiple treatments are often necessary to achieve pain relief and the obliteration of neovessels. A study aiming to investigate whether higher concentrations of polidocanol would lead to fewer treatments found that increasing the concentration of polidocanol from 5 to 10 mg/ml did not reduce the number of injections required [39]. Larger randomised controlled trials of this technique are still required, and the long-term consequences of this approach remain unclear.
Other sclerosing solutions have also been used in the treatment of chronic Achilles tendinopathy. Hyperosmolar dextrose is a mild vascular sclerosant, though its potential effect in tendinopathy is not well understood. This agent is inexpensive and more widely available than polidocanol. Although we have successfully used hyperosmolar dextrose in this context, at present the evidence for its use as a sclerosant is limited to anecdote.
Electrocoagulation
Electrocoagulation of neovessels is based on the same rationale as sclerotherapy, but is potentially more destructive, so fewer treatments may be required. Boesen and colleagues [40] evaluated colour Doppler ultrasound-guided electrocoagulation for the treatment of painful chronic mid-portion Achilles tendinopathy. The needle was placed against the neovessels entering the ventral portion of the Achilles tendon, the same site as used during sclerosing therapy, and coagulation carried out at 20–25 W until the intratendinous Doppler signal had disappeared. At 6 months’ follow-up, electrocoagulation reduced tendon pain during rest and activity in 10 out of 11 patients. Interestingly, although there was immediate disappearance of intratendinous hyperaemia, the hyperaemia reappeared 2 weeks after treatment and persisted throughout the follow-up period despite cessation of pain. The authors suggested that this indicated that mechanisms other than mere changes in vascularity were responsible for the beneficial effects, such as the destruction of nerves accompanying the vessels.
High-volume injections
Chan and colleagues [41] hypothesised that high-volume Achilles tendon injections of normal saline would produce local mechanical effects causing neovessels to break or occlude. Neural ingrowth accompanying the neovessels would also be damaged, thus decreasing the amount of pain perceived in patients with resistant Achilles tendinopathy. Using ultrasound guidance to place the needle between the anterior aspect of the Achilles tendon and Kager’s fat pad, a mixture of 10 ml of 0.5% bupivacaine hydrochloride and 25 mg of hydrocortisone acetate was injected, followed by 40 ml of injectable normal saline. They found that high-volume injections significantly reduced pain and improved function in 30 patients with chronic Achilles tendinopathy both at short-term follow-up (2 weeks) and in the longer term (30.3 weeks).
Stimulation of healing response
The pathophysiological basis of Achilles tendinopathy may be explained as a disordered wound-healing response. Failure to adapt to recurrent excessive loads may result in release of cytokines by tenocytes, leading to an imbalance between matrix degeneration and synthesis, resulting in degradation of the extracellular matrix network and eventual tendinopathy [42]. This model is supported by histological features of haphazard healing. Therapies that directly target the abnormal areas of tendon aim to interrupt the degenerative cycle by initiating a wound-healing cascade. It is hypothesised that the inflammatory response induces the formation of granulation tissue, ultimately leading to improved tendon strength and clinical outcomes [43].
Intratendinous injection of hyperosmolar dextrose
Hyperosmolar dextrose has been used as part of prolotherapy regimens for the treatment of chronic tendon pain since as early as the 1940s. Prolotherapy is a technique that involves injecting small volumes of an irritant solution (proliferant) at multiple sites around a tendon insertion. The injected solution is thought to initiate a local inflammatory response, causing fibroblast proliferation and subsequent collagen production, resulting in increased tendon strength [44]. It is possible that the sclerosing effect of hyperosmolar dextrose may partly be responsible for any benefit. Using a modification of this technique, Maxwell et al. [45] performed intratendinous injections of hyperosmolar dextrose under ultrasound guidance, targeting the abnormal anechoic and hypoechoic areas in the tendon. Their pilot study showed a significant reduction in tendon pain at rest and during tendon-loading activities.
Dry needling and autologous blood injection
Dry needling is the repeated lancing of an abnormal area of tendon in order to incite internal haemorrhage. It is hypothesised that the consequent inflammatory response induces the formation of granulation tissue, which strengthens the tendon. Autologous whole blood and the blood product platelet-rich plasma have been used as injectates for tendinopathy with the aim of providing cellular and humoral mediators to promote healing in areas of degeneration. Ultrasound-guided dry needling followed by autologous blood injections has shown promise as a treatment for patients with refractory epicondylitis and patellar tendinosis [46, 47]. The technique described in the study by James et al. involved dry-needling the site of patellar tendinosis for 1 min, then slowly injecting up to 3 ml of autologous blood drawn from the antecubital vein into the substance of the abnormal tendon. Treatments performed on two occasions 4 weeks apart led to improved clinical and ultrasound outcomes in 41 out of 44 patients after a mean follow-up of 14.8 months. As yet, however, no studies of this technique have been published in patients with Achilles tendinopathy.
Percutaneous tenotomy
Open longitudinal tenotomy is a well-established surgical option if conservative treatment for chronic Achilles tendinopathy fails. The operation aims to promote wound repair by modulation of the tendon cell–matrix environment. However, the complication rate after open surgical treatment for chronic Achilles tendinopathy can be high. Testa and associates [48] evaluated ultrasound-guided percutaneous longitudinal tenotomy in 75 athletes with unilateral chronic Achilles tendinopathy. The technique involved inserting a scalpel blade under ultrasound control parallel to the long axis of the tendon fibres in the centre of the tendinopathic area. A series of longitudinal tenotomies were made through the initial skin incision by passively flexing the ankle and sequentially altering the angle of the blade. At the time of their best outcome, 62 out of 75 subjects (83%) reported symptomatic benefit and at final follow-up (mean 51 months) 55 out of 63 patients continued to report improvement in symptoms. In most instances, the treated tendons remained thickened and the ultrasound appearances remained abnormal at final follow-up, although clinical results were comparable to those reported using more extensive procedures.
Other treatments
Brisement
Adhesive tendinopathy (paratenonitis) may result from chronic inflammation of the paratenon leading to fibrosis, in which adhesions form between the paratenon and the Achilles tendon. Brisement, also known as volume adhesiotomy or paratenon stripping, refers to the injection of a dilute local anaesthetic or saline solution under pressure into the tendon/peritenon interspace to break up adhesions. In our practice, we place the needle under ultrasound guidance just deep to the paratenon using a lateral approach. A 50:50 combination of normal saline and 1% lidocaine is gently injected until the inflamed paratenon is lifted off the tendon. Brisement injections have long been noted to provide relief for mild paratenonitis, especially with local anaesthetic. However, although there is anecdotal evidence of ultrasound-guided volume adhesiotomy, no studies specifically evaluating this technique can be found in the literature.
Aprotinin injection
Aprotinin is a generalised serine protease inhibitor that may reduce collagen degradation in tendinopathy by antagonising collagenases. A randomised controlled trial showed aprotinin injections to be efficacious in patellar tendinopathy [49]. Brown and associates [50] performed a double-blind placebo controlled trial in patients with Achilles tendinopathy, comparing aprotinin (30,000 kIU) + local anaesthetic injections and eccentric exercise with saline (0.9%) + local anaesthetic injections and eccentric exercises. Twenty-six patients (33 affected tendons) were treated with three blind peritendinous injections, each a week apart. In this study, aprotinin was not shown to offer any statistically significant benefit over placebo.
Low-dose heparin injection
Low-dose heparin has been used in the management of Achilles tendinopathies, with the aim of limiting the formation of adhesions. However, there is some evidence that there is no beneficial effect and it has been suggested that heparin, in itself, might cause a degenerative tendinopathy [51].
Implications of the existing literature
This review outlines the spectrum of ultrasound-guided interventional therapies that have been described for chronic Achilles tendinopathy (Table 1). Such minimally invasive treatments, which range from the simple (e.g. corticosteroid injections) to the more involved (e.g. sclerotherapy regimens), may prevent the need for surgery when conservative measures have failed. When performed by experienced practitioners, most of the therapies considered in this review have generally shown beneficial effects in small studies. However, at present only polidocanol sclerotherapy can be supported by data from randomised controlled trials. The other techniques considered in this review are currently limited by a lack of definitive trials, despite positive results being reported in small prospective studies or from anecdotal experience. In the United Kingdom, the National Institute for Clinical Excellence (NICE) reflected this position in recent guidelines on the use of autologous blood injections for tendinopathy, stating that “the current evidence on the safety and efficacy of autologous blood injection for tendinopathy is inadequate in quantity and quality [52]”.
Given the paucity of scientific evidence, the optimal therapeutic strategy has not yet been determined. Alfredson proposed a treatment algorithm for managing chronic Achilles tendinopathy based on the available evidence, with stepwise progression through conservative measures to polidocanol injections to surgery [7]. In patients presenting with chronic achillodynia, an initial period of eccentric rehabilitation training for at least 3 months is generally recommended, together with a combination of other conservative approaches, including orthotic measures (e.g. change of shoes, correction of malalignments) and non-invasive treatments (e.g. cryotherapy, shockwave lithotripsy) [15, 16, 19]. NICE has also recently published guidelines on extracorporeal shockwave therapy for Achilles tendinopathy and, whilst recognising that the treatment is safe, acknowledges that current evidence of the efficacy of the procedure is inconsistent [53]. In patients refractory to conservative treatments, ultrasound-guided sclerosing therapy is appropriate, particularly if tendon neovascularity is prominent. The Umeå researchers advocate small volumes (0.1–0.2 ml, in total 1–2 ml) of polidocanol (5 mg/ml) injected into areas of neovascularisation anterior to the Achilles tendon, followed by a graded rehabilitation programme. The rehabilitation after sclerosing injection treatment includes a period of rest (1–3 days), then gradually increasing tendon-loading activities during the first 2 weeks. Maximum tendon loading (jumping, fast runs, heavy strength activities) is only allowed after 2 weeks. A second injection is warranted after 6–8 weeks if there is residual pain or neovascularity. Since polidocanol is not widely available in some countries, alternative agents such as hyperosmolar dextrose have been used, although there is no evidential basis for this. Surgery provides a final option if these treatments fail.
Conclusion
Several minimally invasive interventional treatments are available for the management of chronic Achilles tendinopathy, some of which appear to have disease-modifying potential. Encouraging results have been reported in small non-randomised and randomised case series. Future studies of sufficient sample size and using validated clinical and radiological measures are warranted to compare efficacies of different ultrasound-guided therapeutic techniques in order to achieve a consensual management strategy.
References
Speed CA. Corticosteroid injections in tendon lesions. BMJ. 2001;323:382–6.
Paavola M, Kannus P, Järvinen TA, et al. Achilles tendinopathy. J Bone Joint Surg Am. 2002;84:2062–76.
Maffulli N, Barrass V, Ewen SW. Light microscopic histology of Achilles tendon ruptures. A comparison with unruptured tendons. Am J Sports Med. 2000;28:857–63.
Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology. 2006;45:508–21.
Maffulli N, Sharma P, Luscombe LJ. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–6.
Bude RO, Adler RS, Bassett DR. Diagnosis of Achilles tendon xanthoma in patients with heterozygous familial hypercholesterolemia: MR vs sonography. AJR Am J Roentgenol. 1994;162:913–7.
Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med. 2007;41:211–6.
Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608–15.
Aström M, Gentz CF, Nilsson P, et al. Imaging in chronic Achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol. 1996;25:615–20.
Kainberger FM, Engel A, Barton P, et al. Injury of the Achilles tendon: diagnosis with sonography. Am J Roentgenol. 1990;155:1031–6.
Archambault JM, Wiley JP, Bray RC, et al. Can sonography predict outcome in patients with achillodynia? J Clin Ultrasound. 1998;26:335–9.
Movin T, Kristoffersen-Wiberg M, Shalabi A, et al. Intratendinous alterations as imaged by ultrasound and contrast medium-enhanced magnetic resonance in chronic achillodynia. Foot Ankle Int. 1998;19:311–7.
Zanetti M, Metzdorf A, Kunert HP, et al. Achilles tendons: clinical relevance of neovascularisation diagnosed with power Doppler US. Radiology. 2003;227:556–60.
Bjur D, Alfredson H, Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320:201–6.
Gross ML, Davlin L, Evanski PM. Effectiveness of orthotic shoe inserts in the long-distance runner. Am J Sports Med. 1991;19:409–12.
Knobloch K, Grasemann R, Jagodzinski M, et al. Changes of Achilles midportion tendon microcirculation after repetitive simultaneous cryotherapy and compression using Cryo/Cuff. Am J Sports Med. 2006;34:1953–9.
Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. A randomized, double-blind, placebo-controlled trial. J Bone Joint Surg. 2004;86:916–22.
Hunter G. The conservative management of Achilles tendinopathy. Phys Ther Sport. 2000;1:6–14.
Fridman R, Cain JD, Weil Jr L, et al. Extracorporeal shockwave therapy for the treatment of Achilles tendinopathies: a prospective study. Am Podiatr Med Assoc. 2008;98:466–8.
Alfredson H, Lorentzon R. Chronic Achilles tendinosis: recommendations for treatment and prevention. Sports Med. 2000;29:135–46.
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg. 2005;87:187–202.
Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–90.
Maffulli N, Binfield P, Moore D. Surgical decompression of chronic central core lesions of the Achilles tendon. Am J Sports Med. 1999;27:747–52.
Paavola M, Kannus P, Orava S, et al. Surgical treatment for chronic Achilles tendinopathy: a prospective seven month follow up study. Br J Sports Med. 2002;36:178–82.
Speed CA. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol. 2007;21:333–47.
Eustace JA, Brophy DP, Gibney RP, et al. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56:59–63.
Zhingis C, Failla JM, van Holsbeeck M. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surg. 1998;23:89–96.
Reach JS, Easley ME, Chuckpaiwong B, et al. Accuracy of ultrasound guided injections in the foot and ankle. Foot Ankle Int. 2009;30:239–42.
Lucas PE. Imaging-guided interventions in the ankle and foot. Tech Foot Ankle Surg. 2008;7:176–87.
Shrier I, Matheson G, Kohl G. Achilles tendinitis: are corticosteroid injections useful or harmful? Clin J Sports Med. 1996;6:245–50.
Fredberg U, Bolvig L, Pfeiffer-Jensen M, et al. Ultrasonography as a tool for diagnosis, guidance of local steroid injection and, together with pressure algometry, monitoring of the treatment of athletes with chronic jumper’s knee and Achilles tendonitis: a randomized, double-blind, placebo-controlled study. Scand J Rheumatol. 2004;33:94–101.
Daftary A, Adler RS. Sonographic evaluation and ultrasound-guided therapy of the Achilles tendon. Ultrasound Q. 2009;25:103–10.
Fredberg U, Ostgaard R. Effect of ultrasound-guided, peritendinous injections of adalimumab and anakinra in chronic Achilles tendinopathy: a pilot study. Scand J Med Sci Sports. 2009;19:338–44.
Koenig MJ, Torp-Pedersen E, Qvistgaard L, et al. Preliminary results of colour Doppler-guided intratendinous glucocorticoid injection for Achilles tendonitis in five patients. Scand J Med Sci Sports. 2004;14:100–6.
Öhberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36:173–7.
Öhberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain—results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2003;11:339–43.
Alfredson H, Öhberg L. Sclerosing injections to areas of neo-vascularisation reduced pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13:338–44.
Lind B, Öhberg L, Alfredson H. Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14:1327–32.
Willberg L, Sunding K, Öhberg L, et al. Sclerosing injections to treat midportion Achilles tendinosis: a randomised controlled study evaluating two different concentrations of Polidocanol. Knee Surg Sports Traumatol Arthrosc. 2008;16:859–64.
Boesen MI, Torp-Pedersen S, Koenig MJ, et al. Ultrasound guided electrocoagulation in patients with chronic non-insertional Achilles tendinopathy: a pilot study. Br J Sports Med. 2006;40:761–6.
Chan O, O’Dowd D, Padhiar N, et al. High volume image guided injections in chronic Achilles tendinopathy. Disabil Rehabil. 2008;30:1697–708.
Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202.
Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg Am. 2003;28:272–8.
Liu YK, Tipton CM, Matthes RD, et al. An in situ study of the influence of a sclerosing solution in rabbit medial collateral ligaments and its junction strength. Connect Tissue Res. 1983;11:95–102.
Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:215–20.
Rabago D, Best TM, Zgierska AE, et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet rich plasma. Br J Sports Med. 2009;43:471–81.
James SL, Kaline A, Pocock C, et al. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med. 2007;41:518–22.
Testa V, Capasso GM, Benazzo F, et al. Management of Achilles tendinopathy by ultrasound-guided percutaneous tenotomy. Med Sci Sports Exerc. 2002;34:573–80.
Capasso G, Testa V, Maffulli N, et al. Aprotinin, corticosteroids and normosaline in the management of patellar tendinopathy in athletes: a prospective randomised study. Sports Exerc Inj. 1997;3:111–5.
Brown R, Orchard J, Kinchington M, et al. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2006;40:275–9.
McLaughlan GJ, Handoll HH. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;2:CD000232.
NICE. Autologous blood injection for tendinopathy. National Institute for Health and Clinical Excellence. 2009;IPG279.
NICE. Extracorporeal shockwave therapy for refractory Achilles tendinopathy. National Institute for Health and Clinical Excellence. 2009;IPG312.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wijesekera, N.T., Chew, N.S., Lee, J.C. et al. Ultrasound-guided treatments for chronic Achilles tendinopathy: an update and current status. Skeletal Radiol 39, 425–434 (2010). https://doi.org/10.1007/s00256-009-0873-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0873-9