Abstract
Objective
To evaluate the short-term and long-term effects of fluoroscopically guided caudal epidural steroid injection (ESI) for the management of degenerative lumbar spinal stenosis (DLSS) and to analyze outcome predictors.
Materials and methods
All patients who underwent caudal ESI in 2006 for DLSS were included in the study. Response was based on chart documentation (aggravated, no change, slightly improved, much improved, no pain). In June 2009 telephone interviews were conducted, using formatted questions including the North American Spine Society (NASS) patient satisfaction scale. For short-term and long-term effects, age difference was evaluated by the Mann–Whitney U test, and gender, duration of symptoms, level of DLSS, spondylolisthesis, and previous operations were evaluated by Fisher’s exact test.
Results
Two hundred and sixteen patients (male:female = 75:141; mean age 69.2 years; range 48 ∼ 91 years) were included in the study. Improvements (slightly improved, much improved, no pain) were seen in 185 patients (85.6%) after an initial caudal ESI and in 189 patients (87.5%) after a series of caudal ESIs. Half of the patients (89/179, 49.8%) replied positively to the NASS patient satisfaction scale (1 or 2). There were no significant outcome predictors for either the short-term or the long-term responses.
Conclusion
Fluoroscopically guided caudal ESI was effective for the management of DLSS (especially central canal stenosis) with excellent short-term and good long-term results, without significant outcome predictors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Degenerative lumbar spinal stenosis (DLSS) is a common disease in the elderly population, and one, which is rapidly increasing with the rise in the number of elderly persons [1, 2]. DLSS is defined as a narrowing of the spinal canal, the lateral nerve root canals or the intervertebral neural foramina due to progressive hypertrophy of any of the surrounding osteocartilaginous and ligamentous elements. It may result in neurogenic or vascular compression of the contents of the spinal canal at one or more levels [1, 3, 4]. DLSS results in low back and leg pain [5], and anatomic classification of DLSS refers to central canal stenosis, lateral recess stenosis or neural foraminal stenosis [1–3, 6]. DLSS is commonly seen on magnetic resonance imaging (MRI) scans of elderly populations, but the symptoms are not correlated with the degree of spinal stenosis [3].
Currently, the use of epidural steroid injection (ESI) for managing low back pain and radicular pain is increasing [7]. For the lumbar spine, there are three approaches for ESI: transforaminal, interlaminar, and caudal [6]. Caudal ESI is usually performed for central canal stenosis in DLSS [8]. Among nonsurgical methods for DLSS, ESI is used frequently to control more severe symptoms in patients who have responded poorly to drug therapy and who are either poor surgical risks or have refused surgery [5, 9].
There is still some controversy regarding the role of caudal ESI in DLSS [1, 6]. Some studies have reported that caudal ESI was not very effective in managing DLSS [10, 11], in which the caudal ESIs were performed without fluoroscopic guidance. According to Boswell et al. in their systematic review in 2007 [6] and Abdi et al. in their systematic review in 2005 [12], evidence of successful caudal ESI in the management of spinal stenosis is limited. However, in systematic reviews, the only article supporting the use of caudal ESI in DLSS management was by Ciocon et al. [9]. They reported that caudal ESI was effective in managing DLSS at up to 10 months’ follow-up.
By searching the PubMed database, we found only three studies on fluoroscopically guided caudal ESI for the management of DLSS [8, 13, 14]. Barre et al. [13] reported the long-term efficacy of fluoroscopically guided caudal ESI for DLSS, but this retrospective study was of patients who underwent caudal ESI from 1995 to 2002. Patients who had undergone ESI over a long time span might not have been a good study population for analysis of the percentage of good responders and outcome predictors. In the remaining two studies, the follow-up period was up to 12 months [8, 14]. To the best of our knowledge, there have been no reports on the long-term results of fluoroscopically guided caudal ESI over more than 2 years for large numbers of patients who underwent caudal ESI for DLSS in the same year. Our study was designed to analyze the data of patients who had undergone fluoroscopically guided caudal ESI for DLSS in the same year and to follow up those patients after more than 2 years by telephone interview.
The purpose of our study was to evaluate the short-term and long-term effects of fluoroscopically guided caudal ESI for the management of DLSS (especially central canal stenosis) and to analyze outcome predictors.
Subjects and methods
Patient selection
Institutional review board approval was obtained, and informed consent was not required for the retrospective review of medical records. All patients who had undergone at least one caudal ESI in 2006 were identified from a computerized database at our hospital. In 2006 a total of 707 caudal ESIs was performed in 554 patients in our department. Caudal ESI was considered in our center for patients whose symptoms originated from central canal stenosis at the lower lumbar level [13]. For neural foraminal stenosis, transforaminal ESI was usually chosen initially, and, for stenosis of the upper lumbar central canal, interlaminar ESI was the first choice of treatment. If there was no improvement after caudal ESI, either interlaminar or transforaminal ESI was chosen, according to the patient’s symptoms: transforaminal for unilateral and interlaminar for bilateral.
The inclusion criteria were (1) presence of low back pain or leg pain; (2) clear evidence of DLSS (especially central canal stenosis) on cross-sectional images such as those from computed tomography (CT) or MRI, which was identified by radiologic reports; (3) presence of follow-up medical records after caudal ESI. The exclusion criteria were (1) unclear description of symptoms; (2) absence of evidence of DLSS (especially central canal stenosis) on cross-sectional images; (3) absence of follow-up data.
Caudal ESI technique
All caudal ESIs were performed by two radiologists who had experience of more than 1,000 ESIs. A uniplanar (Integris Allura Xper FD 20; Philips) digital subtraction angiography unit was used for fluoroscopy. The patients were asked to lie prone on the fluoroscopy table. After sterilization, a 22G spinal needle was inserted through the sacral hiatus. Sacral cornua were used as landmarks to identify the sacral hiatus, as the sacral hiatus is bounded bilaterally by sacral cornua. The ideal point-of-needle penetration was the midline cranial area of the sacral hiatus. Anteroposterior (AP) and lateral views were frequently checked by rotation of the fluoroscopy tube during the procedure. After the needle had penetrated the sacral hiatus, it was advanced into the sacral canal to the S3 level, and contrast agent [Omnipaque 300 (iohexol, 300 mg iodine per milliliter); Amersham Health, Princeton, NJ< USA] was injected. The epidural space was identified by examination of the contrast pattern: an irregular margin, internal and heterogeneous small filling defects on anterior posterior view and no ventral layering of the contrast agent and no fluid level on lateral view. If the contrast agent was not spreading to the lower lumbar level, the needle was carefully advanced toward the cranial side. Intermittent injection of the contrast agent following a slow advance was repeated until the contrast agent had spread toward the lumbar epidural space (Fig. 1). Then, a mixture of 40 mg (1 ml) triamcinolone acetonide suspension [Tamceton (40 mg per ml); Hanall Pharmaceutical, Seoul, Korea] and 4 ml of normal saline solution and a mixture of 0.5 ml bupivacaine hydrochloride (0.5 ml/0.5%; Marcaine Spinal 0.5 % Heavy; AstraZeneca, Westborough, MA, USA] and 4.5 ml of normal saline solution were injected into the epidural space. The steroid mixture was injected first, and the anesthetic mixture was injected sequentially.
a, b Central canal stenoses are shown at the levels of L2/3, L3/4, and L4/5 in an 80-year-old man with pain in both legs. Because of degenerative scoliosis, a midsagittal view was not the same as the sagittal plane for L4/5 (a) and L3/4 (b). The patient received caudal ESI 13 times between February 2006 and March 2009. After each caudal ESI, he experienced no pain for approximately 2 months. On AP (c) and lateral (d) spot radiographs during caudal ESI, the spinal needle is located in the sacral canal, and the contrast agent is spreading into the epidural space of the lumbar spine
Before injection of the drug, the operator carefully checked if the needle had penetrated the dura, resulting in a subarachnoid injection. Subarachnoid injection of the contrast agent shows the contrast pattern seen in myelography: a smooth outline, ventral layering of the contrast agent with the fluid level, visualization of the root sleeve, and immediate movement of the contrast agent during injection. If the operator noted that there had been a subarachnoid injection, the procedure was stopped and the drugs were not injected. If local anesthetic were to be accidentally injected into the subarachnoid space, spinal anesthesia could result, leading to hypotension and transient paraplegia. To manage that, hydration is essential to maintain blood pressure.
Follow-up principle
Follow-up after caudal ESI was scheduled for 2 weeks. At follow-up, the outcome was measured on a 5-point patient satisfaction scale (no pain, much improved, slightly improved, no change, and aggravated) and was recorded on the medical chart. If there was no pain, observation without repeat ESI was considered.. If we decided on observation without further ESI, the next follow-up was scheduled for 2 months later. We advised patients to return to our hospital immediately, even before the routine follow-up date, if symptoms recurred. We also told the patients that they could postpone the scheduled follow-up if their symptoms were still tolerable and to return to our hospital when the symptoms recurred. In accordance with the guidelines of the American Society of Interventional Pain Physicians, ESI was performed a maximum of six times per year [15].
Review of medical records
Items for review from the medical records were identified by three spine radiologists, two orthopedic surgeons and two neurosurgeons in consensus. The items are shown in Table 1. The retrospective review of the patients’ medical records was conducted by one spine radiologist in April and May 2009.
Single or multiple levels of central canal stenosis and the presence of spondylolisthesis were determined by radiologic reports from MRI or CT. Response after an initial caudal ESI or an initial series of caudal ESIs was based on chart documentation and determined by the 5-point patient’s satisfaction scale (aggravated, no change, slightly improved, much improved, no discomfort). Management after the first caudal ESI was classified as follows: observation, repeat caudal ESI, interlaminar ESI, transforaminal ESI, operation, and others such as medication or physical therapy. An initial series of caudal ESIs was considered when the patients had repeat caudal ESI only. Among the patients who showed improvement (slightly improved, much improved, no discomfort) after an initial series of caudal ESIs, the recurrence was evaluated by chart documentation. We recorded the revisit date if there was any symptom recurrence as ‘revisit date due to symptom recurrence’. For the patients whose symptoms did not recur, the last follow-up date was recorded as ‘symptom controllable last follow-up date’ for the statistical analysis of symptom-free interval.
Categorization of the patients
The 5-point patient’s satisfaction scale was also regrouped as follows: (1) improvement (no pain, much improved, slightly improved) or not; (2) excellent improvement (no pain, much improved) or not. Those patients who showed improvement after an initial series of caudal ESIs but later had symptom recurrence were grouped as follows: less than 30 days; 31 ∼ 60 days; 61 ∼ 90 days; 91 ∼ 180 days; 181 ∼ 365 days; more than 366 days.
Telephone interviews
In June 2009 two researchers performed telephone interviews using the formatted questions shown in Table 2 under the supervision of a radiologist. Eight questions were asked for each patient, and the replies were recorded.
Statistical analysis for outcome predictors
To evaluate outcome predictors at short-term response, we divided the patients into two groups according to their response to an initial caudal ESI: improvement (slightly improved, much improved, no pain) or not (not improved or aggravated). For improvement at short-term follow-up, age differences were evaluated by Mann–Whitney U test, and other outcome predictors, such as gender, duration of symptoms (less or more than 1 year), level of central canal stenosis (single or multiple), spondylolisthesis (presence or absence), and previous operations, were evaluated by Fisher’s exact test.
To evaluate outcome predictors for long-term improvement, we grouped the long-term results into two, using the North American Spine Society (NASS) patient satisfaction scale: positive satisfaction (NASS 1 or 2) or negative satisfaction (NASS 3 or 4). Age differences were evaluated by Mann–Whitney U test. Other outcome predictors, such as gender, duration of symptoms (less or more than 1 year), level of central canal stenosis (single or multiple), spondylolisthesis (presence or absence), and previous operations, were evaluated by Fisher’s exact test.
To find the median symptom-free interval after improvement from an initial series of caudal ESIs, we used the Kaplan–Meir method. ‘Symptom controllable last follow-up date’ or ‘revisit date due to symptom recurrence’ were used for the statistical analysis of symptom-free interval. Instead of the recurrence date, we used the date of the revisit to our hospital due to symptom recurrence, because the onset of symptom recurrence was vague in most patients with DLSS.
Results
Pre-injection data
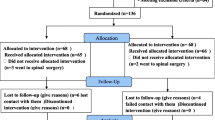
In total, 216 patients (male:female = 75:141; mean age 69.2 years; standard deviation 8.7 years, range 48 ∼ 91 years) were included in this study. Central canal stenosis was diagnosed by MRI in 187 patients and by CT alone in 29 patients. The level of central canal stenosis was single in 45 (45%) and multiple in 171 (79.2%). Among single level central canal stenosis cases, the L3/4 was affected in four patients, L4/5 in 39, and L5/S1 in two patients. Spondylolisthesis was seen in 68 patients (31.5%). Pain in both legs was present in 140 (64.8%) patients, pain in one leg in 74 (34.3%), and back pain only in two (0.9%). Symptom duration was less than 1 year in 125 patients (57.9%) and more than 1 year in 91 (42.1%). Previous operations had been performed on 20 patients (9.3%).
Response after an initial caudal ESI
The initial follow-up after an initial caudal ESI was done on average after 18.4 days (standard deviation 5 days; range 10 ∼ 31 days). Response according to the 5-point patient satisfaction scale is shown in Table 3. Improvement (including slightly improved, much improved, no pain) was seen in 185 patients (85.6%). Excellent improvement (including much improved, no pain) was seen in 103 patients (47.7%).
Response after an initial series of caudal ESIs
Caudal ESIs were repeated according to the patient’s response and willingness and administered at 2-week intervals. For the initial series of caudal ESIs, the injections were given three times to 16 patients (7.4%), twice to 21 patients (9.7%), and once to 179 patients (82.9%). After the initial series of caudal ESIs, follow-up was done at a mean 17.7 days (standard deviation 9.4 days; range 2 ∼ 77 days). The number of patients and their responses according to the 5-point patient satisfaction scale after caudal ESI are shown in Table 3. Improvement (including slightly improved, much improved, no pain) was seen in 189 patients (87.5%), and excellent improvement (including much improved, no pain) was seen in 119 patients (55.1%).
Follow-up and recurrence after a series of caudal ESIs
Among 189 patients who showed improvement after a series of caudal ESIs, 132 (69.8%) showed symptom recurrence. The recurrence dates were grouped and are shown in Table 4, which includes those groups that experienced no recurrence and no improvement. Among 132 patients, recurrence was noted in 38 (28.8%) in fewer than 60 days, in 53 (40.1%) between 60 and 180 days, and in 41 (31.1%) after more than 180 days. The median symptom-free interval for those patients who showed improvement after a series of caudal ESIs was 139 days [95% confidence interval (95% CI) 116.4 ∼ 161.54 days]. Of a total of 216 patients, 17.5% (38) reported fewer than 60 days of pain relief, 55.8% (151) reported more than 60 days of pain relief, and 12.4% (27) reported no pain relief. Thirty-six patients (16.7%) underwent operations after caudal ESI.
Total numbers of ESIs from 2006 to May 2009
The mean total number of ESIs was 3.7, ranging from 1 to 16. ESI was performed fewer than three times in 145 patients (67.1%). Most patients received ESI fewer than six times (190/216, 88%). These results are shown in Table 5.
Telephone interviews
Out of 216 patients, telephone interviews were possible with 179 patients (82.9%) (Tables 6, 7, 8, 9, 10 and 11). For the remaining 37 patients, telephone interviews were attempted but failed because of death (three patients) or change of telephone number (34 patients). The results are summarized in Tables 7, 8, 9, 10, 11 and 12. Out of 179 patients, 118 patients (65.9%) replied that caudal ESI was effective (completely improved, much improved, slightly improved) at that time and 71 patients (39.7%) replied that caudal ESI was still effective. Approximately half of patients (94/179, 52.5%) replied that they wanted to have repeat injections for similar symptom, and about two-thirds (115/179, 64.2%) replied that they would recommend caudal ESI to others. On the NASS 5-point patient satisfaction scale, approximately half the patients (89/179, 49.8%) replied positively to the scale (1 or 2), which meant that caudal ESI had met their expectations or that they would undergo the same procedure again for the same results.
Possible outcome predictors
Possible outcome predictors are shown in Tables 12 and 13. For short-term response after an initial caudal ESI, there were no significant outcome predictors. These results are summarized in Table 12. According to the NASS patient satisfaction scale at long-term follow-up, there was also no significant outcome predictor. These results are summarized in Table 13. Although there was no statistical significance, out of 17 patients who had been operated on, only five (29.4%) replied positively to the NASS patient satisfaction scale (1 or 2), but 84 (51.9%) out of 162 patients who had not been operated on before caudal ESI, replied positively to the scale (1 or 2).
Discussion
Our results showed that approximately 85% of patients showed improvement after an initial caudal ESI; approximately 55% of patients showed excellent improvement after a series of caudal ESI, about 70% of patients showed symptom recurrence, with 139 days’ median symptom-free interval, and about half of the patients expressed good satisfaction at long-term follow up.
Nonsurgical treatment for spinal stenosis varies, but it includes bed rest, nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, oral administration of corticosteroids, physical therapy, and epidural steroid injection [5]. According to a cohort study by Simotas et al. of nonsurgically treated patients with lumbar spinal stenosis [5], 3 years after treatment, nine of the 49 patients had undergone surgical interventions. Twelve of the 40 patients not operated on also had no or only mild pain. The authors concluded that aggressive nonoperative treatment for spinal stenosis remains a reasonable option [5].
Epidural steroid injection is used frequently to control more severe symptoms for patients who are either poor surgical risks or who have refused surgery [5, 9]. Corticosteroids have been shown to be able to block nociceptive C-fiber conduction and also inhibit prostaglandin synthesis [8, 16, 17]. Spinal stenosis is a condition in which there is usually an intermittent compression of the nerve roots, and this could lead to hyperemia, venous congestion, and, perhaps, leakage of neurotoxic substances. Therefore, the rationale for corticosteroid use in epidural injections for spinal stenosis is to impair prostaglandin synthesis, block nociceptive C-fiber conduction, and, possibly, alter the flow of nerve root blood and chemotoxic mediators [8].
Manchikanti et al. [14] reported that significant pain relief (≥50%) was demonstrated in 55–65% of the patients with spinal stenosis after the use of caudal ESI. Botwin et al. [8] reported that 65% of patients after 6 weeks, 62% after 6 months, and 54% after 12 months had achieved a successful outcome, reporting at least a >50% reduction in visual analog pain scores after caudal ESI for DLSS. These results were similar to ours, which showed excellent improvement (much improved or no pain) in 55.1% patients after a series of caudal ESIs.
According to a study by Delport et al. [18], of the 140 participants who underwent transforaminal or caudal ESI for DLSS, 32% reported more than 2 months of pain relief, 39% reported less than 2 months of pain relief, and 29% reported no relief from the injection. Twenty percent subsequently had surgery. Our results were better than those: 17.5% reported less than 2 months of pain relief, 55.8% reported more than 2 months of pain relief and 12.4% reported no pain relief. Sixteen percent subsequently had surgery.
According to the study by Barre et al. [13], a positive NASS satisfaction score was seen in 42% of the patients after caudal ESI. In our study, 49.8% replied positively to the NASS satisfaction score (1 or 2) in long-term follow up. These results are similar.
Barre et al. [13] reported that the concurrent presence of degenerative spondylolisthesis was the only variable which was found to have a significant positive correlation with successful outcomes after caudal ESI for DLSS. However, in our study, the presence of degenerative spondylolisthesis was not related to successful outcomes.
The results of caudal ESI for chronic low back pain without stenosis are poor [19]. Southern et al. [19] reported that at greater than 2-year follow-up, the efficacy of fluoroscopically guided caudal ESI in patients with chronic lumbar discogenic pain without spinal stenosis was poor.
For unilateral radiculopathy in DLSS, transforaminal ESI is known to be effective. According to a study by Botwin et al. [20], of 34 patients who had unilateral radiculopathy from DLSS, 75% had a successful long-term outcome after fluoroscopically guided lumbar transforaminal ESI. Therefore, transforaminal ESI and caudal ESI could be used interchangeably, according to the patient’s response and change of symptoms.
Fukusaki et al. [21] reported that ESI had no beneficial effect on the pseudoclaudication associated with spinal canal stenosis. However, according to our study, 60.4% of patients replied that their walking ability had improved after caudal ESI at their long-term follow-up interview. This is similar to the findings of the study by Botwin et al. [20], which reported that 64% of patients had achieved improved walking tolerance after caudal ESI.
Our study had some limitations. First, it was not a prospective study, and follow-up was not regularly designed. However, considering periodic symptom aggravation in DLSS, it is difficult to formulate a regular schedule for ESI. We think that it is better for patients to have ESI when symptoms appear or worsen. Second, long-term follow-up was determined by retrospective chart review and telephone interview. However, practicably, it was difficult for us to ask the patients to revisit the hospital only for the study if they had no pain or discomfort. Third, telephone interviews were conducted after 3 years, which could have resulted in memory errors by the patient.
In conclusion, fluoroscopically guided caudal ESI was effective for the management of DLSS (especially central canal stenosis), with excellent short-term and good long-term results, without significant outcome predictors for the short-term and long-term results.
References
Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80:1053–66.
Arbit E, Pannullo S. Lumbar stenosis: a clinical review. Clin Orthop. 2001;384:137–43.
Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008;17:679–85.
Postacchini F. Surgical management of lumbar spinal stenosis. Spine. 1999;24:1043–7.
Simotas AC, Dorey FJ, Hansraj KK, Cammisa F Jr. Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25:197–203; discussion 203–4.
Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7–111.
Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32:1754–60.
Botwin K, Brown LA, Fishman M, Rao S. Fluoroscopically guided caudal epidural steroid injections in degenerative lumbar spine stenosis. Pain Physician. 2007;10:547–58.
Ciocon JO, Galindo-Ciocon D, Amaranath L, Galindo D. Caudal epidural blocks for elderly patients with lumbar canal stenosis. J Am Geriatr Soc. 1994;42:593–6.
Cuckler JM, Bernini PA, Wiesel SW, Booth RE Jr, Rothman RH, Pickens GT. The use of epidural steroids in the treatment of lumbar radicular pain. A prospective, randomized, double-blind study. J Bone Joint Surg Am. 1985;67:63–6.
Rosen CD, Kahanovitz N, Bernstein R, Viola K. A retrospective analysis of the efficacy of epidural steroid injections. Clin Orthop. 1988;228:270–2.
Abdi S, Datta S, Lucas LF. Role of epidural steroids in the management of chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician. 2005;8:127–43.
Barre L, Lutz GE, Southern D, Cooper G. Fluoroscopically guided caudal epidural steroid injections for lumbar spinal stenosis: a restrospective evaluation of long term efficacy. Pain Physician. 2004;7:187–93.
Manchikanti L, Cash KA, McManus CD, Pampati V, Abdi S. Preliminary results of a randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 4—Spinal stenosis. Pain Physician. 2008;11:833–48.
Boswell MV, Shah RV, Everett CR, Sehgal N, McKenzie Brown AM, Abdi S, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician. 2005;8:1–47.
Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34:335–8.
Kantrowitz F, Robinson DR, McGuire MB, Levine L. Corticosteroids inhibit prostaglandin production by rheumatoid synovia. Nature. 1975;258:737–9.
Delport EG, Cucuzzella AR, Marley JK, Pruitt CM, Fisher JR. Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil. 2004;85:479–84.
Southern D, Lutz GE, Cooper G, Barre L. Are fluoroscopic caudal epidural steroid injections effective for managing chronic low back pain? Pain Physician. 2003;6:167–72.
Botwin KP, Gruber RD, Bouchlas CG, Torres-Ramos FM, Sanelli JT, Freeman ED, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81:898–905.
Fukusaki M, Kobayashi I, Hara T, Sumikawa K. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14:148–51.
Acknowledgement
We thank Hye Eun Han and Jung Min Choi for their assistance in telephone interview and chart review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.W., Myung, J.S., Park, K.W. et al. Fluoroscopically guided caudal epidural steroid injection for management of degenerative lumbar spinal stenosis: short-term and long-term results. Skeletal Radiol 39, 691–699 (2010). https://doi.org/10.1007/s00256-009-0860-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0860-1