Abstract
Objective
To determine the prevalence and appearance of magnetic resonance imaging (MRI) signal changes that occur in local bone marrow after radiation therapy (RT) and/or chemotherapy for extremity soft tissue sarcoma (STS).
Materials and methods
Seventy patients with primary STS at the level of a long bone who also had undergone pretreatment MRI and at least one post-treatment MRI of the tumor bed were identified. MRIs of each patient were retrospectively reviewed for new changes in marrow signal in the region of the tumor bed and for the morphology, relative signal intensities, heterogeneity, and progression or regression of changes over time.
Results
Focal signal changes in marrow were observed in 26/70 patients (37%) at a median of 9.5 months after RT and/or chemotherapy and diffuse changes in seven (10%) at a median of 8 months. Patients who received neither RT nor chemotherapy did not develop marrow changes. Mean RT doses in patients with changes and those without were 5,867 and 6,076 cGy, respectively. In most patients with focal changes, changes were seen in all sequences and were linear–curvilinear, patchy, or mixed at the level of the tumor bed. Predominant signal intensity of changes was between muscle and fat at T1WI and between muscle and fluid at fat-saturated T2WI or short tau inversion recovery. Most focal changes enhanced heterogeneously and increased or fluctuated in size over time.
Conclusion
Changes in MRI appearance of long bone marrow frequently are evident after combined RT and chemotherapy for STS and most commonly increase or fluctuate in size over time. These changes have various non-mass-like configurations and often show signal intensities similar to those of red marrow and thus should not be mistaken for metastases. The marrow changes might represent an early stage of gelatinous transformation of marrow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fatty replacement of bone marrow, as visualized at magnetic resonance imaging (MRI), is a well-recognized and common sequela of radiation therapy (RT) for treatment of tumors in or around the spine or bony pelvis [1–6]. Other alterations of bone marrow signal do occur at MRI after treatment of soft tissue sarcoma (STS) in an extremity, but they have received scant attention in the radiology literature [7–9]. We have observed post-treatment signal changes in the marrow in the general region where a STS had been present. These signal changes are often focal, and in some cases, they increase in size and number at subsequent follow-up imaging. A new or enlarging marrow lesion may raise concern for osseous metastasis, and because the development of such metastasis could significantly alter both the therapy and the prognosis for the patient, differentiating these post-treatment changes from metastasis at MRI is essential.
The aim of this study was to assess the prevalence and appearance of signal changes in marrow of long bones at MRI in the region of a STS in an extremity after treatment (including surgery, chemotherapy, and/or radiation therapy).
Materials and methods
Patients
This retrospective study was conducted after the need for informed consent was waived by our institutional review board. A computer search of the electronic medical records at our institution was performed to identify patients with a histopathologically proven, primary STS in an upper or lower extremity. The electronic records were then reviewed to select those patients whose tumors were at the level of a long bone in an extremity and who also had undergone both a pretreatment MRI examination and at least one post-treatment (i.e., after surgery, chemotherapy, or RT) MRI examination of the primary tumor bed. An MRI examination was considered evaluable based on criteria listed in “MRI examinations” (below).
Eighty such patients received the diagnosis of STS in an extremity during the period March 2000 through May 2007. Ten of the 80 patients were subsequently excluded from the study because the sarcoma was recurrent or because the tumor represented a metastasis, Hodgkin lymphoma, or dedifferentiation of a primary sarcoma, rather than a primary STS. The study group thus consisted of 70 patients [male, 36; female, 34; mean age, 47 years (range, 3–80 years)]. Nine of the patients were less than 20 years old.
The histologic types of STS were liposarcoma (n = 25), malignant fibrous histiocytoma (n = 9), myxofibrosarcoma (n = 7), synovial sarcoma (n = 6), Ewing family tumor (n = 4), leiomyosarcoma (n = 4), fibrosarcoma (n = 3), extraskeletal chondrosarcoma (n = 3), rhabdomyosarcoma (n = 3), malignant peripheral nerve sheath tumor (n = 3), neuroblastoma (n = 1), poorly differentiated round cell sarcoma (n = 1), and alveolar soft part sarcoma (n = 1).
Except for three patients (two with Ewing family tumors and one with rhabdomyosarcoma), all patients underwent radical surgical resection of the primary STS. The mean tumor size was 9.5 cm in greatest diameter. Nine STS were located in an upper extremity and 61 in a lower extremity. At initial diagnosis, eight (11%) of 70 patients had distant metastasis (in lung or lymph node). Twenty-three patients underwent only surgery, 20 underwent RT only, 23 underwent RT and chemotherapy, and four underwent chemotherapy only.
The mean RT dose, including any boost dose, in 41 patients was 5,808 cGy (range, 4,140–7,020 cGy); the doses administered to two patients at an outside institution were not available. RT and/or chemotherapy were given before and/or after surgical resection of the primary STS.
MRI examinations
MRI examinations, whether performed at our institution or elsewhere, were included in the study if they contained at least the following sequences, obtained in any plane: T1-weighted and either fat-saturated T2-weighted or short tau inversion recovery (STIR). For MRI examinations that met this minimum requirement, the following sequences were evaluated: T1-weighted, fat-saturated T2-weighted and/or STIR, and, when available, post-gadolinium T1-weighted. A total of 367 MRI examinations were reviewed (median of four examinations per patient; range, 2–16 examinations). At least one MRI for each patient included gadolinium-enhanced images. Two hundred eighty-seven of the examinations (78%) were performed at our institution, and 80 (22%) were performed at other facilities and entered into our PACS system.
The mean follow-up interval from pre-treatment baseline with MRI was 28.9 months (range, 4–74 months). The median interval from treatment to first follow-up MRI was 3.5 months (range, 0.5–22.6 months); the median interval from treatment to last follow-up MRI was 24.4 months (range, 3.8–76.5 months).
MRI interpretations
All MRI examinations of a given patient were retrospectively reviewed in temporal sequence by one of three pairs of attending radiologists who subspecialize in musculoskeletal tumor imaging; one radiologist (S.H.) was a member of each of the pairs to help maintain consistency of the approach to subjective classifications. The readers worked in consensus and were blinded with respect to the specific histologic diagnoses and treatment histories of the patients.
The following features were analyzed in each MRI examination for each sequence: the development and extent (focal or diffuse) of new changes in the marrow signal within the region of the tumor bed; the morphology of signal changes (linear-curvilinear, patchy, or nodular); the relative intensity of signal changes (compared to bone cortex, muscle, fat, and fluid); overall heterogeneity of lesion enhancement (homogeneous or heterogeneous) on available post-gadolinium images; and progression or regression of signal changes over time.
The extent of signal change was considered focal when present predominantly at the level of the tumor bed and diffuse when present throughout a much larger portion of the imaged bone, including above and below the tumor bed. The pattern of signal changes was classified as patchy if the changes showed a diffuse or poorly defined border and nodular if they had a well-defined round or oval shape. The maximum length of focal changes was measured in available coronal or sagittal sequences. Signal changes smaller than 0.5 cm were excluded to avoid overinterpreting minimal or equivocal findings (ditzels). When focal changes were seen in two bones on either side of a joint, the lengths of changes in both bones were added. When focal changes were seen in two parallel bones (e.g., tibia and fibula), the larger length was recorded to reflect the extent of the changes. A note was made if the STS invaded subjacent bone. The presence and location of any fracture was noted.
Using all the evaluated MRI sequences, the readers then recorded their overall assessment of the likelihood (yes, no, or indeterminate) that the observed marrow signal changes represented a metastasis.
Clinical data review
Demographic and clinical information for each of the 70 patients was recorded at a later time, including date of birth, patient sex, pathologic diagnosis, region of the primary tumor, initial tumor size, stage at initial diagnosis, dates of operations, whether periosteal stripping was performed, dates and types of RT (external beam and brachytherapy), total RT dose administered, administration of chemotherapy or granulocyte colony-stimulating factor (G-CSF), and local tumor recurrence.
Data analysis
Results of the MR image analysis were stratified according to the extent of marrow changes and type(s) of treatment received. The number of findings was insufficient to allow meaningful statistical comparisons across subgroups.
Results
Frequency and timing of marrow changes relative to therapy
Signal changes in marrow developed (subsequent to the baseline MRI) in 33 of 70 patients (47%; Figs. 1, 2, 3, 4, 5, 6, and 7, Table 1). The changes were focal in 26 patients (37%) and diffuse in seven (10%). Focal changes were most commonly seen in patients who received both RT and chemotherapy [15 of 26 patients (58%)]; diffuse changes also were most common in patients who received both therapies [five of seven patients (71%)]. Marrow changes developed in 20 of 23 patients (87%) who received both RT and chemotherapy but in only nine of 20 (45%) who received only RT.
Signal changes in marrow at MRI stratified by type of therapy. RT Radiation therapy, Chemo chemotherapy. Superscript 1 Two patients with Ewing family tumor did not undergo surgical resection; one patient received RT and chemotherapy, and the other received only chemotherapy. Superscript 2 One patient with rhabdomyosarcoma did not undergo surgical resection, and received RT and chemotherapy
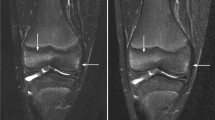
A 36-year-old woman with malignant peripheral nerve sheath tumor in posterior thigh and linear–curvilinear marrow changes. a Six months after surgical resection of tumor, sagittal T1-weighted image shows unremarkable signal intensity in marrow of femoral shaft. b, c New linear–curvilinear changes (arrows) are evident in femoral marrow at MRI obtained 1 year after patient had received chemotherapy and RT to surgical bed. The changes show intermediate to high signal intensity compared to that of muscle in b sagittal T1-weighted image, and high signal intensity in c sagittal fat-saturated T2-weighted image. Configuration and signal intensity of individual foci resemble those of avascular necrosis, with peripheral low-signal-intensity rim surrounding a central region of high T1 signal intensity. Asterisk Postoperative fluid collection
A 33-year-old man with myxoid chondrosarcoma in forearm and patchy marrow changes. a, b Patchy signal changes developed in marrow of radius at level of tumor (T) after preoperative chemotherapy, showing intermediate to high signal intensity (arrow) in a sagittal T1-weighted image and intermediate to high signal intensity in b sagittal fat-saturated T2-weighted image. Note that patient received G-CSF 1 month prior to this MRI and for an additional month afterward. These changes were not present on pretherapy MRI (not shown). c, d Patient received RT 8 months prior to these images, after the marrow changes had already occurred. Interestingly, this follow-up MRI (obtained 12 months after a, b) showed marked reduction in extent of marrow changes in radius in both c T1-weighted and d fat-saturated T2-weighted images. This change is possibly related to “healing” with reconstitution of fatty marrow
A 76-year-old woman with leiomyosarcoma in upper arm, metastatic to lung and liver. Nodular marrow changes first became evident 9 months after RT and 4 months after first chemotherapy (without G-CSF). Patient also has had cancers of lung, breast, colon, and bladder. a Axial T1-weighted image demonstrates nodular foci of intermediate to high signal intensity (arrow) in humeral head. b The nodular foci (arrow) show heterogeneously high signal intensity in axial fat-saturated T2-weighted image. These nodular foci were considered by the readers as being indeterminate for metastasis. Subsequent follow-up MRI did not show evidence of metastasis in this region
A 77-year-old man with high-grade liposarcoma of posterior thigh and fluctuations in size and extent of focal marrow changes. After surgical resection, the patient also received RT to the surgical bed. a Two years after RT, linear–curvilinear changes (arrowheads) are evident in marrow of femoral shaft in sagittal T1-weighted image. Asterisk Superior and inferior extents of the surgical bed. b Sagittal T1-weighted image obtained 6 months after a shows an interval decrease in size and extent of linear–curvilinear changes (arrowheads). c Sagittal T1-weighted image obtained 6 months after b shows interval development of signal changes (arrows) in femoral marrow distal to the previous changes (arrowheads)
An 80-year-old woman with myxoid liposarcoma of calf and infarct-like pattern in marrow. Patient received RT 33 months prior to this MRI; no chemotherapy was given. Coronal fat-saturated T2-weighted image demonstrates linear–curvilinear high and low signal changes (arrowheads) in marrow of distal femur and proximal tibia, mimicking double-line sign of bone infarct. Additional small nodular changes (arrows) are evident in marrow of tibial spine and lateral portion of proximal tibia. Myxoid liposarcoma subsequently recurred locally in soft tissues of ipsilateral thigh, but no bone metastasis developed in these regions of marrow changes
A 36-year-old woman with malignant peripheral nerve sheath tumor in posterior thigh (same patient as in Fig. 2) and heterogeneous enhancement of marrow. a, b Axial fat-saturated T1-weighted images obtained a before and b after intravenous administration of gadolinium contrast material show heterogeneous enhancement (arrow) of anterior portion of femoral marrow in b. Interpreted in isolation, this image could result in misdiagnosis of metastasis; however, correlation with the longitudinal images (Fig. 2b) allows confident diagnosis of focal changes due to treatment
In two of 26 patients (8%), focal marrow changes remained unchanged over time. In five patients (19%), focal marrow changes decreased and then remained unchanged, and in eight patients (31%), focal marrow changes increased over time. In eight patients (31%), focal marrow changes fluctuated over time (Fig. 5). Three patients (12%) did not undergo additional follow-up MRI examinations after marrow changes were first observed.
The mean RT dose in the group of patients with marrow changes (mean age, 50 years) was 5,867 cGy (range, 4,140–6,660 cGy) and in the group without marrow changes (mean age, 56 years), 6,076 cGy (range, 5,000–7,020 cGy). All patients who underwent RT received external beam radiation [mean dose, 5,938 cGy (range, 4,140–7,020 cGy)]; brachytherapy was not given in any patient.
The lowest RT dose that resulted in focal marrow changes was 4,140 cGy (in a patient who also received chemotherapy), and the minimum dose for diffuse changes was 5,040 cGy. The lowest RT dose that resulted in focal marrow changes in a patient who did not receive chemotherapy was 5,000 cGy.
Periosteal stripping was performed during tumor resection in two patients; no marrow changes were seen at subsequent MRI. G-CSF was administered within a year prior to marrow changes seen at MRI in five of 26 patients (19%) with focal marrow changes and in five of seven patients (71%) with diffuse changes (p = 0.015, Fischer exact test). One patient developed focal marrow changes, and two patients developed diffuse changes before the administration of G-CSF.
MRI features of marrow changes
In 25 of the 26 patients (96%) with focal marrow changes, the changes were seen in all sequences (T1-weighted, fat-saturated T2-weighted, and/or STIR). They appeared linear–curvilinear, nodular, patchy, or a mixture of these at the level of the tumor bed (Figs. 2, 3, and 4; Table 2). The predominant signal intensity was between that of muscle and fat in T1-weighted sequences and between that of muscle and fluid in fat-saturated T2-weighted or STIR sequences. One patient who had received RT showed an infarct-like pattern in a 33-month follow-up MRI (the earliest obtained after the baseline examination; Fig. 6). In fact, the configuration and signal intensity of individual foci of focal marrow changes often resembled those of avascular necrosis, with a peripheral low-signal-intensity rim surrounding a central region of high T1 signal intensity (Fig. 2b). No gross difference in the MRI appearance of the marrow changes was evident based on the type(s) of treatment(s) received.
Around joints, the changes occasionally were present in two bones. Of patients who had received gadolinium contrast material, the marrow in 22 of 25 (88%) with focal changes showed heterogeneous enhancement (Fig. 7) and, in three (12%), showed no enhancement. The median maximum length of focal marrow lesions was 5 cm on T1-weighted sequences (range, 1–17 cm) and 6.5 cm on fat-saturated T2-weighted or STIR sequences (range, 1–21 cm).
In one patient with diffuse marrow change (fatty replacement), the initial tumor focally invaded the subjacent proximal femur at MRI. Five of eight patients (63%) with distant metastasis at presentation showed focal marrow changes, two (25%) had diffuse changes, and one (13%) showed no change.
Focal marrow changes in one patient (Fig. 4) and diffuse changes in another patient were judged to be indeterminate for metastasis by the readers; all other focal and diffuse marrow changes were considered likely to be benign. No marrow changes were considered to be clearly compatible with metastasis.
Clinical course
Tumor recurred locally in two patients: in one with high grade myxofibrosarcoma of the thigh and in another with myxoid liposarcoma in the knee (Fig. 6). Ipsilateral inguinal adenopathy recurred in a patient with monophasic synovial sarcoma of the posterior proximal thigh. No bone metastasis developed in the region of the initial STS in any of the 70 patients.
Insufficiency fractures developed in four patients (6%) at a median time of 18.5 months (range, 8–47 months) after therapy; two of those patients had received RT, and the other two had received both RT and chemotherapy.
One patient with diffuse marrow changes at MRI subsequently developed acute myelogenous leukemia, proven at bone biopsy. Interestingly, the diffuse marrow changes had decreased at the last follow-up MRI, obtained 2 weeks before the bone biopsy.
Discussion
The results of our study show that, after treatment of STS with RT, chemotherapy, or a combination of both, changes are commonly evident at MRI in the marrow of extremity long bones; focal changes were evident in 37% of patients and diffuse changes in 10%. A variety of other changes in irradiated osseous structures have been reported at radiologic examination, including disturbances in growth, osteonecrosis, pathologic fracture, and radiation-induced sarcomas [5, 7]. Those other changes have well known, distinct clinical and radiologic features that are readily distinguishable from the subtler changes manifested in patients treated for STS of the extremity.
Unlike diffuse fatty replacement of marrow in the spine, focal marrow changes in the extremity manifest in various shapes. The configurations of most of the changes were linear or curvilinear, patchy, or a mixture of these, rather than nodular (Figs. 2, 3 and 4). This variable appearance is in contrast to the band of peripheral, intermediate signal changes in the spine observed in the late MRI pattern after RT [2]. The signal intensity of the changes in our study was predominantly intermediate compared to that of muscle at T1-weighted imaging and intermediate to high in signal at fat-saturated T2-weighted or STIR imaging, resembling the signal of red marrow.
It was also interesting to observe the wide range of time intervals for changes to develop (1–42 months for focal changes; Table 1) and the fluctuation in size of focal changes over time (Fig. 5). Some portion of this apparent variability undoubtedly is the result of the nonstandardized time interval between imaging examinations. Focal marrow changes most often increased or fluctuated in size over time. In the few cases in which marrow changes resolved (Fig. 3), it is possible that they “healed,” with reconstitution of fatty marrow.
Focal and diffuse marrow changes were most commonly observed after RT, whether or not chemotherapy was also given; this finding may be at least partly because the majority of patients (43/70, 61%) received RT (Fig. 1). Only four patients were treated with chemotherapy but no RT; yet, all four developed marrow changes (two focal and two diffuse). Of note, no marrow changes were observed in the 23 patients (33% of the 70 patients) who received neither RT nor chemotherapy. Marrow changes developed in 20 of 23 patients (87%) who received both RT and chemotherapy but in only nine of 20 patients (45%) who received only RT. These findings strongly suggest (but do not prove) a causative role of therapy—particularly the combination of RT and chemotherapy—in the development of marrow changes.
Focal changes were first observed at an earlier median time in patients who had received either RT or chemotherapy (9 and 1 month, respectively) than they did in patients who had received both therapies (13 months). The median age of patients who had received both RT and chemotherapy was younger (42 years) than of those who had received either RT (50 years) or chemotherapy (50.5 years). The younger age of the combined-therapy group could have played a role in delaying the onset of focal changes, as the marrow of younger patients is known to have a better ability to regenerate and repair [10]. However, because follow-up MRI examinations were not obtained at standardized, short time intervals, the marrow changes could have appeared earlier than when first observed at MRI.
Although no histopathologic correlation was obtained for the marrow changes in the patients included in our study, we postulate that the observed focal changes might represent an early stage of radiation-induced osteonecrosis [5]. Furthermore, after the study was completed, we encountered a patient with malignant peripheral nerve sheath tumor of the thigh who underwent preoperative radiation therapy. The follow-up MRI (Fig. 8) showed new femoral marrow changes identical in appearance to those observed in our study population. The patient subsequently underwent hip disarticulation, and histopathologic examination of that region of the femoral marrow showed marked adipocyte atrophy with accumulation of intercellular myxoid material and scattered histiocytes. No features of marrow infarction or necrosis were seen, and no tumor cells were evident. The histopathologic appearance was similar to that of “gelatinous (or serous) transformation” of marrow, which is typically seen in AIDS, starvation, anorexia nervosa, cachexia, alcoholism, and other disorders [11–13]. However, the MRI features reported in gelatinous transformation of marrow are generally diffuse because they are caused by systemic processes and consist of low to intermediate signal intensity on T1-weighted images and very high signal intensity on T2-weighted images [12, 13]. In contrast, the marrow changes observed at MRI in our study and in the patient in Fig. 8 did not resemble the extensive, diffuse serous changes reported in anorexia nervosa [12, 13]. Perhaps the marrow changes observed after therapy for STS are a less-advanced or less-extensive stage of such gelatinous transformation; further study is needed to elucidate the exact nature of the underlying pathologic process.
A 38-year-old man with malignant peripheral nerve sheath tumor in anterior thigh, who received 5,000 cGy of preoperative radiotherapy. Tumor enlarged rapidly during therapy, largely due to necrosis, ultimately necessitating hip disarticulation. a Pretreatment sagittal T1-weighted MR image shows unremarkable marrow in femur. T Tumor, b, c sagittal T1-weighted MR images obtained 9 days before and 25 days after the end of RT, respectively, show new and increasing focal marrow changes (arrows) at the level of the tumor. d Histopathologic examination of the region of abnormal marrow in b, c showed marked adipocyte atrophy, with accumulation of intercellular myxoid material and scattered histiocytes. No infarcted or necrotic marrow was evident. No tumor cells were seen. The histopathologic appearance is that of gelatinous (serous) transformation of marrow (hematoxylin and eosin, ×100)
Diffuse changes were less common than focal changes. They often occurred in patients who had received chemotherapy, suggesting that they may be caused by red marrow reconversion related to recovery of marrow after therapy-induced suppression and/or to the administration of hematopoietic growth factors (such as G-CSF) given to permit intensification of chemotherapy and to limit the occurrence of chemotherapy-associated infection.
No clear correlation between the RT dose and the development of marrow changes was observed; in fact, the mean RT dose was slightly higher in patients without marrow changes (6,076 cGy) than in those with marrow changes (5,867 cGy). Although the chronological evolution of changes in marrow after RT and chemotherapy has been extensively studied in the spine and pelvis, information about focal changes at MRI in the long bones of the extremities after therapy has received scant attention [7–9].
Our study has several limitations. The study was retrospective, resulting in non-uniform MRI technique (such as image planes, section thickness, pulse sequence parameters, and field of view). Not all MRI examinations included gadolinium-enhanced images, but the marrow changes also were visible on all other series in nearly every patient. The shape of focal marrow changes and the extent of focal and diffuse changes were difficult to classify in some patients because of variability in planes of imaging and section thickness; however, we do not believe that the results or message of the study are substantially affected by this issue. The timing of follow-up MRI examinations was variable, limiting analysis of the time course for development of marrow changes. The various parameters of the radiation therapy (including the dose and its distribution) were tailored to the particular tumor location for each patient; as a result, a wide range of doses was delivered to the adjacent bones, undoubtedly affecting the incidence and severity of any resultant marrow changes at MRI. The patients underwent a variety of chemotherapy regimens, which could affect the frequency of marrow changes observed. Because the marrow changes in the study population were not biopsied (due to lack of a clinical indication for such), we can only speculate about their nature. Although readers in the study were blinded to whether a patient had received any therapy, they might have deduced that a patient had received radiation therapy if typical high-signal changes at fat-saturated T2-weighted or STIR imaging were evident in the soft tissues and subcutaneous fat; however, similar changes also may result from extensive surgery. These particular readers, working in a tertiary care cancer center, already were quite aware of the occurrence of these changes in marrow at MRI based on experience and thus were less likely than a less-experienced interpreter to consider the changes suspicious for metastasis.
In conclusion, changes in the appearance of marrow in the long bone of an extremity frequently are evident at MRI after chemoradiation for STS in that extremity and commonly increase or fluctuate in size over time. These changes have various non-mass-like configurations and show signal intensities similar to those of red marrow and, therefore, should not be confused for interval development of osseous metastases. Given that local bone metastases are quite uncommon in STS of the extremities, it is important for radiologists to avoid “overcalling” these marrow changes. The marrow changes observed at MRI after therapy for STS might represent an early stage of gelatinous transformation of marrow, but further study is needed to elucidate the underlying pathologic process.
References
Ramsey RG, Zacharias CE. MR imaging of the spine after radiation therapy: easily recognizable effects. Am J Roentgenol 1985;144: 1131–1135.
Stevens SK, Moore SG, Kaplan ID. Early and late bone-marrow changes after irradiation: MR evaluation. Am J Roentgenol 1990;154: 745–750.
Yankelevitz DF, Henschke CI, Knapp PH, Nisce L, Yi Y, Cahill P. Effect of radiation therapy on thoracic and lumbar bone marrow: evaluation with MR imaging. Am J Roentgenol 1991;157: 87–92.
Blomlie V, Rofstad EK, Skjonsberg A, Tvera K, Lien HH. Female pelvic bone marrow: serial MR imaging before, during, and after radiation therapy. Radiology 1995;194: 537–543.
Mitchell MJ, Logan PM. Radiation-induced changes in bone. RadioGraphics 1998;18: 1125–1136.
Otake S, Mayr NA, Ueda T, Magnotta VA, Yuh WTC. Radiation-induced changes in MR signal intensity and contrast enhancement of lumbosacral vertebrae: do changes occur only inside the radiation therapy field? Radiology 2002;222: 179–183.
Mullen LA, Berdon WE, Ruzal-Shapiro C, et al. Soft-tissue sarcomas: MR imaging findings after treatment in three pediatric patients. Radiology 1995;195: 413–417.
Kransdorf MJ, Peterson JJ, Bancroft LW, Buskirk SJ, O’Connor MI. Extremity marrow changes following radiation therapy for soft tissue sarcoma [abstract]. Skeletal Radiol 2006;35: 333–334.
Kransdorf MJ, Murphey MD. Imaging of soft tissue tumors. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2006: 63–64.
Sacks EL, Goris ML, Glatstein E, Gilbert E, Kaplan HS. Bone marrow regeneration following large field radiation: influence of volume, age, dose, and time. Cancer 1978;42: 1057–1065.
Böhm J. Gelatinous transformation of the bone marrow: the spectrum of underlying diseases. Am J Surg Pathol 2000;24: 56–65.
Vande Berg BC, Malghem J, Devuyst O, Maldague BE, Lambert MJ. Anorexia nervosa: correlation between MR appearance of bone marrow and severity of disease. Radiology 1994;193: 859–864.
Vande Berg BC, Malghem J, Lecouvet FE, Lambert M, Maldague BE. Distribution of serouslike bone marrow changes in the lower limbs of patients with anorexia nervosa: predominant involvement of the distal extremities. Am J Roentgenol 1996;166: 621–625.
Acknowledgment
We thank Murray Brennan, MD, for his insightful observations and discussions, and Mark Edgar, MD, for providing the pathologic correlation in Fig. 8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, S., Lefkowitz, R., Landa, J. et al. Local changes in bone marrow at MRI after treatment of extremity soft tissue sarcoma. Skeletal Radiol 38, 11–19 (2009). https://doi.org/10.1007/s00256-008-0560-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-008-0560-2