Abstract
Introduction
Sacral insufficiency fractures are a well recognised cause for low back, buttock and groin pain in the elderly. However, over a 4 year period, four patients have presented with symptoms of cauda equina syndrome, who were found on investigation to have acute sacral insufficiency fracture without any other aetiological spinal abnormality.
Patients and method
Four patients who presented to the spinal surgeons of our institution with symptoms of cauda equina syndrome were referred for spinal MR. Sagittal and axial T1 and T2 weighted turbo spin echo sequences of the lower thoracic and lumbar spine were performed on all patients. Subsequent studies included MR of the sacrum supplemented where appropriate by CT and technetium MDP bone scintigraphy.
Results
No evidence of a compressive lesion of the lower thoracic or lumbar spine was present in any of the four patients. Dedicated MR examination of the sacrum in these patients revealed unilateral acute insufficiency fractures involving zone 1 from S1 to S3 extending from the sacro-iliac joint to the lateral margin of the sacral foramen. There was no evidence of compression of the sacral nerve roots. The possible mechanism for the symptomatic presentation is discussed.
Conclusion
Sacral insufficiency fractures should be excluded in elderly or osteoporotic patients presenting with cauda equina syndrome who have no evidence of compression in the thoraco-lumbar MR studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sacral insufficiency fractures, first identified in the early 1980s [1, 2], are a well described cause of low back, buttock and groin pain in elderly patients [3]. They are difficult to diagnose as there is usually no significant history of trauma, and radiographs of the sacrum do not demonstrate them adequately [4]. Delayed neurologic complications during the course of conservative treatment in patients with insufficiency fractures of the sacrum have been rarely reported [5]. Over a period of 2 years, four patients, who were referred with a clinical diagnosis of cauda equina syndrome, were found to have acute sacral insufficiency fractures, without any other spinal abnormality identified on MR of the thoracolumbar spine to explain the clinical presentation. We describe the imaging features, possible pathomechanisms of neurological dysfunction and management implications of these four patients.
Materials and methods
The four cases were prospectively identified over a 2 year period. All were female patients with ages of 45, 58, 72 and 80 years. They had all been previously diagnosed as having osteoporosis and presented with a short history of back and buttock pain, with bowel and bladder symptoms and were referred to this department with suspected cauda equina syndrome. The 58 year old patient suffered from rheumatoid arthritis and was on steroid therapy. Magnetic resonance (MR) of the lower thoracic and lumbar spine on a 1.5 T system with sagittal and axial T1 and T2 weighted turbo spin echo sequences was performed on all patients. Supplementary imaging included radiography, radionuclide bone scan, MR and computed tomography (CT) of the sacrum. The site of the insufficiency fracture was evaluated according to the zone of the sacrum [6].
Zone 1 is the ala lateral to the neural elements; zone 2 transects the sacral foramen; zone 3 involves the central canal of the sacrum.
Results
No significant compressive lesion of the dural sac was demonstrated on routine lumbar spine MR examination in any of the four patients. However, dedicated MR examination of the sacrum in these patients revealed unilateral acute sacral insufficiency fractures. The common feature of all these fractures was involvement of zone 1 from S1 to S3, extending from the sacroiliac joint to the lateral margin of the sacral foramen. There was no significant cortical break, translation or angulation, and no haematoma either sub-periosteal in the canal or in the foramen. There was no obvious compression on the emerging sacral nerve roots. All four responded to conservative management and gained full recovery.
Clinical findings are summarised in Table 1. Case 2 suffered form rheumatoid arthritis and had been treated with steroids. Cases 3 and 4 had multiple vertebral osteoporotic fractures with no spinal canal compression.
Detailed illustrative case report
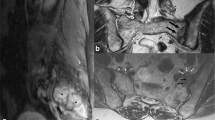
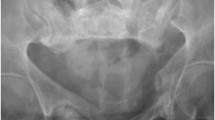
The 72-year-old woman who was on steroid therapy (8 mg a day for 8 years) for polymyalgia rheumatica, gave a 4 day history of pain in the bottom of the back. Sitting and getting up was extremely painful and she could only walk with difficulty if she kept her back straight. There was no history of trauma. Her past history included stent insertion for thoracic aortic aneurysm, osteoporosis, hypothyroidism and angina pectoris. The pain increased in severity, and micturition and bowel evacuation became increasingly difficult over the next 4 days. She had paraesthesia in the feet and ankles with weakness in both legs. On examination, her vital signs and general examination were within normal limits. Neurologically, bilateral ankle dorsiflexion and plantar flexion were weak and sensation diminished to pinprick below L3 and in the saddle area, more on the left than the right. Vibration sense was lost to the level of the ribs. A compressive spinal lesion was suspected and MR of the spine was urgently performed. Although multilevel degenerative changes in the intervertebral discs and facet joints were present in the lumbar spine, there was no significant compressive lesion of the cauda equina (Fig. 1). In the most distal axial images through the L5/S1 disc, marrow oedema was noted in the right side of the sacrum. A separate MR examination of the sacrum was performed. On MRI, oedema was confirmed in zone 1 of the right sacral ala from the promontory to the S3 level with an ill defined adjacent low T1/T2 fracture line entering the sacroiliac joint (Fig. 2). Radiograph of the sacrum showed ill defined sclerosis of the lateral aspect of the right sacral ala with blurring of the arcuate lines, suggestive of an insufficiency fracture. This was confirmed on CT scan which demonstrated increased sclerosis within the right half of the sacrum, as well as along the subchondral outline of the right sacroiliac joint (Fig. 3). Vacuum phenomenon was noted within the sacroiliac joint with minimal sclerosis on the iliac aspect of the sacroiliac joint. The cortical outline and foraminal contours of the sacrum were preserved. The patient was treated conservatively by bed rest, with gradual recovery of bladder and bowel control, and resolution of lower limb neurological signs over a 4 week period.
Discussion
Polyradicular symptomatology of the cauda equina syndrome results from a dysfunction of sensory, motor and autonomic components of the lumbosacral nerve roots, presenting as low back pain, saddle anaesthesia, bilateral sciatica, motor weakness of the lower extremities or chronic paraplegia and bladder dysfunction [7]. A recent review of the cauda equina syndrome [8] covered compressive and non compressive causes in the lumbar spine, but did not highlight sacral insufficiency fractures in the list of aetiologies. However, cauda equina syndrome in high energy sacral trauma is well documented [6, 9–11], but there are no published reports of cauda equina as a presenting feature of sacral insufficiency fractures. Delayed neurological complications in patients with diagnosed sacral insufficiency fractures have been rarely reported. In their review of the literature, Finiels et al. [5] reviewed the literature and identified 11 cases of neurological deficit in 493 patients of sacral insufficiency fractures and added three of their own, giving a 2.8% incidence of neurological compromise. They found no differences between patients with and without neurological complications as regards age (mean age 83 years; range 75–92 years), sex distribution (female to male ratio of 11:1), presence of trivial trauma (5 cases), or risk factors for bone insufficiency (osteomalacia in two cases and pelvic irradiation in one case) [5]. The presenting feature in all of these cases was low back or perineal pain and/or referred pain to the buttock or hips. In contrast to our series, in none of these cases was cauda equina syndrome identified as the presenting clinical feature, and neurological symptoms were delayed after initial presentation in five cases [5]. In decreasing order, the most common neurological abnormalities included sphincter dysfunction (11 cases), distal paraesthesia (7 cases) and leg weakness (5 cases) [5]. The main objective neurological findings were decreased anal sphincter tone and hypaesthesias in the distribution of the sacral nerve roots [5].
The sacral spinal canal is capacious with ample space for the cauda equina. The L4 and L5 lumbar anterior rami combine anterior to the middle of sacral ala just superior to the S1 foramen, before continuing laterally on the surface of the ala close to the sacroiliac joint [12]. The anteriorly exiting S1 roots occupies up to one-third of the foraminal exit area, with progressive decrease to the S4 nerve root which occupies one-sixth of the anterior foraminal area [7]. Each of the large four sacral ventral rami are closely adherent to the anterior osseous surface of the sacrum and cover almost all of it lateral to the foramen from the S2 foramen in a distal direction [12]. Despite this close association, nerve root deficit is a rarely reported complication of sacral insufficiency fracture.
L5 and S1 nerve root injuries are readily clinically identified, but injury to roots S2 through S5 are frequently overlooked at neurological examination due to the lack of obvious sensorimotor signs in the lower limbs [7]. The S2 root plays a major role in genital, bladder and anorectal function as a main constituent of the pudendal nerve. This nerve also receives a contribution from the ventral rami of S3 and S4, and innervates the striated musculature that forms the external urethral and external anal sphincters. Sphincter disturbances can be caused by unilateral sacral disease, as shown by clinical studies of spinal dysraphism and in patients undergoing major resection of sacral neoplasms [7]. It is therefore possible that the incidence of sacral nerve root deficit in insufficiency sacral fractures is higher, but not recorded clinically unless a detailed neurological examination is carried out.
Co-ordinated emptying of the bladder and rectum depends also on input from the autonomic nervous system [7]. The pelvic splanchnic nerves, which arise as fine branches in the ventral rami of S2 through S4 [7], are distributed to the bladder and rectum and their afferent fibres are mainly responsible for the normal awareness of vesical and ampullary filling, whereas their efferent fibres initiate detrusor and rectal contraction. Local sympathetic input to the inferior hypogastric plexus derives from the small sacral splanchnic nerves arising from the S2 and S3 sympathetic ganglia, which lie just medial to their respective ventral foramina [7]. The sympathetic afferents are responsible for pain and thermal sensibilities, and the sympathetic efferent limb causes contraction of the internal urethral and anal sphincters, and inhibits contraction within the muscular walls of the associated viscus [7]. Disruption of this neural balance by the sacral fracture causing sphincter disturbances was the most common problem encountered in the delayed complications previously reported in sacral insufficiency fractures [5].
Imaging of patients presenting acutely with the cauda equina syndrome focuses on the thoracolumbar spine using MR to exclude compressive aetiologies like herniated lumbosacral discs, spinal stenosis and neoplastic lesions. Only the central portion of the sacrum is seen on the sagittal MR sequences that are programmed to examine the spinal canal. The sacral ala are not examined and only the most superior aspect is included in the axial L5/S1 disc images. None of our four cases who clinically presented with acute cauda equina syndrome demonstrated any significant pathology on imaging of the lumbar spine and sacral insufficiency fracture was revealed only on dedicated imaging of the sacrum. Given the close relationship of the sacrum with the sensorimotor and autonomic nervous system, it is imperative to image the sacrum in cases of acute cauda equina syndrome, if no cause is established clinically, biochemically or on lumbar imaging.
The characteristics of sacral osteoporotic fractures do not seem to reliably predict the risk of neurological complications and the pathophysiology of neurological complications in insufficiency fractures in the elderly remain unclear [5]. Finiels et al. [5] suggest that stretching of nerves is unlikely, because of the absence of significant trauma, except in those cases with displacement, as in the case report by Jones [13]. They speculate that compression of the nerve root at the foramina due to a bone fragment or haematoma could theoretically occur in zone 2 fractures, suggesting it as a possible cause in Lock and Mitchell’s case report [14]. Finiels et al. [5] also suggest the possibility of the extra-spinal segment of the nerve root being compressed anterior to the sacrum. Although they had no case to substantiate this postulation at the time of their publication, a case report of left L5 radiculopathy, secondary to a left sacral stress fracture, with periosteal reaction involving the nerve root anterior to the sacral ala has been subsequently published [15]. In their two cases of delayed onset of neurological manifestation, Stabler et al. noted raised erythrocyte sedimentation rate and cerebrospinal fluid protein which suggested an inflammatory or post-inflammatory process [16]. However, they ascribed the cause to presumably mechanical effects on the nerve roots. It is not clear from the report if these patients had received previous radiation therapy.
The cauda equina syndrome presentation in our four cases is also unique in that the fractures were unilateral in zone 1, with no evidence of nerve root traction, avulsion or compression. There was no involvement of the sacral foramen or spinal canal, nor any haematoma or periosteal reaction. However, the fracture did extend to the sacroiliac joint. In their experimental study on rats, Murata et al. have shown that the sacroiliac joint has a dual innervation at the ventral aspect [17, 18]. Extension of the insufficiency fracture to the sacroiliac joint could theoretically cause neurological dysfunction through either periradicular oedema, haemorrhage or inflammation of the autonomic nervous pathway. The possible pathomechanisms are summarised in Table 2.
The vast majority of sacral insufficiency fractures probably have no untoward neurological complications. When present, however, although initially alarming, the clinical course of these patients is usually uneventful with a good recovery and outcome. Given the small number of reported cases, paucity of detail in the reports and uncertainty regarding pathophysiology of the neurological symptoms, it is difficult to make management recommendations [19]. From our cases, we support Finiels et al.’s [5] recommendation that if there is no evidence of significant displacement or nerve root compression, bed rest alone until the pain subsides may be the best option, as in uncomplicated osteoporotic sacral fractures [5].
Conclusion
In evaluating an elderly patient or subjects known to be osteoporotic presenting with acute cauda equina syndrome, it may not be sufficient to merely image the thoracolumbar spine. Dedicated imaging of the entire sacrum may be required, especially when a compressive lesion in the spine is not present, in order not to miss a sacral insufficiency fracture as a cause for the symptoms.
References
Lourie H. Spontaneous osteoporotic fracture of the sacrum. An unrecognised syndrome of the elderly. JAMA 1982;248:715–7.
Ries T. Detection of osteoporotic sacral fractures with radionuclides. Radiology 1983;146:783–5.
Cooper KL, Beabout JW, Swee RG. Insufficiency fractures of the sacrum. Radiology 1985;156:15–20.
Peh WC, Khong PL, Ho WY, Yeung HW, Luk KD. Sacral insufficiency fractures. Spectrum of radiological features. Clin Imaging 1995;19:92–101.
Finiels PJ, Finiels H, Strubel D, Jacquot JM. Spontaneous osteoporotic fractures of the sacrum causing neurological damage. Report of three cases. J Neurosurg 2002;97 3 Suppl:380–5.
Vaccaro AR, Kim DH, Brodke DS, Harris M, Chapman J, Schildhauer T et al. Diagnosis and management of sacral spine fractures. J Bone Joint Surg [Am] 2004;86-A:166–75.
Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res 1988;227:67–81.
Byrne TN. Disorders of the spinal cord and cauda equina. Curr Opin Neurol Neurosurg 1993;6:545–8.
Orendacova J, Cizkova D, Kafka J, Lukacova N, Marsala M, Sulla I et al. Cauda equina syndrome. Prog Neurobiol 2001;64:613–37.
Schmidek HH, Smith DA, Kristiansen TK. Sacral fractures. Neurosurgery 1984;15:735–46.
Bonin JG. Sacral fractures and injuries to the cauda equine. J Bone Joint Surg 1945;27-B:113–27.
Esses SI, Botsford DJ, Huler RJ, Rauschning W. Surgical anatomy of the sacrum. A guide for rational screw fixation. Spine 1991;16 6 Suppl:S283–8.
Jones JW. Insufficiency fracture of the sacrum with displacement and neurologic damage: a case report and review of the literature. J Am Geriatr Soc 1991;39:280–3.
Lock SH, Mitchell SC. Osteoporotic sacral fracture causing neurologic deficit. Br J Hosp Med 1993;49:210.
Aylwin A, Saifuddin A, Tucker S. L5 radiculopathy due to sacral stress fracture. Skeletal Radiol 2003;32:590–3.
Stabler A, Beck R, Bartl R, Schmidt D, Reiser M. Vacuum phenomena in insufficiency fractures of the sacrum. Skeletal Radiol 1995;24:31–5.
Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Sensory innervation of the sacroiliac joint in rats. Spine 2000;25:2015–9.
Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Origin and pathway of sensory nerve fibres to the ventral and dorsal sides of the sacroiliac joint in rats. J Orthop Res 2001;19:379–83.
Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil 2000;79:404–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukumar, T., Butt, S.H., Cassar-Pullicino, V.N. et al. Cauda equina syndrome presentation of sacral insufficiency fractures. Skeletal Radiol 36, 309–313 (2007). https://doi.org/10.1007/s00256-006-0239-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-006-0239-5