Abstract
Objective
To compare the magnetic resonance (MR) imaging findings of a group of patients with clinically diagnosed peroneal tendonopathy and peroneal tenosynovitis with the MR imaging findings of a control group of patients with no clinical evidence of peroneal tendon disorder.
Subjects and methods
The MR examinations of 24 patients with symptomatic peroneal tendinopathy or peroneal tenosynovitis and 70 patients with no clinical evidence of peroneal tendon disorder were retrospectively reviewed to determine the presence or absence of four MR imaging findings: 1) predominantly or uniform intermediate signal intensity within the peroneal tendons on one or more axial proton density-weighted images, 2) predominantly or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted images, 3) intermediate T2 signal intensity within the peroneal tendons, and 4) circumferential fluid within the common peroneal tendon sheath greater than 3 mm in maximal width. The sensitivity and specificity of these MR imaging findings for determining the presence or absence or symptomatic peroneal tendinopathy or peroneal tenosynovitis were calculated.
Results
The sensitivity of MR imaging findings 1, 2, 3, and 4 for determining the presence of peroneal tendinopathy or peroneal tenosynovitis were 92%, 92%, 50%, and 17% respectively. The specificity of MR imaging findings 1, 2, 3, and 4 for determining the absence of peroneal tendinopathy or peroneal tenosynovitis were 57%, 79%, 93%, and 100% respectively.
Conclusion
The presence of predominantly or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted images is a highly sensitive and moderately specific indicator of symptomatic peroneal tendinopathy. The presence of intermediate T2 signal within the peroneal tendons, and the presence of circumferential fluid within the peroneal tendon sheath greater than 3 mm in maximal width, are highly specific indicators of peroneal tendinopathy and peroneal tenosynovitis respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a wide spectrum of pathologic conditions of the peroneal tendons, which include tendinopathy, tenosynovitis, tendon tear, and tendon subluxation or dislocation. Peroneal tendon disorders were once thought to be uncommon. However, with the advent of magnetic resonance (MR) imaging, disorders of the peroneal tendons are being reported with increased frequency as a source of chronic lateral ankle pain [1–3].
MR imaging is commonly used to evaluate patients with peroneal tendon disorders. Multiple previous studies have documented the ability of MR imaging to diagnose complete rupture of the peroneal longus and peroneal brevis tendons, longitudinal split tears of the peroneal brevis tendon, and peroneal tendon subluxation and dislocation [4–15]. However, few previous studies have reported the MR imaging findings in patients with peroneal tendinopathy and peroneal tenosynovitis [12–15].
The diagnosis of patients with peroneal tendinopathy and peroneal tenosynovitis using MR imaging is often difficult. Increased signal intensity within a tendon is a common MR imaging finding in patients with tendinopathy. However, increased signal intensity within the peroneal tendons as they course around the tip of the lateral malleolus may be seen in asymptomatic individuals secondary to the “magic angle” phenomenon [16, 17]. Fluid within a tendon sheath is a common MR imaging finding in patients with tenosynovitis. However, fluid within the peroneal tendon sheath has also been described in asymptomatic individuals [17, 18].
There is a wide overlap between the MR imaging findings in patients with peroneal tendinopathy and peroneal tenosynovitis and patients with no clinical evidence of peroneal tendon disorder. For this reason, well-defined MR diagnostic criteria are needed to determine the presence or absence of these pathologic conditions of the peroneal tendons. Thus, this study was performed to retrospectively summarize the MR imaging findings in a group of patients with clinically diagnosed peroneal tendinopathy and peroneal tenosynovitis, and to compare these MR imaging findings to the findings of a control group of patients with no clinical evidence of peroneal tendon disorder.
Subjects and methods
Study group
The study was performed in compliance with HIPPA regulations, and with approval from our Institutional Review Board. A waiver of informed consent was obtained prior to performing the study.
A musculoskeletal MR database was used to identify 49 consecutive patients who were referred for MR imaging of the ankle at our institution between July 2001 and September 2005 to rule out peroneal tendon disorder. The MR examinations of all 49 patients were retrospectively reviewed by a fellowship-trained musculoskeletal radiologist with 4 years of clinical experience. Twenty-two patients were found to have morphologic changes in the peroneus brevis tendon or peroneal longus tendon, characteristic of a peroneal tendon tear [4–8]. Three patients were found to have a tear of the superior peroneal retinaculum with associated peroneal tendon subluxation or dislocation [9]. These 25 patients were excluded from the study. The remaining 24 patients had clinical signs and symptoms of peroneal tendon disorder, and had intact and normally located peroneal tendons on MR imaging. For this reason, it was assumed that their clinical signs and symptoms were due to peroneal tendinopathy or peroneal tenosynovitis.
The study group was comprised of these 24 patients with clinically diagnosed peroneal tendinopathy or peroneal tenosynovitis. There were 12 women and 12 men in the study group. The patients were between 17 years and 59 years of age, with an average age of 35.1 years. All 24 patients were referred for MR imaging of the ankle by an orthopedic surgeon or sports medicine specialist. All patients complained of chronic posterior lateral ankle pain at the time of presentation to their physician, with duration of symptoms ranging from 4 months to 3 years. All patients had point tenderness over the peroneal tendons on physical examination. All patients had an initial clinical diagnosis of peroneal tendon disorder and had intact and normally located peroneal tendons on MR imaging. Eighteen patients had a final diagnosis of peroneal brevis tendinopathy, two patients had a final diagnosis of peroneal brevis and peroneal longus tendinopathy, two patients had a final diagnosis of peroneal tenosynovitis, and two patients had a final diagnosis of peroneal brevis tendinopathy and peroneal tenosynovitis. The final diagnosis of each patient was made by the referring physician based upon the clinical history, physical examination, and MR imaging findings.
The same musculoskeletal MR database was used to identify a control group of 70 consecutive patients with no clinical signs or symptoms of peroneal tendon disorder who were referred for MR imaging of the ankle at our institution between August 2004 and September 2005. The 70 patients in the control group were selected based upon a retrospective review of their clinical notes, without knowledge of their MR imaging findings. There were 36 women and 34 men in the control group. The patients were between 16 years and 69 years of age, with an average age of 37.6 years. All 70 patients were referred for MR imaging of the ankle by an orthopedic surgeon or sports medicine specialist. Thorough retrospective review of clinic notes revealed that none of the 70 patients in the control group complained of posterior lateral ankle pain either prior to or at the time of presentation to their physician. Furthermore, none of the patients had point tenderness over the peroneal tendons on physical examination. Twenty-four patients had a final diagnosis of posterior tibialis tendon dysfunction, 13 patients had a final diagnosis of anterior talofibular ligament sprain, ten patients had a final diagnosis of deltoid ligament tear, four patients had a final diagnosis of os trigonum syndrome, six patients had a final diagnosis of plantar fasciitis, six patients had a final diagnosis of degenerative joint disease, and seven patients had a final diagnosis of Achilles tendon tear. A Fisher’s test and a two-sampled Student’s t-test showed no statistically significant difference at the p<0.05 level in the sex and mean age respectively of patients in the study group and control group.
MR Examination
An MR examination of the ankle was performed on all 24 patients in the study group and all 70 patients in the control group. All MR examinations were performed on the same General Electric 1.5 Tesla field strength magnet (General Electric Medical Systems, Milwaukee, WI, USA) using a phased array extremity coil. The MR examinations included axial and coronal proton density-weighted fast spin-echo sequences (TR/TE: 2000–4000 msec/15–30 msec, echo train length of 4) and fat-suppressed T2-weighted fast spin-echo sequences (TR/TE: 2000–4000 msec/60–80 msec, echo train length of 8). The fat-suppressed T2-weighted fast-spin echo sequences were performed using a frequency selective chemical presaturation pulse (ChemSat; General Electric Medical Systems, Milwaukee, WI, USA) to suppress signal from adipose tissue. All MR examinations were performed with a 14-cm field of view, a slice thickness between 3 mm and 4 mm with an interslice gap of 1 mm, a matrix of 256×192 or 256×256, and two excitations.
Review of MR examinations
The MR examinations of all 24 patients in the study group and all 70 patients in the control group were retrospectively reviewed by a fellowship-trained musculoskeletal radiologist with 4 years of clinical experience. The radiologist was unaware of the clinical findings of the patients when reviewing their MR examination.
The peroneal tendons were evaluated on four consecutive axial proton density-weighted and T2-weighted MR images through the ankle from the level of the tip of the lateral malleolus proximally to the level of the peroneal tubercle distally. The signal intensity of the peroneus brevis tendon and peroneus longus tendon on each axial proton density-weighted MR image was classified into one of three generalized categories: 1) uniform low signal intensity, 2) predominantly low signal intensity with focal areas of intermediate signal intensity comprising less than 50% of the cross-sectional area of the tendon, and 3) predominantly intermediate or uniform intermediate signal intensity with focal areas of low signal intensity comprising less than 50% of the cross-sectional area of the tendon. The signal intensity of the peroneus brevis tendon and peroneus longus tendon on each axial T2-weighted MR image was classified into one of two generalized categories: 1) uniform low signal intensity or 2) partial or uniform intermediate signal intensity. Intratendinous signal intensity was defined as being low on proton density-weighted and T2-weighted MR images if it was of the same intensity as cortical bone. Intratendinous signal intensity was defined as being intermediate on proton density-weighted and T2-weighted MR images if it was the same intensity as muscle. The number of consecutive axial proton density-weighted and T2-weighted MR images in which each type of signal intensity was present within the peroneal brevis tendon and peroneal longus tendon was recorded.
Thickening of the peroneal brevis tendon and peroneus longus tendon on the axial proton density-weighted and T2-weighted MR images was also documented. The peroneal tendons were considered to be thickened when they were the same size or larger than the posterior tibialis tendon.
The presence of fluid within the common peroneal tendon sheath on axial and coronal T2-weighted MR images was also documented. When fluid was present in the common peroneal tendon sheath, the fluid was described as being circumferentially or non-circumferentially distributed around the peroneal tendons. The amount of fluid within the common peroneal tendon sheath was determined by measuring the maximum width of the fluid at any point around the peroneal tendons on axial and coronal T2-weighted MR images. The maximal width of the fluid was measured from the surface of the peroneal tendons to the outer margin of the common peroneal tendon sheath. When fluid was present in the common peroneal tendon sheath, the integrity of the calcaneofibular ligament and the presence of fluid within the ankle joint were documented. This was done to determine whether fluid within the common peroneal tendon sheath may have been secondary to a tear of the calcaneofibular ligament and the resultant communication of the tendon sheath with the ankle joint . When documenting the presence of ankle-joint fluid, no attempt was made to differentiate a physiologic from a non-physiologic amount of fluid within the ankle joint.
Statistical analysis
The sensitivity and specificity with 95% confidence intervals of six MR imaging findings for determining the presence or absence of clinically diagnosed peroneal tendinopathy or peroneal tenosynovitis were calculated. These MR imaging findings included: 1) peroneal tendon thickening, 2) any intermediate signal intensity within the peroneal tendons on one or more axial proton density-weighted MR images, 3) predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on one or more axial proton density-weighted MR images, 4) predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on two consecutive axial proton density-weighted MR images, 5) predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted MR images, 6) intermediate signal intensity within the peroneal tendons on one or more axial T2-weighted MR images, and 7) circumferential fluid within the common peroneal tendon sheath greater than 3 mm in maximal width. McNemar’s chi-square tests were used to compare the sensitivity and specificity of the MR imaging findings [19]. A difference in sensitivity and specificity between the MR imaging findings was considered to be statistically significant if the p-value was less than 0.05. The statistical software program SAS (Version 8.2, SAS Inc., Cary, NC, USA ) was used to perform the statistical analysis.
Positive and negative odds ratios of the six MR imaging findings were also calculated. The positive odds ratio indicated the increase in odds that a patient had peroneal tendinopathy or peroneal tenosynovitis if a specific MR imaging finding was present. The negative odds ratio indicated the decrease in odds that a patient had peroneal tendinopathy or peroneal tenosynovitis if a specific MR imaging finding was absent [20]. The statistical software program SAS (Version 8.2, SAS, Inc., Cary, NC, USA) was used to perform the statistical analysis.
Results
MR imaging findings
Two of the 24 patients in the study group had thickening of the peroneal tendons on MR imaging. None of the 70 patients in the control group had thickening of the peroneal tendons.
The signal intensity within the peroneal brevis tendon and peroneal longus tendon for patients in the study group and control group on axial proton density-weighted and T2-weighted MR images are summarized in Tables 1 and 2. Twenty-two of the 24 patients in the study group had predominantly intermediate or uniform intermediate signal intensity within the peroneus brevis tendon on three consecutive proton density-weighted MR images. (Figure 1) Twelve of these patients also had intermediate signal intensity within the peroneal brevis tendon on one or more T2-weighted MR images. (Figure 2) Fifteen of the 70 patients in the control group had predominantly intermediate or uniform intermediate signal intensity within the peroneus brevis tendon on three consecutive proton density-weighted MR images. Five of these patients also had intermediate signal intensity within the peroneal brevis tendon on one or more T2-weighted MR images (Fig. 3).
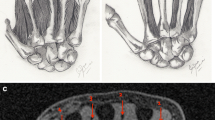
43-year-old male with symptomatic peroneal tendon disorder. (a) Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the tip of the lateral malleolus. The peroneal brevis tendon (arrow) is of predominantly intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of predominantly low signal intensity. (b) Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the previous figure. The peroneal brevis tendon (arrow) is of predominantly intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of predominantly low signal intensity. (c) Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the previous figure. The peroneal brevis tendon (arrow) is of uniform intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of uniform low signal intensity
35-year-old male with symptomatic peroneal tendon disorder. (a) Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the tip of the lateral malleolus. The peroneal brevis tendon (arrow) is of predominantly intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of predominantly low signal intensity. (b) Corresponding axial fat-suppressed T2-weighted fast spin-echo image of the ankle. The peroneal brevis tendon (arrow) is of uniform intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of uniform low signal intensity
22-year-old male college track athlete with no clinical evidence of peroneal tendon disorder. a Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the tip of the lateral malleolus. The peroneal brevis tendon (arrow) is of predominantly intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of predominantly low signal intensity. b Corresponding axial fat-suppressed T2-weighted fast spin-echo image of the ankle. The peroneal brevis tendon (arrow) is of predominantly intermediate signal intensity, while the peroneal longus tendon (arrowhead) is of uniform low signal intensity
Four of the 24 patients in the study group had circumferential fluid within the common peroneal tendon sheath which measured greater than 3 mm in maximal width. Two of these four patients had uniform low signal intensity within the peroneus brevis tendon and peroneus longus tendon on four consecutive axial proton density-weighted and T2-weighted MR images. (Figure 4) These two patients were given a final diagnosis of peroneal tenosynovitis. All four patients with fluid within the peroneal tendon sheath had fluid within the ankle joint and an intact calcaneofibular ligament.
47-year-old female with symptomatic peroneal tendon disorder. a Axial proton density-weighted fast spin-echo MR image of the ankle just distal to the tip of the lateral malleolus. The peroneal brevis tendon (arrow) and peroneal longus tendon (arrowhead) are of uniform low signal (arrowhead) intensity. b Corresponding axial fat-suppressed T2-weighted fast spin-echo image of the ankle. The peroneal brevis tendon (small arrow) and peroneal longus tendon (arrowhead) are of uniform low signal (small arrowhead) intensity. Note the large amount of circumferential fluid within the common peroneal tendon sheath (large arrowhead) which measured 3.2 mm in maximal width
Fourteen of the 70 patients in the control group had fluid within the peroneal tendon sheath. Twelve of these patients had non-circumferential fluid within the common peroneal tendon sheath which measured less than 1.5 mm in maximal width. All of these patients had fluid within the ankle joint and an intact calcaneofibular ligament. Two patients had circumferential fluid within the common peroneal tendon sheath which measured 2.2 mm and 2.6 mm in maximal width. Both of these patients had large ankle-joint effusions and complete tears of the calcaneofibular ligament.
Statistical analysis
The sensitivity and specificity with 95% confidence intervals of the seven MR imaging findings for determining the presence or absence of clinically diagnosed peroneal tendinopathy or peroneal tenosynovitis are listed in Table 3. MR imaging findings 2, 3, 4, and 5 had the highest sensitivity for determining the presence of peroneal tendinopathy or peroneal tenosynovitis. The differences in the sensitivity of these MR imaging findings and the sensitivity of MR imaging findings 1, 6, and 7 were statistically significant at the p<0.05 level. MR imaging findings 1 and 7 had the highest specificity for determining the absence of peroneal tendinopathy or peroneal tenosynovitis. The differences in the specificity of these MR imaging findings and the specificity of MR imaging finding 2 and 3 were statistically significant at the p< 0.05 level. The difference in the specificity of these MR imaging findings and the specificity of MR imaging findings 4, 5, and 6 were not statistically significant (p-values=0.09–0.67).
The positive odds ratios and negative odds ratios with 95% confidence intervals of the seven MR imaging findings for determining the presence or absence of peroneal tendinopathy or peroneal tenosynovitis are listed in Table 3. The positive odds ratio for MR imaging findings 1 and 7 could not be calculated due to their perfect specificity.
Discussion
Peroneal tendinopathy represents a non-inflammatory degenerative process within the peroneal tendons. Peroneal tendinopathy is most commonly seen in athletes involved in sporting activities such as running, competitive walking, and ballet dancing which place repetitive stress upon the lateral ankle tendons. However, the condition has also been described in elderly individuals, diabetics, patients with inflammatory arthritis, and individuals with displaced fractures of the lateral malleolus and calcaneus [1–3].
Previously described MR imaging findings in patients with peroneal tendinopathy have included thickening of and increased signal intensity within the peroneal tendons [12–15]. In our study, thickening of the peroneal tendons was an infrequent finding which was present in only two patients in the study group. However, increased signal intensity within the peroneal tendons on axial proton density-weighted MR images was noted in 22 of the 24 patients in the study group. These patients were given a final diagnosis of peroneal tendinopathy.
However, increased signal intensity within the peroneal tendons was a nonspecific finding in our study, and was also commonly seen in patients in the control group, with no clinical evidence of peroneal tendon disorder. In our study, predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons was present on one or more axial proton density-weighted MR images in 30 of 70 patients in the control group. Previous studies have also described increased signal intensity within the peroneal tendons on axial T1-weighted and proton density-weighted MR images in asymptomatic individuals. This increased signal intensity has been attributed to the “magic angle” phenomenon [4–6, 9–15]. The “magic angle” phenomenon is caused by changes in the dipolar interactions between water hydrogen protons that are loosely bound to collagen fibrils in organized tissue such as tendons. When tendons are oriented 55 degrees relative to the external magnetic field, as are the peroneal tendons as they course distally around the lateral malleolus, their T2 relaxation time is lengthened, which results in focal increased signal intensity on short echo time MR images [16].
Our study suggests that factors other than the “magic angle” phenomenon may also be responsible for increased signal intensity within the peroneal tendons in asymptomatic individuals on short-echo time MR images. The peroneal tendons have almost parallel courses as they pass distally around the lateral malleolus. If the “magic phenomenon” was the only process involved in the formation of increased signal intensity within the peroneal tendons in asymptomatic individuals, it would be expected that the incidence of this finding would be identical for the peroneal brevis tendon and peroneal longus tendon. However, in our study, increased signal intensity on axial proton density-weighted MR images in patients in the control group was much more common within the peroneal brevis tendon. For this reason, it is our belief that increased signal intensity within the peroneal tendons in asymptomatic individuals may also be secondary to other factors such as asymptomatic tendon degeneration. Asymptomatic tendon degeneration leading to increased signal intensity on short echo time MR images has also been described in the Achilles tendon, the common extensor tendon, and the common flexor tendon [21–24]. The higher incidence of increased signal intensity within the peroneal brevis tendon than within the peroneus longus tendon on axial proton density-weighted MR images in both symptomatic and asymptomatic patients in our study likely reflects the fact that the peroneus brevis tendon is more prone to attrition within the common peroneal tunnel. Within the common peroneal tunnel, the peroneal brevis tendon is subjected to repetitive dynamic compression against the retromalleolar groove by the overlying peroneal longus tendon during ankle dorsiflexion [7].
Due to the high incidence of increased signal intensity within the peroneal tendons in asymptomatic individuals on short echo time MR images, many authors have recommended using only T2-weighted images to evaluate the signal intensity of the peroneal tendons [4–6, 9]. In our study, intermediate signal intensity within the peroneal tendons on axial T2-weighted images was a much more specific MR imaging finding for determining the absence of symptomatic peroneal tendon disorder than intermediate signal intensity within the peroneal tendons on axial proton density-weighted images. However, intermediate signal intensity within the peroneal tendons on axial T2-weighted MR images was present in only 12 of 22 patients in the study group with a final diagnosis of peroneal tendinopathy. Thus, increased T2 signal intensity within the peroneal tendons is a highly specific but relatively insensitive MR imaging finding in patients with peroneal tendinopathy.
In our study, the best MR imaging finding for determining the presence or absence of peroneal tendinopathy was predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted MR images. This MR imaging finding had a sensitivity of 92% for detecting the presence of clinically diagnosed peroneal tendon disorder in patients with intact and normally located peroneal tendons. Only two of the 24 patients in the study group did not have predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted MR images. Both patients had a large amount of fluid within the peroneal tendon sheath, and were given a final diagnosis of peroneal tenosynovitis. If these two patients in the study group were excluded from the statistical analysis, the sensitivity of this MR imaging finding for determining the presence of clinically diagnosed peroneal tendinopathy would increase to 100%. Predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted MR images also had a moderate specificity of 79% for determining the absence of symptomatic peroneal tendon disorder.
Peroneal tenosynovitis represents inflammation of the common synovial sheath surrounding the peroneal brevis and peroneus longus tendons. Peroneal tenosynovitis is commonly seen in ballet dancers and other athletes involved in sports activities which place repetitive stress upon the lateral ankle tendons. The condition has also been described in individuals with anatomic variants such as an enlarged peroneal tubercle, os perineum, peroneus quartus muscle, or low lying peroneus brevis muscle belly, and in individuals following ankle inversion injuries and displaced fractures of the lateral malleolus and calcaneus. Peroneal tenosynovitis may also be secondary to infection or inflammatory processes such as rheumatoid arthritis, seronegative spondyloarthropathies, gout, and systemic lupus erythematosis [1–3].
Previously described MR imaging findings in patients with peroneal tenosynovitis have mainly consisted of fluid within the common peroneal tendon sheath [12–15]. In our study, fluid was present within the common peroneal tendon sheath in four of 24 patients in the study group. These patients were given a final diagnosis of peroneal tenosynovitis. Two of the four patients also had predominantly intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted MR images, and were given a final diagnosis of both peroneal tendinopathy and peroneal tenosynovitis.
However, fluid within the common peroneal tendon sheath was a nonspecific finding in our study, and was also commonly seen in patients in the control group with no clinical evidence of peroneal tendon disorder. Previous studies have also described fluid within the common peroneal sheath in asymptomatic individuals [17, 18]. In our study, fluid was present within the common peroneal tendon sheath in 14 of 70 patients in the control group. However, all four patients in the study group and none of the 14 patients in the control group with fluid within the common peroneal sheath had circumferential fluid which measured more than 3 mm in maximal width. For this reason, the MR imaging finding of circumferential fluid within the common peroneal tendon sheath greater than 3 mm in maximal width may be used to differentiate between patients with symptomatic peroneal tenosynovitis and patients with no clinical evidence of peroneal tendon disorder.
The MR imaging findings in patients with peroneal tendinopathy and peroneal tenosynovitis have been described previously in the radiology literature. However, all previous descriptions are found in review articles or small case reports [12–15]. Our study is the first to summarize the MR imaging findings in a large group of patients with clinically diagnosed peroneal tendinopathy and peroneal tenosynovitis, and to compare these MR imaging findings to the findings of a control group of patients with no clinical evidence of peroneal tendon disorder.
In our study, no MR imaging finding in our study was 100% sensitive and specific for determining the presence or absence of clinically diagnosed peroneal tendinopathy and peroneal tenosynovitis. Nevertheless, MR imaging is useful for evaluating patients with clinically diagnosed peroneal tendon disorder. MR imaging can exclude the presence of peroneal tendon tears and peroneal tendon subluxation or dislocation. MR imaging can also detect changes in the morphology and signal intensity of the peroneal tendons, and identify fluid within the common peroneal tendon sheath that can lead to a definitive diagnosis of peroneal tendinopathy or peroneal tenosynovitis.
The major limitation of our study was that the MR imaging findings of patients in both the study group and the control group were not correlated with surgical and pathologic findings. All patients in our study were successfully treated with conservative therapy. As a result, surgical and pathologic correlation was not available. It is possible that some of the patients in the study group were misdiagnosed by their physician, or had subtle tears of the peroneal tendons which were not identified by the musculoskeletal radiologist reviewing the MR examinations. It is also possible that the clinical diagnosis of patients in the control group was incorrect, and that some of these individuals actually had peroneal tendon disorder. Another limitation of our study was the presence of selection bias. Not all patients with symptomatic peroneal tendinopathy and peroneal tenosynovitis at our institution were evaluated with MR imaging.
In conclusion, our study has shown that the presence of predominately intermediate or uniform intermediate signal intensity within the peroneal tendons on three consecutive axial proton density-weighted images is a highly sensitive and moderately specific MR imaging finding for diagnosing peroneal tendinopathy. In contrast, the presence of increased T2 signal intensity within the peroneal tendons is a highly specific but relatively insensitive MR imaging finding for diagnosing peroneal tendonopathy. Our study has also shown that the presence of circumferential fluid within the common peroneal tendon sheath greater than 3 mm in maximal width is a highly specific MR imaging finding for diagnosing peroneal tenosynovitis.
References
Clarke H, Kitaoka H, Ehman R. Peroneal tendon injuries. Foot Ankle Inter 1998;19:280–8.
Sammarco G. Peroneal tendon injuries. Orthop Clin North Am 1994;25:135–45.
Sammarco G, DiRaimondo C. Chronic peroneus brevis tendon lesions. Foot Ankle 1989;9:163–70.
Khoury N, el-Khoury G, Saltzman C, Kathol M. Peroneus longus and brevis tendon tears: MR imaging evaluation. Radiology 1996;200:833–41.
Rosenberg Z, Beltran J, Cheung Y, Colon E, Herraiz F. MR features of longitudinal tears of the peroneus brevis tendon. AJR Am J Roentgenol 1997;168:141–7.
Schweitzer M, Eid M, Deely D, Wapner K, Hecht P. Using MR imaging to differentiate peroneal tendon splits from other peroneal disorders. AJR Am J Roentgenol 1997;168:129–33.
Sobel M, Bohne W, Markisz J. Cadaver correlation of peroneal tendon changes with magnetic resonance imaging. Foot Ankle 1991;11:384–8.
Rademaker J, Rosenberg Z, Delfaut E. Tear of the peroneus longus tendon: MR imaging features in nine patients. Radiology 2000;214:700–4.
Major N, Helms C, Fritz R, Speer K. The MR imaging appearance of longitudinal split tears of the peroneus brevis tendon. Foot Ankle Inter 2000;21:514–9.
Rosenberg Z, Bencardino J, Astion D, Schweitzer M, Rokito A, Sheskier S. MRI features of chronic injuries of the superior peroneal retinaculum. AJR Am J Roentgenol 2003;181:1551–7.
Shellock F, Feske W, Frey C, Terk M. Peroneal tendons: use of kinematic MR imaging of the ankle to determine subluxation. J Magn Reson Imaging 1997;7:451–4.
Bencardino J, Rosenberg Z, Serrano L. MR imaging features of diseases of the peroneal tendons. Magn Reson Imaging Clin N Am 2001;9:493–505.
Ton E, Schweitzer M, Karasick D. MR imaging of peroneal tendon disorders. AJR Am J Roentgenol 1997;168:135–40.
Mota J, Rosenberg Z. Magnetic resonance imaging of the peroneal tendons. Top Magn Reson Imaging 1998;9:273–85.
Yao L, Tong G, Cracchiola A. MR findings in peroneal tendonopathy. J Comput Assist Tomogr 1995;19:460–64.
Erickson S, Cox I, Hyde J, Carrera G, Strandt J, Estkowski L. Effect of tendon orientation on MR imaging signal intensity: a manifestation of the “magic angle” phenomenon. Radiology 1991;181:389–92.
Rosenberg Z, Bencardino J, Mellado J. Normal variants and pitfalls in magnetic resonance imaging of the foot and ankle. Top Magn Reson Imaging 1998;9:262–72.
Schweitzer M, van Leersum M, Ehrlich S, Wapner K. Fluid in normal and abnormal ankle joints: amount and distribution as seen on MR images. AJR 1994:162:111–4.
Hawass N. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol 1997;70:360–6.
Simel D, Samsa G, Matcher D. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 1991;44:763–70.
Haims A, Schweitzer M, Patel R, Hecht P, Wapner K. MR imaging of the Achilles tendon: overlap of findings in symptomatic and asymptomatic individuals. Skeletal Radiol 2000;29:640–5.
Martin C, Schweitzer M. MR imaging of epicondylitis. Skeletal Radiol 1998;27:133–8.
Mackay D, Rangan A, Hide G, Hughes T, Latimer J. The objective diagnosis of early tennis elbow by magnetic resonance imaging. Occup Med 2003;53:309–12.
Kijowski R, De Smet A. Magnetic resonance imaging findings in patients with medial epicondylitis. Skelet Radiol 2005;34:196–202.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kijowski, R., De Smet, A. & Mukharjee, R. Magnetic resonance imaging findings in patients with peroneal tendinopathy and peroneal tenosynovitis. Skeletal Radiol 36, 105–114 (2007). https://doi.org/10.1007/s00256-006-0172-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-006-0172-7