Abstract
Objective
Chordoma is a rare malignant bone tumor that arises from notochord remnants. This is the first trial to investigate the utility of 11C-methionine (MET) positron emission tomography (PET) in the imaging of chordoma before and after carbon-ion radiotherapy (CIRT).
Design and patients
Fifteen patients with chordoma were investigated with MET-PET before and after CIRT and the findings analyzed visually and quantitatively. Tumor MET uptake was evaluated by tumor-to-nontumor ratio (T/N ratio).
Results
In 12 (80%) patients chordoma was clearly visible in the baseline MET-PET study with a mean T/N ratio of 3.3±1.7. The MET uptake decreased significantly to 2.3±1.4 after CIRT (P<0.05). A significant reduction in tumor MET uptake of 24% was observed after CIRT. Fourteen (93%) patients showed no local recurrence after CIRT with a median follow-up time of 20 months.
Conclusion
This study has demonstrated that MET-PET is feasible for imaging of chordoma. MET-PET could provide important tumor metabolic information for the therapeutic monitoring of chordoma after CIRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chordoma is a rare malignant neoplasm representing less than 3% of all primary bone tumors. It usually has a benign histological appearance and a slow growth rate. It has little metastatic potential, but considerable local destructiveness [1]. Since almost all the lesions arise in the spine or skull base with about 50% of chordomas of sacrococcygeal origin [2], a small difference in the tumor extent can have profound clinical consequences. Efforts to increase local control have included aggressive surgery, radiation therapy or combined approaches, but locally-free survival rates remain relatively low and have not exceeded 30% at 5 years [3]. As total surgical resection is often not feasible, other management strategies must frequently be considered [4, 5, 6]. Traditionally, this tumor has been considered to be “radioresistant” to standard-dose radiation therapy [7]. To improve the radiobiological effect, fractionated irradiation with charged particles has been introduced in our institute and reported with encouraging results [8, 9, 10]. Since chordoma is slow-growing and most of the treated patients show no significant decrease in tumor volume, the early response of chordoma after charged particle therapy may be difficult to evaluate by anatomical imaging modalities alone. Furthermore, it is crucial that the active and aggressive regions of tumor are defined accurately both before charged particle therapy and for follow-up of treatment, to form the basis for further accurate treatment planning and delivery of radiation therapy.
It has been shown that 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is valuable for the detection, staging, grading and monitoring of therapy of various tumors [11, 12], although the usefulness of FDG-PET in the early evaluation of chemotherapy or radiotherapy is still a matter of debate, since increased FDG uptake can occur in macrophages, neutrophils, fibroblasts and granulation tissue immediately after therapy [13, 14, 15]. However, as an essential amino acid, l-methionine plays a central role in the altered metabolism of cancer cells [16]. l-[methyl-11C]-methionine (MET) uptake correlates with cell proliferation in cell culture, Ki-67 expression and proliferating cell nuclear antigen expression, indicating its role as a marker for active tumour proliferation [17]. Autoradiographic findings reported by Kubota et al. [18] demonstrate that the level of increased MET uptake correlates with the number of tumor cells, whereas no significant MET uptake occurs in chronic inflammatory or radiogenic lesions. Preclinical studies validating the possible use of MET in the evaluation of chemo- or radiotherapy generally show that MET uptake is reduced rapidly—more rapidly than that of FDG [19, 20, 21]—and that MET uptake correlates better than FDG with tumor proliferative activity in squamous cell head and neck cancer cell lines [22]. In addition, increased uptake of MET as measured by PET has been suggested to reflect increased transport, transmethylation rate and protein synthesis of malignant tissue [22, 23, 24, 25], and is less influenced by radiation-induced inflammatory reaction after radiotherapy compared with FDG [21]. We therefore chose MET-PET to evaluate the early response of chordoma after charged particle carbon ion radiotherapy (CIRT). Our hypothesis was that chordoma shows high amino acid metabolism that decreases within a few days after CIRT, thus providing the potential for imaging and monitoring of chordoma treated by CIRT. To our knowledge, no other studies have reported the imaging of chordoma with MET-PET. In this paper, we report our initial experience in the imaging of chordoma with MET-PET before and after CIRT.
Patients and methods
Patients
This study comprised a total of 30 MET-PET scans carried out on15 chordoma patients (11 men, 4 women) with a mean age of 66 years (SD ±13). Table 1 presents the patient characteristics and the results of the MET-PET study. All patients had a complete history and physical examination, including radiography, computed tomography (CT) or magnetic resonance imaging (MRI), prior to MET-PET. Based on the clinical findings, tumor was considered to be present in all patients without distant metastases. Tumors were grossly measured, but size did not exceed 15 cm. Tumors were judged to be unresectable by the referring surgeon, or the patients were medically inoperable or declined surgery. Histological diagnosis was confirmed by tumor biopsy and histological grading was performed according to the World Health Organization (WHO) classification [26]. After CIRT, invasive biopsy was not performed in order to get an uncontaminated follow-up for the therapeutic response to carbon ion radiotherapy. Patients who had undergone chemotherapy within 4 weeks before carbon ion radiotherapy or those who had prior radiation therapy at the same site were excluded from the study. All patients signed the informed consent form approved by our institutional review board.
PET imaging
11C-MET was prepared by a method modified from the synthesis of Langstrom et al. [27]. Whole-body scanners (ECAT EXACT HR+ and ECAT EXACT 47; Siemens CTI, Knoxville, Tenn.) were used, providing an axial field of view of 15.5 and 16.2 cm, resulting in 63 and 47 transverse slices with a thickness of 2.5 and 3.4 mm, respectively. The spatial resolution of the reconstructed images is 4.2–6.0 mm at full-width half maximum. Transmission scans were performed with germanium-68 rod sources. Emission data corrected for random events, dead time and attenuation were reconstructed by filtered backprojection using a ramp filter with a cutoff frequency of 0.4, followed by the decay correction,
Before initiation of CIRT, a baseline MET-PET was performed and MET-PET was repeated within 1 month after completion of CIRT. Patients fasted for at least 4 h before PET imaging. Before MET injection, all patients underwent a transmission scan for one (ECAT EXACT HR+) or two bed positions (ECAT EXACT 47) including the lesion site, each bed position for 20 and 10 min for ECAT EXACT HR+ and ECAT EXACT 47, respectively. Static emission data for the same positions were obtained from 23 min after the intravenous administration of approximately 740 MBq of MET [28]. Because of the difference in sensitivity of the PET scanners, static emission scans were performed for 30 min in ECAT EXACT HR+ and 15 min in ECAT EXACT 47 for each bed position, respectively.
Image analysis
Tomographic images displayed as coronal, sagittal and transaxial slices were viewed on a workstation. Two experienced nuclear medicine physicians (H.Z. and K.Y.) evaluated the PET images for the presence of regional metabolic activity. For quantitative evaluation, regions of interest (ROI) with a diameter of 1 cm were manually drawn over these heterogeneous tumors in the transaxial slice with maximum MET uptake on the baseline scan to reflect the most metabolically active area of the tumor. The most metabolically active areas are thought to reflect tumor regions with more aggressive tumor. The inherent assumption was made that the overall behavior of the tumor is predicted by the activity of the most aggressive regions. The ROIs for the background radioactivity measurement were drawn on the homologous contralateral or surrounding normal tissue. Tumor-to-nontumor ratio (T/N ratio) was calculated using the following formula: T/N ratio = mean counts per pixel of tumor ROI/mean counts per pixel of normal tissue ROI. The percentage reduction in the T/N ratio (%T/N ratio) after CIRT was calculated as:
Statistics
Data were expressed as mean±SD. Student’s t-test was used for the statistical analysis. A difference with P<0.05 was considered significant.
Results
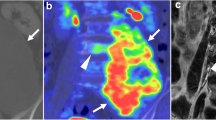
Table 1 shows the characteristics of the patients and the results of the MET-PET studies. Eleven tumors were in Gx and four were recurrent tumors. No patient had received chemotherapy over the 4 weeks prior to the initiation of CIRT. Variable baseline MET uptakes were observed in tumors, the T/N ratio ranging from 0.8 to 7.1 with a mean value of 3.3±1.7. The mean T/N ratio was 2.8±1.4 (range 0.8–5.4) for primary tumors and 4.7±1.8 (range 3.0–7.1) for recurrent lesions. It was found that MET uptake in recurrent lesions was significantly higher than that of primary tumors (P<0.03). All four recurrent lesions were identified on MET-PET imaging, while eight of 11 (73%) primary tumors could be visualized and three (27%) primary lesions were negative. Overall, tumor area was clearly visible in the baseline PET study in 12 of 15 (80%) patients. Typical examples are shown in Figs. 1 and 2. It should be noted that inhomogeneous MET uptake could be observed in some cases, which reflected the variety of the components, such as fluid and gelatinous mucoid substance, associated with recent and old hemorrhages, and necrotic areas within chordoma, and as well as calcification and sequestered bone fragments. Delineation of one tumor (patient 9) was difficult because of the low MET uptake. However, the tumor could still be delineated from the image when the anatomical location of the tumor was known by the authors before image interpretation.
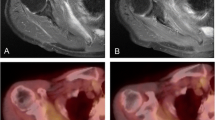
Case 11. A 65-year-old man who received 70.4 GyE to chordoma in the sacrum. A MR image of the pelvis showed a large heterogeneous mass in the sacrum. B Baseline MET uptake demonstrated an inhomogeneous mass in the same region. C MR image still revealed a large mass after CIRT. D Tumor MET uptake decreased significantly after CIRT
All the patients had a second PET study within 1 month after CIRT. Tumor MET accumulation visibly decreased and the mean T/N ratio decreased to 2.3±1.4 (range 0.9–6.4). The difference between the two mean T/N ratios was significant (P<0.05) (Fig. 3). The reductions in MET uptake by the patients after CIRT is shown in Fig. 4. A mean MET uptake reduction of 24%±24% was observed after CIRT. Eleven of 12 patients with positive initial MET studies had a reduction in MET uptake with a mean value of 33.8%. All 11 patients had an MET uptake reduction of more than 10%, whereas one patient had over a 10% increase and three patients showed no change. Of the 11 patients, seven (64%) showed a significant reduction of more than 20%, and four (36%) showed a reduction of more than 30%. The patients were followed up for up to 5 years for therapeutic evaluation. Only one patient was diagnosed as having a local recurrence after CIRT. Time to local recurrence was 12 months (patient 4). Because of the very small population, the correlation between local recurrence and MET uptake could not be analyzed.
Discussion
CIRT has shown promising local control rates in excess of 73% at 3 years, contrasting with the approximately 35% control rate reported with conventional radiation [10, 29, 30]. As this is a new treatment technique, there is a need for accurate assessment of the early response of tumor as well as the follow-up of the patients. Current imaging methods including CT and MRI have limitations in distinguishing viable tumor from post-therapeutic changes because of changes in normal anatomy, distortion of tissue planes, and lack of distinction between tumor and post-therapeutic tissue [31, 32]. These traditional radiographic methods for assessing response to therapy are poorly suited to evaluating osseous lesions [33]. Although peritumoral edema and in some cases extraosseous soft tissue masses may be reduced around osseous tumors, traditional radiographic studies are unable to distinguish nonresponding from responding lesions [34]. In this study, invasive biopsy was not performed after CIRT in order to obtain an uncontaminated follow-up study of the therapeutic response to CIRT. Our data clearly demonstrate the feasibility of MET-PET, which yielded a sensitivity of 80% (12/15) in the visualization of all lesions and 100% (4/4) for the recurrent tumors. Patients who had recurrent tumors tended to have high baseline MET uptake, which probably reflects the tendency to malignant transformation in progressive tumors. Primary tumors showed variable uptakes in this study, and it was not possible to evaluate therapeutic assessment of chordoma when there was a negative finding on baseline MET-PET image. Chordoma has variable growth rates [4, 5], which may be due to differing metabolic activity and resulted in differing accumulation of MET in our study. Second, all the primary tumors in this study had an undifferentiated pathological grade (Gx), which might include variable malignant cells, which are different for each tumor. This could be a partial explanation for the variable MET accumulations in this study. Factors such as gender, age, tumor size and pre-CIRT therapy were not found to affect MET accumulation statistically.
Tumor MET accumulation was evaluated by the T/N ratio in this study, as it has been shown that semiquantitative analysis by SUV is not as reliable as the tumor-to-liver ratio in evaluation of tumor response to fluorouracil in colorectal cancer liver metastases [35]. This is indicative of the problems of introducing extra measurement variables when trying to establish a semiquantitative method. Inadequate injection with extravasation of some of the MET at the time of intravenous administration most likely led to an underestimate of the tumor SUV. This problem did not affect the tumor-to-background ratio as injected activity is irrelevant. A further potential confounding factor for SUV calculation of MET is that a competing organ such as the liver could take up a greater proportion of the injected MET dose, leaving less for the tumor. If this is present only on the pretreatment or post-treatment scan, it may result in a misinterpretation of the tumor response.
Muhr et al. investigated the metabolic reduction of tumor in connection with the effectiveness of interferon-α (IFN-α) treatment in meningioma [36]. After IFN-α treatment, tumors were observed at biopsy to be changed into fibrous-rich tissue with fewer viable tumor cells but had not decrease in size compared with before treatment [37]. Their study suggested that in tumor treatment it is important to demonstrate decreased tumor metabolism as a sign of decreased tumor growth but not necessarily diminished tumor volume. In this study, our results demonstrated that CIRT was effective in reducing tumor metabolism. The measurements of chordoma size, including intraosseous extent on either T1-weighted or short inversion time inversion recovery (STIR) MRI, did not change significantly after CIRT and were difficult to assess in some patients. Chordomas in this study were mainly located in the sacrum, and the bladder sometimes showed a similar MET uptake compared with tumor tissue. However, due to the anatomical localization of the tumors, this did not interfere with scan interpretation.
It is known that fluid and gelatinous mucoid substance, associated with recent and old hemorrhages, and necrotic areas are found within chordoma, and in some cases calcification and sequestered bone fragments reflect the type of tissue or matrix in the tumor. Different parts within a tumor may have different malignancy grades. In this MET-PET study it was found that MET uptake could be variable within a tumor before CIRT. After CIRT, MET-PET showed peripheral areas of higher metabolic activity and central areas of decreased or no activity, suggestive of tumor necrosis. These important biological characteristics are immediately apparent on MET-PET imaging and available for clinical decision-making. In addition, it would be potentially advantageous to recognize the presence of residual viable tumor in both the planning of post-treatment therapy and during the follow-up phase for demonstration of progressive disease. Thus, MET-PET might provide important information about metabolic activity and might be useful in treatment planning as well as post-therapeutic assessment.
It should be noted that there were several limitations to this study. Firstly, as most of the patients had Gx chordoma, it was impossible to evaluate whether MET-PET could be used for the histological grading of chordoma. Secondly, histological confirmation of suspicious imaging abnormalities should be the gold standard for post-therapeutic assessment, but in clinical practice that is unrealistic. Invasive biopsy after CIRT was not performed in order to avoid interference in the follow-up for the therapeutic response to CIRT. Thus the MET uptake changes in the tumor could not be correlated with histological confirmation after CIRT. Nevertheless, MET-PET could be worthy of a suitable indicator for post-therapeutic assessment, since a significant reduction in MET uptake after CIRT showed a good local control of tumor over a relative long period in this study. Longer follow-up will be required to determine the true significance of MET accumulation and its relationship with prognosis.
In conclusion, this study demonstrated that MET-PET is feasible for imaging of chordoma. MET-PET could provide important tumor metabolic information for the therapeutic monitoring of chordoma after CIRT.
References
Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol 2003; 10:291-296.
Mindell ER. Chordoma. J Bone Joint Surg Am 1981; 63:501–505.
Breteau N, Demasure M, Lescrainier J, Sabbattier R, Michenet P. Sacrococcygeal chordomas: potential role of high LET therapy. Recent Results Cancer Res 1998; 150:148-155.
Watkins L, Khudados ES, Kaleoglu M, Revesz T, Sacares P, Crockard HA. Skull base chordomas: a review of 38 patients, 1958–88. Br J Neurosurg 1993; 7:241-248.
Gay E, Sekhar LN, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 1995; 36:887-897.
Al-Mefty O, Borba LA. Skull base chordomas: a management challenge. J Neurosurg 1997; 86:182-189.
Krayenbuhl H, Yasargil MG. Cranial chordomas. Progr Neurol Surg 1975; 6:380–434.
Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys 1995; 31:577–581.
Castro JR, Collier JM, Petti PL, et al. Charged particle radiotherapy for lesions encircling the brain stem or spinal cord. Int J Radiat Oncol Biol Phys 1989; 17:477–484.
Kamada T, Tsujii H, Tsuji H, et al. Working Group for the Bone and Soft Tissue Sarcomas. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 2002; 20:4466–4471.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Natl Rev Cancer 2002; 2:683–693.
Zhang H, Tian M, Oriuchi N, Higuch, T, Tanada S, Endo K. Oncological diagnosis using positron coincidence gamma camera with fluorodeoxyglucose in comparison with dedicated PET. Br J Radiol 2002; 75:409–416.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33:1972―1980.
Haberkorn U, Strauss LG, Dimitrakopoulou A, et al. PET studies of fluorodeoxyglucose metabolism in patients with recurrent colorectal tumors receiving radiotherapy. J Nucl Med 1991; 32:1485–1490.
Hautzel H, Muller-Gartne, HW. Early changes in fluorine-18-FDG uptake during radiotherapy. J Nucl Med 1997; 38:1384–1386.
Hoffman RM. Unbalanced transmethylation and the perturbation of the differentiated state leading to cancer. Bioessays 1990; 12:163–166.
Kracht LW, Friese M, Herholz K, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 2003; 30:868―873.
Kubota R, Kubota K, Yamada S, et al. Methionine uptake by tumor tissue: a microautoradiographic comparison with FDG. J Nucl Med 1995; 36:484–492.
Schaider H, Haberkorn U, Berger MR, Oberdorfer F, Morr I, van Kaick G. Application of alpha-aminoiosbutyric acid,l-methionine, thymidine and 2-fluoro-2-d-glucose to monitor effects of chemotherapy in a human colon carcinoma cell line. Eur J Nucl Med 1996; 23:55–60.
Higashi K, Clavo AC, Wahl RL. In vitro assessment of 2-fluoro-2-d-glucose, l-methionine, thymidine as agents to monitor the early response of a human adenocarcinoma cell line to radiotherapy. J Nucl Med 1993; 34:773–779.
Kubota K, Ishiwata K, Kubota R, et al. Tracer feasibility for monitoring tumor radiotherapy: a quadruple tracer study with fluorine-18-fluorodeoxyglucose or fluorine-18-fluorodeoxyuridine,l-[methyl-14C] methionine, [6-3H]thymidine, and gallium-67. J Nucl Med 1991; 32:2118–2123.
Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose andl-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med 1‘995; 36:252–258.
Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro 1984; 20:663–670.
Stern PH, Wallace CD, Hoffman RM. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J Cell Physiol 1984; 119:29–34.
Wheatley DN. On the problem of linear incorporation of amino acids into cell protein. Experientia 1982; 38:818–820.
Weiss SW. WHO international histological classification of tumours. Histological typing of soft tissue tumours, 2nd edn. Berlin Heidelberg New York: Springer, 1994.
Langstrom B, Antoni G, Gullberg P, et al. Synthesis ofl-and d-[methy-11C]methionine. J Nucl Med 1987; 19:1037–1040.
Leskinen-Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Joensuu H. Uptake of11C-methionine in breast cancer studied by PET. An association with the size of S-phase fraction. Br J Cancer 1991; 64:1121―1124.
Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175(Suppl 2):57–63.
Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999; 91:432–439.
Verstraete KL, Lang P. Post-therapeutic magnetic resonance imaging of bone tumors. Top Magn Reson Imaging 1999; 10:237–246.
Fletcher BD. Effects of pediatric cancer therapy on the musculoskeletal system. Pediatr Radiol 1997; 27:623–636.
Murphy WA Jr. Imaging bone tumors in the 1990s. Cancer 1991; 67:1169–1176.
Hawkins DS, Rajendran JG, Conrad EU 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer 2002; 94:3277–3284.
Findlay M, Young H, Cunningham D, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 1996; 14:700–708.
Muhr C, Gudjonsson O, Lilja A, Hartman M, Zhang ZJ, Langstrom B. Meningioma treated with interferon-alpha, evaluated with [11C]-l-methionine positron emission tomography. Clin Cancer Res 2001; 7:2269―2276.
Andersson T, Wilander E, Eriksson B, Lindgren PG, Oberg K. Effects of interferon on tumor tissue content in liver metastases of human carcinoid tumors. Cancer Res 1990; 50:3413―3415.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was part of the 10-Year Strategy for Cancer Control of the Japanese Government and was supported by the Ministry of Education, Science and Technology of Japan Government
Rights and permissions
About this article
Cite this article
Zhang, H., Yoshikawa, K., Tamura, K. et al. Carbon-11-methionine positron emission tomography imaging of chordoma. Skeletal Radiol 33, 524–530 (2004). https://doi.org/10.1007/s00256-004-0815-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-004-0815-5