Abstract
Sediment metal profiles were carefully examined for their distribution, chemical fractions, and mobility in three major marine-estuarine transects in the world’s largest mangrove (Sundarban) ecosystem because anthropogenic influences had been very often noticed. The level of pollutants was determined by CF and PLI. Metal contents in the freshly deposited sediment of Hugli transect were relatively high (Pb, Fe, Zn, Mn, Ni, and Co) in comparison to Matla and Saptamukhi due to lithogenic contribution from large catchments area (8 × 105 km2). Principal component analysis and correlation coefficient revealed that the concentrations of metals were mainly governed by the variability of the sediment properties. Sediment was subjected to sequential chemical extractions to delineate metal fractions, namely exchangeable, carbonate, Fe–Mn oxide, organic, and residual. This indicated a valuable insight into the geochemical mode of the metal retention and their environmental behavior. Single extraction did not help significantly to explain the metal profiling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sources of metals in the Sundarban Marine-estuarine (SME) system are numerous. The deltaic riverine system (Hugli–Matla–Saptamukhi), a large section of SME, is largely exposed to anthropogenic influences and thereby metals are accumulated in the river sediments (Tessier et al. 1994; Bryan and Lengston 1992). But metals may not be fixed permanently to sediments rather they may be remobilized through physical, chemical, and biological processes (Salomons et al. 1987). Dissolved metals gradually change their forms in the marine-estuarine physico-chemical processes either through precipitation as oxides and/or carbonates and/or formation of complexes during transportation and mobilization (Salomons and Forstner 1984). Therefore, total content of metals present in the sediment may not provide a clear picture of the metal loads that have been deposited in the marine-estuarine sediments and ultimately play a key role in the environmental behaviour of metals (mobility, pathways, and bioavailability). The metals are often associated with various sediment components (eg carbonates/organics/oxides/hydroxides) in different ways. Various factors such as bioturbation, resuspension, burial, pH, and Eh status generally regulate their mobility and bioavailability (Burton et al. 2005). In these situations the knowledge of fractionation will give a more complete description of environmental risk (Ryssen et al. 1999). Research has been carried out so far to focus only the metal concentration in SME environment (Subramanian et al. 1988; Gibbs 1977; Vaithiyanathan et al. 1993; Panda et al. 1995; Modak et al. 1992). The on-going anthropogenic activities in the upper catchment areas may result the enrichment of metals in the SME system. The present study aimed at the assessment of the spatial distribution of metals in freshly deposited sediment and identification of the governing factors responsible for the variability of metal concentrations in the sediment and their bioavailability (chemical forms). For partitioning many sequential extraction methods for sediments and soils are available in the literature (Tessier et al. 1979; Sposito et al.1982; Welte et al. 1983; Clevenger 1990; Ure et al. 1993; Howard and Vandenbrink 1999; Ianni et al. 2001)—but the method of Tessier et al. (1979) is most widely used to evaluate the possible chemical associations of metals in different geo-chemical fractions in sediments. A chemical fractionation approach has been employed to demonstrate the vulnerability of bio-availability of metals.
Study area
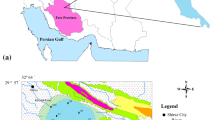
The Sundarbans (426,300 ha), situated at the apex of the Bay of Bengal (between 21°13′ to 22°40′N latitude and 88°03′ to 89°07′E longitude) is the largest world delta complex being controlled by the depositional activities of the Ganges and Brahmaputra. A group of islands and a dense network of rivers, canals, and creeks comprise the area (Fig. 1). In West Bengal the flow of river Ganga (traversing distance ~2,000 km) has bifurcated (Padma Bay of Bengal via Bangladesh; Bhagirathi also called Hugli Bay of Bengal via West Bengal, India) and ultimately meets SME at Bay of Bengal. A number of tributaries (Jalangi, Churni, Ajoya, Damodar, Roopnaryan, and Haldi) have also adorned the area. SME system comprises of Thakuran, Matla, and Saptamukhi. The study area comprises the delta plains fronts and the shallow coasts are with mud flats of soft sediments. The inter-tidal zone registers a gradual change (sandy to silty nature) in the textural characteristics owing to high/low water level. The sand flats (mixed and open-sea inter-tidal zone) consist of 90–95% of sand fine to very fine. The confluence points are heavily silted during monsoon and thereby contributing large amount of micro-pollutants carried out from upland areas (Dutta and Subramanian 1997).
Methods and materials
In this study, 15 sampling stations were selected along an array of three transects from east to west at the mouths of Matla, Saptamukhi, and Hugli estuaries. The five sampling stations (shown in Fig. 1 as S1, S2, S3, S4, and S5) at each transect were fixed at following locations:
-
(1)
Inshore (500 m before coast line),
-
(2)
Close to shore (2 km offshore),
-
(3)
5 km from the coastline,
-
(4)
10 km from the coastline,
-
(5)
22.5 km from the coast line.
Sediment samples were collected from on board CRV (Coastal Research Vessel) Sagar Poorvi during winter using Van-Veen Grab sampler. At each station five sediment samples were collected to maintain the accuracy of the findings. All the samples were stored separately in precleaned polyethylene bags and transported to the laboratory. Samples were air dried in a dust free environment and grounded with pestle and mortar and sieved through a 200 μm sieve and then kept sealed in screw cap polyethylene bottles.
Total metal concentrations: for measurement of total metals, samples were digested with sodium chloride and a mixture of nitric acid and perchloric acid (Thompson and Wood 1982). Few sub samples were digested by Microwave digestion (CEM MDS-2000) using 5 ml aqua regia as per manufacturer’s recommendation.
The single extraction method was carried out with water and EDTA (MAFF 1986 ). A total of 5 g air dried (200 μm fraction) sediment sample was extracted with 50 ml of water and 0.05 M EDTA (disodium salt) solution at pH 7.0, respectively in a shaker for 1 h at 25°C and centrifuged. Sequential extraction was carried out in accordance with procedure used by Tessier et al. (1979) already modified by Baffi et al. (1998) with improvements made according to the European Community Bureau of Reference(BCR 701). A total of 5 g air-dried sediment samples (200 μm fractions) were taken for extraction of metals in different fractions.
The analyses of the entire pretreated sample were performed using Flame AAS of GBC (Avanta PM). For Calibration, the standards traceable to NIST were used. Recalibration check was performed at regular interval. Quality control was carried out using sample replicates, blank and certified reference materials. The total metal concentrations showed good agreement with Microwave digestion method. The accuracy of the total extraction was evaluated using the certified reference material (MBSS) prepared by Akademie der Wissenschafen der DDR, Institut fur Meereskunde and certified by analyzing in 42 laboratories in 18 states. The sequential extraction determined in different fractions was crosschecked by comparing the total concentration and the sum of the five phases of the sequential extraction in few sediments. This comparison reveals very good agreement between total and sum of the fractions. Analytical standards were prepared in the corresponding extract to minimize matrix effects.
The estimation of organic carbon and the determination of percentage of sand, silt, and clay in sediments were carried out in accordance with method described by Gee and Bauder (1986).
In this study, statistical methods such as mean, median, standard deviation, co-efficient of variation, multiple regression analysis, and principal component analysis were employed for interpretation of data.
Contamination Factors (CF) were computed utilizing the metal content in sediment and continenetal shale (Turekian and Wedepohl 1961) as background value to understand the contamination state of the metals in sediment. Pollution Load Index (PLI) was computed as recommended by Tomlinson et al. (1980). The calculation methods are given below.
where,
Results and discussion
Characterisation and distribution of metals
The assemblage of analytical results of SME at all the stations (S1, S2, S3, S4, S5) of each transect were analyzed to determine the mean, co-efficient of variation(CV), median and range of each metal concentrations (Table 1). Simultaneously these statistical parameters of organic matter and sediment texture (sand, silt percent, and clay) are also determined (Table 1). The CV revealed that magnitude of variation of concentration of metals among the stations of each transect was different and in the order of Matla (26-54%) > Saptamukhi (14–52%) > Hugli (9–27%). In Hugli, relatively high mean values with low CV demonstrated that the study stretches of the Hugli transect was more vulnerable in terms of metal accumulation in the freshly deposited sediment. The large number of industries and major towns in the basin of Hugli river may be the reasons of such higher values. In this context it may be mentioned that CV was less than 20% for Co (9%), Mn (14%), and Ni (18%) and less than 30% for other metals. In Matla, average concentrations of Fe, Cu, Mn, Ni, and Co were lower than that of Hugli though average concentrations of Pb and Zn in both the transects were same. But CV revealed wide variations of concentrations of metals among the stations of each transect. Whereas in Saptamukhi, mean values of all the metals were relatively low with respect to Hugli and Matla. The elevated level of Fe in the sediment of all transects indicated a natural enrichment in Fe concentrations mainly due to the high weathering and erosion of the soils in vast catchments area. In general, average concentration of metals considering all the values of each transect were below the World Shale value except Co as shown in Table 1. The minimum and maximum concentration of metals along with organic carbon and sediment texture are presented in Table 1 to show the ranges of their concentrations. But nature of the distribution of sediment texture is unlikely that of metals because there is no close agreement between mean and median. That may be attributed to lack of analytical accuracy. Good agreement between mean and median of the metal concentrations (Table 1) exhibits nature of distribution of the above parameters in the study area. Considering wide variation of all the above parameters, distribution pattern among the stations of each transects are discussed below.
Distribution pattern of heavy metals with the increase of distance from inshore to offshore are graphically presented in Fig. 2 for each transect. The bar diagrams of the two transects (Matla and Hugli) show irregular pattern of distribution with the increase of distance (up to 22.5 km) from the inshore line in the freshly deposited sediment except Pb in Hugli where incline trend was prominent. In Saptamukhi, inclined trend of metal concentrations was mainly observed with the distance from the inshore line. It was interestingly noted that concentrations of all the metals were lowest at the inshore point of Hugli and highest (except Pb) at inshore point of Matla. Such unique feature did not demonstrate the pollutant load to SME system but anthropogenic activities and soil erosion going on in vast catchments area of Hugli were the major source of pollutants. Therefore, distribution pattern can not be simply explained in terms of input of metals from natural and anthropogenic sources. It is interesting to note that the distribution pattern of organic carbon is largely identical with metal distribution pattern in all the stations of each transect. In the light of above, it may be inferred that rate of sedimentation and input of metals to Hugli and Matla are relatively high. It has been reported that these two tributaries bring huge amount of silt during monsoon season (Khan 1995) in addition to other pollutants from upland (Khan 1995). The sediment flux through the SME is one of the highest on a global scale (Dutta and Subramanian 1997). The sediment enriched with metals carried out from these two rivers are continuously deposited over the bottom of entire flat and shallow coast perpendicular to these two rivers. The deposition of fresh sediment at different sampling points may be governed by water circulation pattern and textural characteristics (Sarkar et al. 2002). In Saptatamukhi, both sedimentation rate and metal load are quite low and that led to less deposition of fresh sediment perpendicular to this river. Based on the above observations it may be mentioned that mutual identical behavior of metals, organic carbon, and sediment texture in the freshly deposited sediments as well as highly buffered sea water exhibited crucial role in accumulation of metals in the sediment.
Contamination factor and pollution level index
The average concentration of heavy metals are below the continental Shale as shown in Table 1. The order of prevalence (ranking) of these metals is by and large similar to the order of continental Shale barring nickel (Table 1). However, a little disorder is also observed (Co, Pb, and Cu in Table 1). The results of contamination factor and pollution level index for each metals were tabulated and shown in Table 2. All the values were below except nickel and Co. The order of abundance along with average concentration of metals (CF < 1 and PI < 1) clearly indicated that the SME sedimentary environment was less contaminated in comparison to International Standards. But input of metals to the SME from vast catchments area is not negligible. Therefore, further study is needed to evaluate the fate of these metals in the sediment.
Relationship of metals with sediment properties
The variability of metal concentration in the sediment was found more or less identical when compared with distribution pattern of organics and sediment texture. Considering this fact the correlation coefficients among all the parameters (metals, texture, and organic) are determined to establish their interrelationship among each other. The correlation coefficient explored the significant positive correlation of metals (r > 0.75, and n = 60) with organic (r > 0.75 and n = 60), clay (r > 0.79 and n = 60), and silt (r > 0.71 and n = 60) whereas negative correlation with sand (r > −0.75 and n = 60). Only Pb showed slightly weak correlation with sediment texture (r = 0.3 and n = 60) compared to other metals. However, these close associations of metals with organic, clay, and silt are the indication of strong bindings of metal with sediment. This SME is funnel shaped extensive tidal flats with strong bidirectional current (Thom 1984). The tremendous erosive power of alongshore tidal currents (Sarkar et al. 2002) prevented formation of barrier islands and redistributes sediment at deeper parts. The characteristic smaller grain sizes having large adsorptive reactions may serve as potential trap for metals (Dutta and subramanian 1997). Therefore, concentration of metals in the freshly deposited sediments particularly at tide-dominated coastal waterways are largely controlled by hydrodynamic processes of the marine environment. (Selvam 2003).
Nature of variability of metals
The above discussion clearly showed that variability of metals in the study area was mainly governed by the sediment properties. Therefore, the Principal Component Analysis (PCA) of the data of each transect (Table 3) was done to explain the role of sediment properties and organics in regulating the level of metals in freshly deposited SME sediment. The first component explains the variability of 89.3, 89.2, and 72.9% in Saptamukhi, Matla, and Hugli, respectively. In the second component, the percent of variance was achieved to 95.7, 95.7 and 90.8 in Saptamukhi, Matla, and Hugli, respectively. In Hugli, that value of 95% was achieved in third component. Therefore, two components account for a maximal amount of total variance in the observed variables. Maximum amount of total variance in the first component indicated correlation with most of the observed variables. Therefore, role of sediment properties in governing the metal content in freshly deposited sediment is clearly established. For confirmation variable loading of these two components are shown in Fig. 3 to explain the contribution of the variables in these two components. The component I represented the highest contribution of silt in the sediments of Saptamukhi (Fig. 3a). This means that the local environment and the dynamic process is playing crucial role regarding accumulation of the metals in freshly deposited sediment. Similar situation has been observed in the Hugli river where first component is again silt (Fig. 3e) playing major role in accumulation of metals. The component I in Fig. 3a of Saptamukhi indicated that Fe and Co contributed certain percentage of variance in positive direction and Pb and clay in negative direction. The component I in Fig. 3e of Huglii indicated that Fe and Zn contributed certain percentage of variance in positive direction and Pb, Mn, and clay in negative direction. Bipolar nature of Pb showed uniqueness in behavioral pattern than that of Fe and Co in Saptamukhi(Fig. 3a) and Fe and Zn in Hugli (Fig. 3e). These features indicate the origin of excess load of Pb in the sediment from anthropogenic activities. The component I of Fig. 3c represented the highest contribution of clay in the sediments of the Matla river with negative loading features. The possible reason behind negative loading feature is that the formation of clay is difficult due to physical separation of finer grain size with their long term depositional phenomenon in the sediment because the system behaves dynamically with ebb and tide frequency. Therefore, the physically separated finer grain particles remain in dissolved phase and carried out to the much deeper part of the fore deep. The Matla is maintaining more dynamic flow pattern as well as variation in the bulk distribution of silt and mud. The influence of clay on variability of metals in this transect revealed more input of organic load to river Matla because mangrove forest in the most productive sites produce organic substances in excess of the ecosystem requirement. In the second component again clay was the highest in Matla and Ni in Saptamukhi and Hugli. Though the second component indicated influence of some variables but contribution with respect to total variance was insignificant. In this environment, systematic sorting of sediment due to natural agents causes formation of silt and clay in this area. This condition clearly indicated that occurrences of metals were clearly dependent on the sediment properties. The PCA results in this study not only clearly explained the cause of variability of metals but also support the interrelationship among the metals.
Bioavilable fraction—single extraction
The present study also deals with metal fractionation with the help of single extraction (SE). The SE study (1:EDTA, 2:water in Table 4) reveals that the percentage of metals in EDTA extractant was relatively high and quite variable. It was noted that Mn, Cu, and Pb were found extracted with EDTA in the range of 34–50, 19–28%, and 36–53%, respectively whereas Ni in the range of 3.5–10%. All other metals were found below 3%. Interestingly Ni, Cu, and Mn in EDTA extractant were relatively high in the sediment of Matla and Pb in the sediment of Saptamukhi. On other hand the water extraction is practically negligible and/or absent (BDL). Both the study signifies that single extraction may not be a helpful tool to understand the complete metal profiling in freshly deposited sediment of SME segment. The scanty data profile of water extractant clearly demonstrates that water soluble metals were either absent and/or negligible in quality. The EDTA extraction of Pb and Mn indicates that they have tendency towards formation of metals complex (Mn-EDTA complexity; Pb-EDTA complex). Therefore, the presence of dissolved organic legends (biodegraded product of humic acid from catchments area particularly during flooding) may form metal complex and further transported to deeper areas.
Geochemical fractions—sequential extraction
The major objective of the study is to focus behavior of the seven metals in SME environment by their specific physico-chemical forms rather than simply total concentration. Therefore, all the physico-chemical forms (residual, organic, metal oxide, carbonate, and exchangeable) in freshly deposited sediment of the SME of Sundarbans are carefully determined and shown in Table 5. The major outcome of the study is that the residual fraction is dominating throughout the experiment with the exception of Pb in Saptamukhi and Mn in Hugli. No uniform order of different fractions in respect of seven metals in different transects was observed. Fractionation study showed that residual fraction was dominating at more than 80% level for Fe in all the transects and for Cu, Ni, and Co in Hugli. In Saptamukhi and Matla, more than 70% of Ni was associated with residual fraction. Association of Fe, Cu, Ni, and Co with the residual fractions reflects the holding capacity for a long period of time under the conditions usually encountered in SME system. The transportation of minerals such as appetite, magnetite, cuprite etc. from the vast catchment area(Dutta and Subramanian 1997; Panda et al. 2006; and Yokayama et al. 1990) to SME may accelerate the transformation of the Co, Ni, Fe, and Cu from weakly bound fractions to more strongly bound fractions (Seaman et al. 2001; Knox et al. 2003; Kaplan et al. 2002) particularly in Hugli. These metals in these sediments are not exchangeable because metals sorbed to sediment did not occur via non-specific, outer sphere by electrostatic mechanism (Fujiyoshi et al. 1996). This means that bioavailability of the metals are restricted (low exchangeable from) in SME system and they are mostly arrested in the silicates (residual fraction) and thereby mobility is relatively retarded. The residual fractions of other metals were varying from 33 to 66%. The poor availability of residual fraction of other metals reflects the abundance of other fractions those may not have firm bindings to the sediment. Organic fraction of Zn was quite prominent in all the transects and present in the order of Saptamukhi (36.2%) > Hugli (33.2%) > Matla (27.6%). It was interestingly noticed that the highest (39.4%) association of Pb was found with organic fraction in Saptamukhi followed by Matla (27.5%). On other hand organic fraction of Pb (7.1%) was found relatively low that was compensated by carbonate fraction (14.1%) because carbonate fraction in Saptamukhi and Matla was quite low. Next to Pb and Zn, organic fraction of Ni was quite prominent in Matla (14.2%) and in Saptamukhi (13.1%). In this SME system like other metals, large fraction of Pb may be expected as residual fraction in the form of pyromorphite (Ma et al. 1993) but in reality organic and Fe–Mn oxide fractions were relatively high in this study area. Fe–Mn oxide along with organic matter is generally considered predominant metal sorbent due to its large surface area (Forstner and Wittmann 1979). This clearly reflects the dominance of anthropogenic influence and natural processes. The organic phase of sediments may be the significant scavenger for Pb, Zn, and Ni in this SME system. The organic fraction of Fe, Cu, Mn, and Co was quite low in all transects. The highest association of Pb with Fe–Mn oxide was found in Saptamukhi (22.1%) followed by Hugli (17.1%). Fe–Mn oxide fraction of Co (17–17.8%), and Cu (11–15.6%) was also prominent in Matla and Saptamukhi. Association of Fe and Mn with Fe–Mn oxide was almost similar and in the range of 9.2–12.6%. Another interesting feature in fractionation of Mn in Hugli is that association of Mn with carbonate fraction was ranking first with 44.8% association. The carbonate fraction of this metal in Saptamukhi and Matla was also prominent with 35% association. Next to Mn, the carbonate fraction of Cu and Co in Saptamukhi and Matla and Pb in Hugli was also prominent. This fraction is bioavailable to the biota ingesting sediment. These elevated carbonate fractions indicates that the environment may be enriched with dissolved bicarbonate ion (high alkalinity) and thereby precipitating carbonates in the sediments. Mn, Cu, Pb, and Co has the similarity in their ionic radii to that of calcium. As a result, the precipitation of such carbonate (manganese carbonate, copper carbonate, lead carbonate, and cobalt carbonate slightly soluble in water) are possible in an environment where competitive mechanisms are going on and thereby carbonate of these metals may appear in the freshly deposited sediment. This condition may form Rhodochrosit (manganese carbonate), caledonite (lead copper carbonate) sphaerocobaltite (cobalt carbonate) like minerals. It may also happen that such metal carbonate in particular form may be further mobilized in reducing condition in the various part of SME system. Next to carbonate fraction, Cu was associated with the Fe–Mn oxides because organic fraction was negligible. The exchangeable fraction of Pb in Hugli, Co and Cu in Saptamukhi and Matla was quite prominent though The exchangeable fraction of Mn in all the transects are consistent and available in the range of 2.1–3.2%. Interestingly Zn in all transects and Pb in Saptamukhi and Matla and Ni and Co in Hugli were absent. One interesting feature was noted that prominent association of metals in carbonate and Fe–Mn oxide showed availability of metals in exchangeable form. The Fe–Mn oxide fraction may be released through reductive dissolution of the oxide minerals at Eh less than approximately +250 mv for Mn oxide and +100 mV Fe oxides (Patrick and Jugsujinda 1992). The metals were available as exchangeable fraction may be the cause of concern in terms of bioavalibility.
Conclusion
This study clearly revealed the distribution pattern of seven metals in the world’s largest mangrove (Sundarban) ecosystem. The metal content in sediment of the entire study stretch are far below the continental shale except Co. The loadings of PCA demonstrated that the variability of the metals in this zone was mainly governed by the sediment properties. Bipolar nature of few metals observed in PCA and correlation coefficient among the metals exhibited the distinctive dominance of natural and anthropogenic sources. In this context it may be mentioned that the environmental behavior of Pb, Co, and Zn clearly exhibited anthropogenic sources. The textural composition and organic matter of freshly deposited sediments play crucial role in the sorption and complexation of transition metals. Though anthropogenic inputs persist but estuarine–marine condition (physical and chemical) in this zone prevents enrichment of metals in the sediment. The outcome of sequential extraction revealed a valuable insight into the geochemical mode of the metal retention. The strong association with residual part and poor association with exchangeable portion for few metals at particular transect indicated low availability and thereby bio-availability or mobility was not in alarming level to biotic community. Whereas, exchangeable fraction of few metals those are easily removed and used by organisms was present. This fraction is potentially toxic for the organisms. Also the prominent association of few metals with the phases of organic, carbonate and Fe–Mn oxide in particular transects can be solubilized depending upon physical and chemical parameters, for example oxygen content and redox potential changes, and bacterial activity and may be toxic to the organisms. This study focused that the variation of percentage of metals in different phases in the SME system indicated large heterogeneities in the distribution of metals among phases in the freshly deposited sediments and showed the difficulty of performing a geochemical evaluation in complex environments of SME.
References
Baffi F, Ianni C, Soggia F, Magi E (1998) Evaluation of the acetate buffer attack of a sequential extraction scheme for marine particulate metal speciation studies by scanning electron microscopy with energy dispersive X-ray analysis. Anal Chim Acta 360:27–34
Bryan GW, Lengston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries. Rev Environ Pollut 81:89–131
Burton ED, Phillips JR, Hawker DH (2005) Geochemical partitioning of Cu, Pb and Zn in Benthic and Estuarine sediment profile. J Environ Oval 34:263–275
Clevenger TE (1990) Use of sequential extraction to evaluate the heavy metals in mining wastes. Water Air Soil Pollut 50:241–255
Dutta DK, Subramanian V (1997) Texture and mineralogy of sediments from Ganga–Brahmaputra–Meghna river system in the Bengal Basic, Bangladesh and their environmental implications. Env Geol 30(3–4):181–188
Forstner U, Wittmann GTW (1979) Metal pollution in the aquatic environment. Springer, Berlin, p 386
Fujiyoshi R, Gomei T, Katayama M (1996) Sorption of Zn(II) on marine sediments by a sequential extraction—radiotracer technique. Appl Ratiat Isot 47:165–169
Gee GW, Bauder JW (1986) Method of soil analysis-particulate size analysis-internetional pipette method, part 1, Second edn. Soil Science Society of America, Wisconsin, pp 399–401
Gibbs RJ (1977) Transport phases of transition metals in the Amazon and Yukon rivers. Geol Soc Am Bull 88:829–943
Howard JL, Vandenbrink WJ (1999) Sequential extraction analysis of heavy metals in sediments of variable composition using nitrilotriacetic acid to counteract resorption. Environ Pollut 106:285–292
Ianni C, Ruggieri N, Rivara P, Frache R (2001) Evaluation and comparison of two selective extraction procedures for heavy metal speciation in sediments. Anal Sci 17:1273–1278
Kaplan DI, Knox AS, Coffey C (2002) Reduction of contaminant mobility at the TNX Outfall Delta through the use of apatite and zero-valent iron as sediment amendments. WSRC-TR-2002–00370. Westinghouse Savannah River Company, Aiken
Khan RA (1995) Ecology of the Hugli-Matla estuarine system. Estuarine ecosystem series, Part 2: Hugli–Matla estuary. Zoological Society of India, Calcutta, pp 417–464
Knox SC, Kaplan AS, Adriano DC, Hinton TG (2003) Evaluation of rock phosphate and phillipsite as sequestering agents for metals and radionuclides. J Environ Qual 32:515–525
Ma LQ, Traina SJ, Logan TJ (1993) Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ Sci Technol 27:1803–1810
Ministry of Agriculture, Fisheries, Food (MAFF) (1986) The analysis of agricultural methods: a manual of the analytical methods used by the Agricultural Development and Advisory Service, 3rd edn. Her Majesty’s Stationary Office, London
Modak DP, Singh KP, Chandra H, Ray PK (1992) Mobile and bound forms of metals in sediment of lower Ganges. Water Res 26:1541–1548
Panda D, Subramanian V, Panigrahy RC (1995) Geochemical fractionation of heavy metals in Chilka lake (east coast of India)—a tropical coastal lagoon. Environ Geol 26:199–210
Panda UC, Rath P, Saha KC, Mazumder S, Sundaray SK (2006) Environmental quantification of heavy metals in the Subarnarekha estuary and nearshore environment, east coast of India. Asian J Water Environ Pollut 3(2):85–92
Patrick WH, Jugsujinda A (1992) Sequential reduction and oxidation of inorganic nitrogen, manganese and iron in flooded soil. Soil Sci Soc Am J 56:1071–1073
Ryssen RV, Leermakers M, Bacyam W (1999) The mobilisation potential of trace metals in aquatic sediments as a tool for sediment quality classification. Env Sci Pol 2:75–86
Salomons W, De Rooji NM, Kevdrigk Bril J (1987) Sediments as a source of contaminants. Hydrobiologia 13:30–38
Salomons W, Forstner V (1984) Metals in the hydrocycle. Springer, Berlin, p 349
Sarkar SK, Bhattacharya B, Debnath S, Bandopadhyay, Geri S (2002) Heavy Metals in biota from Sundarban Wetland Ecosystem India: Implications to monitoring and environmental assessment. Aquat Geosyst Health Manage 5(4):467–472
Seaman JC, Arey JS, Bertsch PM (2001) Immobilization of nickel and other metals in contaminated sediments by hydroxyapatite addition. J Environ Qual 30:460–469
Selvam V (2003) Environmental classification of mangrove wetlands of India. Curr Sci 84(6):757–765
Sposito G, Lund J, Chang AC (1982) Trace metal chemistry in arid- zone field soils amended with sewage sludge: Fractionation of Ni, Cu, Zn, Cd and Pb in solid phases. Soil Sci Soc Am J 46:260–264
Subramanian V, Jha PK, Jergrieken R (1988) Heavy metals in the Ganges estuary. Mar Pollut Bull 19:290–293
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of trace metals. Anal Chem 51:844–851
Tessier A, Carignam R, Belzile N (1994) Processes occurring at the sediment- water interface: emphasis on trace elements. In: Buffle J, De Vitre RR (eds) Chemical and biological regulation of aquatic processes. Lewis, Chelsea, pp 137–175
Thom BG (1984) Coastal landforms and geomorphic processes. In: Snednker SC, Snedaker JG (eds) Mangrove ecosystem: research methods. UNESCO, Paris, pp 3–17
Thompson I, Wood S (1982) In: Cantle EJ (ed) Atomic absorption spectrometry. Elsevier, New York, pp 261–284
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgolander Meeresuntersuchungen 33:566–575
Turekian KK, Wedepohl KH (1961) Distribution of the Elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Ure A, Quevaullier Ph, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the CEC. Int Environ Analyt Chem 51:135–151
Vaithiyanathan P, Ramanathan AL, Subramanian V (1993) Transport and distribution of heavy metals in Cauvery river. Water Air Soil Pollut 71:13–28
Welte B, Bles N, Montiel A (1983) Study of the different methods of speciation of heavy metals in the sediments: II Application. Environ Tech Lett 4:223–238
Yokayama K, Amano K, Taira A, Saito F (1990) Mineralogy of silts from the bengal fan proceedings of the Ocean Drilling Programme. Scientific Results 116:59–73
Acknowledgments
Authors acknowledge Central Pollution Control Board to provide all facilities to carry out the above work. We are grateful to Dr M.N. Das, my teacher, for his guidance in statistical interpretation of data and Sri B.R. Naidu, then Incharge of Zonal Office, Central Pollution Control Board for his encouragement towards this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, D., Mukherjee, A. & Kumar, B. Chemical fractionation of metals in freshly deposited marine estuarine sediments of sundarban ecosystem, India. Environ Geol 58, 1757–1767 (2009). https://doi.org/10.1007/s00254-008-1675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1675-4