Abstract
The concurrent use of karst aquifers as drinking water resources and receptors of combined sewer overflow lacking appropriate pre-treatment may cause conflicts between drinking water supply and storm water management. A storm water tank (SWT) for combined wastewater is identified as the source of sporadic contamination of a karst spring (Gallusquelle, “Schwäbische Alb”, SW Germany) used for public water supply. Spring water quality was examined by routine and event sampling and by evaluating physicochemical and microbiological parameters. The total number of microbial colonies growing at 20°C and the number of Escherichia coli colonies rose to values up to four orders of magnitude higher than background, 2–5 days after overflow of the SWT. High concentrations of chloride, sodium, and total organic carbon (TOC) and high values of turbidity coincide with this increase. However, high bacterial contamination is also observed while turbidity and TOC are low. Several wastewater-related organic micro-pollutants such as chlorinated and non-chlorinated organophosphates were detected in the SWT and, depending on their K ow values and their biodegradability, in lower concentrations at the spring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst catchments are predominantly drained via underground channels or conduits created by the widening of fractures in soluble rocks such as limestone or dolomite. As a consequence, surface waters are scarce on karst plateaus. The karst conduit system generally focuses flow to prominent points of resurgence situated in the valleys. These springs are often used for drinking water supply. According to Ford and Williams (2007), karst aquifers provide drinking water for nearly a quarter of the world’s population.
Unfortunately, these important water resources are particularly vulnerable to anthropogenic contamination because of the typically thin soil layers and the high permeability of the karstified host rock, which lead to short residence times and low natural attenuation capacities. Vesper et al. (2001) provided an overview of contaminants and their transport behaviour in karst aquifers. Potential threats to karst waters include non-point sources, such as fertilizers and pesticides (e.g. Ryan and Meiman 1996) and street runoff (e.g. Zhou et al. 2003) as well as point sources, such as contaminated sites, landfills, and wastewater infiltration. Point-source contamination is often related to hazardous events, for example, catastrophic sinkhole collapses in retention basins (Jenkins 1988) and wastewater lagoons (e.g. Memon and Azmeh 2001). Kaçaroğlu (1999) reports that wastewater has been injected into sinkholes and swallow holes. In extension to the above-mentioned studies, this work addresses the adverse effects on drinking water supplies that may result from the approved operation of wastewater utilities in karst areas.
Considering the threats originating from wastewater utilities, the type of wastewater system is of crucial importance. In Germany as in many other industrialized countries a combined wastewater system is prevalent, that is, domestic wastewater and storm water runoff from impervious surfaces (streets, roofs etc.) are collected in the same sewer system. During rain events, the wastewater treatment plants as well as the sewer systems have to cope with a high load of combined wastewater that may exceed the systems’ capacity. Thus, certain volumes of wastewater must be stored or discharged. This may be achieved by storm water overflow basins or storm water tanks (SWT), with both retention and treatment functions (settling of particles). These facilities consist of a concrete basin with an inflow branch for combined wastewater, an outlet to the treatment plant and an overflow for excess wastewater. Usually, SWTs are located near surface waters, where the sewer overflow is discharged and diluted. Due to the lack of surface waters, a less favourable option is implemented in karst regions. The sewer overflow is directly discharged into the aquifer system via sinkholes or dry valleys. These surface depressions are typically closely connected to the karst conduit system and lead to rapid infiltration and transport of polluted water. The overflow of an SWT therefore represents a point source with potentially adverse effects on karst water resources, for instance, contamination of spring waters with suspended solids (turbidity), organic material, faecal bacteria including pathogens, and organic micro-pollutants.
To investigate the adverse effects of sewer overflows on the water quality in karst terrains, water quality changes were monitored at a karst spring (Gallusquelle) on the Jurassic formation of the “Schwäbische Alb” in southwest Germany. At this spring, water quality deteriorations frequently observed after storm events are believed to originate from overflows of an SWT at a distance of 9 km (P. Knaus, water supply company Hermentingen, personal communication). In detail, the objectives of this study are to
-
(1)
quantify the intensity and the time scales of water quality changes at different hydrologic conditions,
-
(2)
identify similarities and differences in the behaviour of various wastewater contaminants such as turbidity, bacteria, and organic micro-pollutants, and
-
(3)
evaluate whether sum parameters such as turbidity or total organic carbon provide sufficient information to monitor and manage spring water quality.
Building on the work of Heinz et al. (2006), who provided a preliminary evaluation focusing on a single overflow, this paper analyses and compares data of five overflow events of the SWT and the subsequent changes in water quality parameters at the spring. Discharge, electrical conductivity and the ions sodium and chloride provide information on the general hydrological conditions in the aquifer; in addition, total organic carbon (TOC), turbidity and the number of colonies at 20°C as well as of Escherichia coli were analysed to evaluate the influence of wastewater.
For one overflow event, the concentrations of organophosphate flame-retardants, polycyclcic musks and the bactericide triclosan were used as marker compounds for wastewater contamination (Andresen et al. 2004; Bester 2003, 2004; Meyer and Bester 2004). The persistent chlorinated organophosphates with low lipophilicity (pK ow 2–3) were used as markers for prevalent conservative transport, for example, tris-(2-chloro-, 1-methyl-ethyl)-phosphate (TCPP), tris-(2-chloroethyl)-phosphate (TCEP), and tris-(2-chloro-, 1-chloromethyl- ethyl)-phosphate (TDCP). The lipophilic polycyclic musks (pK ow 5–6), galaxolide (HHCB) and tonalide (AHTN) were used as markers for sorption processes, triclosan and the non-chlorinated organophosphates tri-n-butylphosphate (TnBP), triphenylphosphate (TPP), and tris(butoxyethyl)-phosphate (TBEP) as markers for biotransformation processes.
Material and methods
Study area
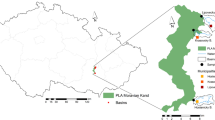
The Gallusquelle karst spring is located on the plateau of the western Schwäbische Alb in southwest Germany. It drains a catchment area of approximately 45 km2 and has an average discharge of 0.5 m3 s−1. The predominant form of land use within the spring catchment is forest (60%). The remaining part is under agricultural (>35%) or urban use (<5%) (Sauter 1992).
The aquifer is formed within the marine sequence of Upper Jurassic carbonates. An SWT with overflow, as described in the Introduction, is located approximately 9 km from the spring and belongs to the combined wastewater drainage and treatment system of two villages of approximately 4,000 inhabitants (Bitz and Freudenweiler). The SWT lies within the Harthauser dry valley that follows the course of the Hohenzollern fault zone (Fig. 1). Long-term observations indicate that overflows occur 9–10 times per year and each of these events releases on average approximately 23,000 m3 of combined wastewater into the valley (Engineering Company Kovacic, Sigmaringen, Germany, personal communication). Both, a sufficiently high rain amount and intensity are required to cause an overflow of the SWT. These conditions are usually reached several times during the summer months. The water released from the tank follows the course of the dry valley over a total distance of up to 1 km through an unsealed trench and further along a forest and meadow terrain. The seepage process is assumed to occur along the entire pathway.
Sampling
Water samples were collected at the spring for analysis of microbiological parameters, TOC content and major ions. Samples were collected once a week during dry periods. After overflow events #1 and #2, samples were collected every 4–6 h to monitor the contaminant breakthrough. After overflow events #3, #4 and #5, only sporadic samples were collected during and after the contaminant breakthrough. Samples for the analysis of major ions were taken manually in 50-mL PET bottles or, during the sampling period with higher frequency, with an automatic water sampler (900 MAX Portable Sampler, American Sigma, Loveland, CO, USA). Samples for microbial analysis were collected in sterilized 250-mL glass bottles. The bottles were transported to the laboratory (1 h) in a cooled box at 8–12°C and stored at 4°C. The majority of 57 samples were analysed within 3 to 8 h after sampling. Eight samples were held between 8 and 18 h and 13 between 18 and 24 h. Bacterial numbers did not change significantly for longer holding times. Opaque, non-sterile glass bottles were filled completely with water samples for organic carbon measurements. The samples for TOC and ion analysis were collected and stored between 3 and 10 weeks at 4°C until analysis. No correlation between holding time and TOC was observed, indicating no major change of the samples during storage. Samples for organophosphates and personal care products (only last overflow event) were collected in 2-L glass bottles with Teflon sealings (Schott) and cooled at 4°C. They were shipped to the xenobiotics laboratory of the University Duisburg-Essen, Germany, and analysed within 8 days after sampling. Additional water samples were occasionally collected directly at the outflow of the SWT.
A stationary multi-parameter monitoring system with internal data logger (MSM-9, UIT, Dresden, Germany) recorded water level, temperature (not shown here), electrical conductivity, and turbidity (given in formazine nephelometric units = FNU). Water level and electrical conductivity data were calibrated by manual measurements once per week (for electrical conductivity: Lf 340/SET hand-held meter, WTW, Weilheim, Germany). Based on the recorded water level, spring discharge was determined using a stage-discharge relationship. Rainfall data were provided by a private meteorological station located in Bitz.
Laboratory methods
TOC was determined with a high temperature TOC analyser (Elementar Analysensystem GmbH, Hanau, Germany). Major ions were analyzed from filtered samples with a standard ion chromatograph (DX-120, Dionex, Sunnyvale, CA, USA).
Organophosphates and personal care products were analysed by means of liquid/liquid extraction using 1 L of unfiltered water and 10 mL of toluene as described by Andresen et al. (2004) and Meyer and Bester (2004). The extracts were separated from the aqueous phase and condensed to 1 mL. They were analysed by GC-MS using a large volume (10 μL) programmed temperature vaporizer injection technique to allow quantification below 1 ng L−1 (Andresen and Bester 2006).
As a measure for microbiological water contamination, the total number of aerobic colonies growing at 20°C and the number of growing Escherichia coli (E. coli) colonies were quantified as indicator parameters, with E. coli representing faecal contamination by humans and endothermic animals. Coliformic bacteria and the number of colonies growing at 36°C were also analysed but data are not shown in this paper. For event #2, all microbiological results are presented in Heinz et al. (2006).
For quantification of the number of colonies growing at 20°C, the plate count method according to the European Drinking Water Directive (European Community 1998) was applied: The medium (ingredients per litre: 8 g nutrient broth, 5 g peptone, 5 g NaCl, 12 g agar) was sterilized at 121°C and 1.2 bar for 25 min and then poured into sterile Petri dishes (polystyrene, 94 mm in diameter, 16 mm high). After a drying period of at least 2 days the plates were used. Two different volumes of each water sample were distributed on the plate for each incubation temperature; each sample was analysed in triplicate. Plates were incubated at 19°C for 68 (±4) h, after which the colonies that developed on the plates were counted. The result was calculated as the average of those plates that showed numbers of colonies between 30 and 300. When numbers where too low, all plates were considered.
The number of E. coli colonies was determined using the Chromocult® Coliform Agar (CCA) method. The CCA medium was sterilised by heating at 96°C for 10 min. During the heating and cooling process, the medium was stirred several times. When higher numbers of bacteria were expected, samples were analysed by the pour plate technique: Three aliquots of each sample, ranging from 0.1 to 5 mL were distributed on three plates. After cooling the medium to 44°C it was poured onto the plate, which was then turned forwards and backwards to mix the sample with the medium. When lower numbers of bacteria were expected, the membrane filter technique was applied: Three aliquots of a water sample (50 or 100 mL) were filtered by a vacuum pump through a sterile glass filter unit with a cellulose acetate membrane filter (diameter 50 mm, pore size 0.45 μm, Sartorius). The filter was placed on a prepared CCA medium plate. All samples were incubated at 36°C for 24 h. After the incubation, pink to red and violet to dark blue colonies were identified as total coliformic bacteria, while the violet colonies represented colonies of E. coli.
Results and discussion
During a summer period, five overflows were observed (Fig. 2). The spring discharge, indicative for the hydrological conditions in the aquifer, was relatively high (∼1 m3 s−1) at the beginning of the investigation period (May 2005) due to sustained snow melting effects. Starting in May, the discharge decreased by 80% over a 3-month recession period. Several heavy rain events (indicated by the rainfall in Bitz, Fig. 2a) with amounts of at least 12 mm per day caused SWT overflows and led to sharp increases in spring discharge. The beginning of the rise in spring discharge indicates the time when the infiltrating water reaches the water table (Williams 1983).
Rainfall at Bitz and water quality development at Gallusquelle after SWT overflows during a 5-month investigation period in 2005: a spring discharge and daily rainfall; high frequency variations of discharge are due to water abstraction by waterworks at night, b electrical conductivity, concentrations of chloride and sodium, c numbers of colonies at 20°C and Escherichia coli (E. coli) (logarithmic scale), d turbidity and concentrations of TOC. Dashed vertical lines indicate time points of SWT overflows
The steep decreases in electrical conductivity are attributed to the mixing of pre-event groundwater and weakly mineralised event water (Williams 1983). After the overflow events #2, #3, #4 and #5, an increase prior to the drop of electrical conductivity was observed (Fig. 2b). After the overflow event #3 this increase occurred twice, indicating two single rain events. Williams (1983) and Sauter (1992) explain such an increase in conductivity by the displacement of higher mineralised water from the subcutaneous zone.
The spring water concentrations of the ions Ca2+ and HCO3 −, which make up the major part of dissolved solids in karst waters, were depleted in response to storm events (data not shown). This causes the drop in electrical conductivity. In contrast, slightly increased concentrations of Cl− and Na+ after all overflow events except for Cl− after the events #4 and #5 (Fig. 2b) were observed. The analysis of one sample from the SWT (during overflow event #1) showed low electrical conductivity (166 μS cm−1), typical for storm water, but relatively elevated concentrations of Cl− (22.08 mg L−1) and Na+ (21.27 mg L−1), which may explain their increased concentrations in the spring water. Common salt from excretions in domestic wastewater and street de-icing salts, transferred by street runoff and soil infiltration, represent potential sources.
Results from the microbiological analyses of all events are presented on a logarithmic scale, considering the differences of several orders of magnitude in the concentrations measured during dry and rainy periods (Fig. 2c). The values between 12 July and 25 July 2005, a relatively dry period, probably represent the background concentration. Distinct reactions to the overflow events, indicated by steeply rising total numbers of colonies and E. coli colonies parallel to the turbidity changes (Fig. 2d) were observed for all events (for event #1, E. coli CFUs were not quantified). The total number of colonies showed maxima in the range of 103 to 104 CFU mL−1 and the concentration of E. coli reached from 103 to almost 104 CFU per 100 mL with a time lag of 2–5 days after the overflow events. These values are considerably higher than that required by the official standards for drinking water quality (e.g. European Community 1998), which require the absence of coliformic bacteria and E. coli in a water sample of 100 mL.
TOC was elevated after the overflow events, with the most significant increase after the events #3, #4 and #5 (Fig. 2d). The peaks of TOC, turbidity, bacterial contamination, and Cl− and Na+ were reached concurrently. Changes in turbidity ranged from small deflections of about 1 FNU after event #1 to an increase by three orders of magnitude after overflow events #4 and #5 (Fig. 2d).
The above-described observations illustrate the typical response of a karst spring to heavy rainfall. The spring discharge responds almost directly to rainfalls and overflows, while the spring water quality, for example, represented by a decreasing electrical conductivity, responds with a time lag of approximately 2–5 days. The changes in chemical and microbiological parameters as well as in turbidity may partly result from the input of sediments and organic material mobilised by heavy rains within the entire catchment. However, the parallel development of turbidity, TOC, number of colonies at 20°C, the potential wastewater components Cl− and Na+, and of E. coli as an indicator for faecal contamination strongly suggest a significant influence of the localised infiltration of overflow from the SWT.
Low-flow versus high-flow conditions
The overflow #2 on 25 July 2005 (Fig. 2) occurred at low-flow conditions mainly as a result of a technical failure in the sewer system (water supply company Hermentingen, personal communication) and to a lesser extent due to rainfall. Heinz et al. (2006) provided a detailed discussion of this overflow event, clearly showing the adverse effects of wastewater input for the spring water quality at low-flow conditions. The purpose of this section is to compare the effects of this rather unusual overflow at low-flow conditions to those of the more common overflows triggered by heavy rainfalls (overflows #1, #3, #4, and #5 in Fig. 2). The situation most contrary to event #2 was encountered after multiple intense rain events between 9 and 11 September 2005, which caused two overflows of the SWT (#4 and #5) at high-flow conditions (Fig. 3).
Rainfall at Bitz and water quality development at Gallusquelle after SWT overflows on 9 and 11 September (events #4 and #5): a spring discharge and daily rainfall; high frequency variations of discharge are due to water abstraction by waterworks at night, b electrical conductivity, concentrations of chloride and sodium, c numbers of colonies at 20°C and E. coli, d turbidity and concentrations of TOC. Dashed lines indicate time points of SWT overflows
Overflows caused by heavy rainfalls are typically associated with significant increases in spring discharge (Fig. 2). In contrast, the slight increase in spring discharge after overflow #2 shows that groundwater recharge was relatively low (Fig. 2a). Thus dilution of contaminants by event water plays a minor role in this case. The electrical conductivity increased significantly after overflow #2, suggesting that a large proportion of subcutaneous water that was displaced before the event water reached the aquifer (Fig. 2b). The subsequent decrease in electrical conductivity is less pronounced compared to the other overflow events that were associated with stronger rainfalls. This indicates that the proportion of event water reaching the spring after overflow #2 is lower than that after the other events.
As opposed to the minor changes of conductivity and spring discharge, the contamination parameters such as number of colonies at 20°C and E. coli colonies show more pronounced increases with higher amplitudes after overflow #2 than those observed after overflow events #1 and #3 (Fig. 2c). The peaks coincide at a time of 110 h after the overflow. In contrast, a more rapid spring response is observed at high-flow conditions (overflow #5: 38 h; overflow #1: 60 h; overflow #4: 65–75 h; overflow #3: 81 h). Evidently, the low spring discharge after the long recession period preceding overflow #2 indicates a low hydraulic gradient in the aquifer resulting in slow transport of the diluted wastewater. “Contaminant transport velocities” discusses the relationship of spring discharge and travel time in more detail.
It is noteworthy that after overflows #2, # 3, #4 and #5, turbidity slightly increased before the electrical conductivity of the spring water increased, that is, before the subcutaneous water reached the spring. Thus, at both low-flow and high-flow conditions the turbidity of the spring water may increase prior to the arrival of event water at the spring. Following Sauter (1992), this increase in turbidity stems from particulate matter that was resuspended within the aquifer due to the high flow velocity. Field and Pinsky (2000) discussed the coexistence of mobile- and immobile-fluid regions causing deposition of sediments, which can be remobilised later by more rapid water. In any case, the detailed sampling after overflow #2 reveal elevated bacterial concentrations in the spring water prior to the arrival of the subcutaneous water (Fig. 2c). Both, the total number of colonies and the number of E. coli colonies increased by at least one order of magnitude suggesting a considerable fraction of the bacteria to be associated with the resuspended material.
In general, the major peaks of bacterial numbers and turbidity occurred at the same time. However, bacterial numbers appeared to decrease more slowly afterwards (Fig. 2). After overflow #2, for instance, turbidity returned to background level approximately 6 days after the maximum had been recorded, while bacterial numbers were still one order of magnitude higher than the pre-event values over a period of 11 days. This might suggest a delayed release of the bacterial contamination from the aquifer matrix due to kinetic desorption.
Low background values as observed before overflow #2 indicate that after sufficiently long residence times and no additional overflow events, colony numbers of suspended, allochthonous bacteria such as E. coli decline almost completely in the spring water. In addition to desorption, cell die-off plays a role, because enteric bacteria, poorly adapted to environmental conditions, die within days or weeks (e.g. Taylor et al. 2004).
At low-flow conditions, Na+ and Cl− concentrations at the spring show a distinct response to overflows of the SWT (#2 and #3 in Fig. 2). Heinz et al. (2006) demonstrated that these responses could be used to derive estimates of the overflow volume if detailed concentration data is available (event #2). As opposed to SWT overflows at low-flow conditions, the overflows #4 and #5 (Fig. 3) at high-flow conditions caused only minor changes of Na+ and Cl− concentrations in the spring water. The maximum concentration for Na+ was found to be only slightly higher (approx. 5.5 mg L−1), the Cl− content even lower (approx. 13 mg L−1) than the pre-event values, suggesting reduced influence of de-icing salts or a smaller proportion of municipal wastewater in the sewer overflow as well as in the total recharge to the karst aquifer.
The two peaks in spring discharge observed during events #4 and #5 (Fig. 3a), occurred 38 h after overflow #4 and 17 h after overflow #5, respectively. Electrical conductivity showed the initial slight increase indicating the contribution of subcutaneous water (Fig. 3b) followed by a sharp decrease in conductivity, which is interrupted by a half-day partial recovery. This allows separation of two independent rain events as already indicated by the discharge signal.
Similar to electrical conductivity, turbidity data also indicate an overlap of at least two recharge events (Fig. 3d). The two peaks with a maximum between 20 and 30 FNU (approx. 65 and 75 h after overflow #4) occurred during the decrease in electrical conductivity, that is, before the first event water reached its maximum proportion in spring water. At the same time, bacterial concentrations, in particular the number of E. coli colonies, were also very high (Fig. 3c), suggesting a response to overflow #4. The third and highest peak in turbidity is most likely the response to overflow #5, although it occurred only 37 h later (see “Contaminant transport velocities ”).
The period with turbidity values of more than 20 FNU was characterized by visibly grey and turbid water at the spring. At the turbidity maximum with values of up to 113 FNU, foam bubbles on the water surface were observed probably indicating detergent residues. The corresponding microbiological analysis yielded the highest total number of colonies during the entire investigation period. Yet the concentration of E. coli cells was lower relative to the foregoing sample despite maximum turbidity. This suggests more efficient dilution of faecal contamination by storm water under the high-flow conditions following overflows #4 and #5. The maximum TOC was higher than after overflow event #2, which corresponds to the higher cell numbers and turbidity values (Figs. 2, 3). Nevertheless the duration of the main recovery period appears to be similar to event #2. Thus, the high discharge probably benefited a rapid outwash of the contaminated water, although the water quality deterioration during the breakthrough of the contaminants was severely strong.
Do turbidity and TOC correlate to bacterial contamination?
The above-discussed results demonstrate that, as a consequence of the concentrated input of sewer overflow into the aquifer, several indicative parameters for contamination behave in a similar way. The time interval between overflow and maximum values of turbidity, total numbers of colony forming units and colony forming E. coli cells, and chemical parameters such as concentrations of TOC, Cl− and Na+ coincide. Since turbidity is a water quality parameter that can be monitored easily, it may serve as a promising indicator for microbial contamination of wells and springs, although its reliability has been discussed controversially (Ryan and Meiman 1996; Mahler et al. 2000; Pronk et al. 2006, 2007). The present data illustrate that bacterial concentrations are related to the corresponding turbidity values for all observed overflow events and the dry period between overflow events #1 and #2 (Fig. 4). High turbidity values are found to signal high numbers of bacterial colonies. However, this is not always true vice versa. Event #2, in particular, shows that low turbidity values may coincide with high bacterial contamination, for example, concentrations of E. coli up to 3,000 CFU per 100 ml with turbidity still below 1 FNU. This overflow event was triggered by a technical failure and thus occurred during low-flow conditions with relatively low turbidity. This suggests that the transport of bacterial cells is not necessarily associated with sediment transport and thus confirms that turbidity is not always a reliable indicator for low amounts of cells. Although less obviously, this can also be seen from the other events where the correlation of bacterial contamination and turbidity appears to be better defined with increasing turbidity values. The hysteresis loops shown in Fig. 4, in particular, suggest that bacterial contamination may still remain high after turbidity has returned to background. This means that with low turbidity the health risk resulting from already small concentrations of bacteria in drinking water cannot be excluded. Similar conclusions were drawn by Pronk et al. (2006), based on observations at karst springs with microbial contamination resulting from agricultural land use. These authors, therefore, examined the suitability of dissolved organic carbon (DOC) as indicator of bacterial contamination. As DOC often makes the major contribution to TOC (Thurman 1985), the latter can be expected to behave in a similar way. In fact, Fig. 5 shows that in the case of an overflow event caused by heavy rainfalls (events #4 and #5) the behaviour of TOC is similar to that observed by Pronk et al. (2006) and also similar to the above described behaviour of turbidity; the increase in TOC points to the increase in bacterial contamination but TOC appears to return more rapidly to background than bacteria. However, in the case of the event #2, caused by a technical failure at low-flow conditions, TOC totally fails to indicate the bacterial contamination. Thus, this parameter must be considered even less indicative for bacterial contamination than turbidity.
Total number of colonies (a) and E. coli colonies (b) in relation to turbidity for overflow events #1 to #5 and the dry period between events #1 and #2.Values for event #2 are depicted in grey colour; this event was triggered by a technical failure and thus represents a situation where the SWT most likely provides the major input to the aquifer. Where the rising limb differs from falling limb, the graph is presented as a line, with arrows indicating the temporal sequence
Contaminant transport velocities
In 1963, the Geological State Office conducted a tracer test with 10 kg of sodium fluorescein (Uranin) in the upper course of the Harthauser valley close to the present day SWT. A time interval between tracer injection and peak tracer concentration of 11.8 days was observed (Kiderlen 1963). Using this time interval t p and the linear distance x s to the karst spring, a dominant tracer velocity v = x s /t p = 32 m h−1 is obtained (Heinz et al. 2006). The spring discharge at that time was 0.125 m3 s−1. Thus, the tracer test provides information about solute transport under low-flow conditions, where the karst flow system may be expected to be nearly at steady state. In contrast, the present study evaluates the consequences of sporadic but very intense input of contaminated water into the aquifer. Thus, the dominant velocities of contaminants, represented by the peaks of colony numbers and turbidity in the spring water, were calculated and compared with the tracer test result. In general, solutes (Uranin) and particulate matter (bacteria, turbidity) may travel at different velocities. However, this difference is believed to be small in karst systems with flow through large-sized solution conduits (Taylor et al. 2004).
Figure 6 shows that the dominant velocities resulting from both the 1963 tracer breakthrough and the peaks of bacteria and turbidity can be approximated by the same linear relationship to spring discharge at low-flow and medium-flow conditions. Similarly, Sauter (1992) found a linear relationship between discharge and velocities obtained from lag times between maximum discharge and minimum electrical conductivity after storm events. This suggests that the cross-sectional area of flow is constant, which applies to phreatic (i.e. entirely water filled) conduit systems. The existence of a phreatic conduit system was also claimed by Sauter (1995), based on analyses of water level records and on the geological and geomorphological history of the area.
It is noteworthy that other tracer experiments with injection locations closer to the Gallusquelle yield significantly lower velocities at a given discharge, that is, fall below the straight line shown in Fig. 6. Tracer tests at a distance of 3 km from the spring, for instance, yield velocities between 60 and 70 m h−1 at spring discharges between 0.375 and 0.430 m3 s−1 (Birk et al. 2005; Geyer et al. 2007). A likely explanation is the specific geological setting at the location of the SWT within the Harthauser dry valley. This dry valley follows a fault zone (Fig. 1), which might favour higher flow velocities.
It should further be noted that the linear relationship fails to predict transport velocities at high-flow conditions. This was also observed by Sauter (1992) in the aforementioned evaluation of lag times between discharge and conductivity peaks. The velocity resulting from the evaluation of event #1 is lower than expected, which might result from an increase of the cross-sectional area at high-flow conditions. Event #5, on the contrary, yields an unexpectedly high velocity. This event overlapped with the preceding one, which might have led to an inaccurate velocity estimate.
Despite the deviations at high-flow conditions, Fig. 6 demonstrates that groundwater tracing provides useful information for the assessment of transport velocities and related lag times of spring water contamination caused by point sources such as SWTs.
Organic micro-pollutants
Organic micro-pollutants representing markers for wastewater were detected in the SWT samples as well as in the spring water following overflow events #4 and #5 (Table 1). However, the concentrations found in the SWT samples were lower than those normally occurring in wastewater (Andresen et al. 2004; Meyer and Bester 2004; Bester 2003, 2004). This is probably due to dilution of domestic wastewater by rainwater.
Table 1 reveals that the spring water concentrations c are generally lower than the concentrations c 0 in the sewer overflow (SWT sample). The relative concentrations c/c 0 are different for the diverse compounds. The non-chlorinated organophosphate TiBP exhibits the highest relative concentration in spring water (c/c 0 = 22% on 12 September). The spring water concentrations of TCEP, considered to be a marker for conservative transport, were similarly high (c/c 0 = 14% and 12% on 12 and 13 September, respectively). Given that the sewer overflow most likely represents the only source of these organic micro-pollutants in the spring catchment, these relative concentrations provide lower bounds on the proportion of sewer overflow in the spring water at the sampling times.
Lower relative concentrations were determined for TCPP (c/c 0 = 7 and 3%) and TDCP (c/c 0 = 3 and 1%). As these compounds exhibit slightly higher values of the octanol-water partition coefficient K ow (Table 1), they are likely to sorb slightly better to the soil material than TCEP. The concentrations of galaxolide (HHCB) and tonalide (AHTN) were about 1% of those found in the SWT. Triclosan (c/c 0 = 3 and 2%) and TnBP (c/c 0 = 2 and <1%) were still found, but some elimination occurred.
The higher relative concentrations of TCEP, TCPP, TDCP and triclosan indicate that these compounds are less efficiently retained in this karst system than in other, soil-water systems such as bankfiltration. Andresen and Bester (2006) for instance, observed an effective elimination of the same compounds after slow underground passage through other soil and aquifer materials, provided the residence time was long enough (10–15 days). In karst areas, however, the presence of sinkholes and vertical shafts enables rapid and concentrated infiltration into underground channels draining toward the spring. In this study, the total travel time from the SWT to the Gallusquelle, indicated by the lag time between overflow and water quality deterioration at the spring, has been found to be only a few days. The fact that triclosan, galaxolide (HHCB), and tonalide (AHTN) are nevertheless retained appears to be related to their high K ow values (see Table 1; Fig. 7): the higher the K ow values for the respective compounds, the higher the elimination of it on its way from the SWT to the spring. This finding indicates sorption processes to organic material.
However, sorption cannot exclusively account for the low relative concentrations of some compounds. Despite comparatively low K ow values, most non-chlorinated organophosphates were detected in the spring water at relatively low concentrations (TnBP, TPP) or were below detection limit (TBEP, EHDPP). Thus, processes other than sorption, such as biotransformation, must be involved in the elimination of these compounds. Following Andresen et al. (2004) and Meyer and Bester (2004), the non-chlorinated organophosphates are more likely to be degraded more easily than the chlorinated ones.
Conclusions
In karst areas, combined sewer overflows represent a serious threat to groundwater resources, as they can release large amounts of faecal bacteria and wastewater components during and after heavy rainfalls. To characterize the adverse effects of such point sources on the water quality, the temporal changes of various contamination parameters at a karst spring were evaluated. The results suggest that overflows of an SWT at a distance of 9 km from the spring represent an important source of contamination. The water quality of the spring is found to be significantly affected in the days after sewer overflows. In general, high numbers of faecal bacteria, high turbidity, and high TOC were observed at the same time. However, free faecal bacteria were also found while the other parameters were at uncritical levels. This represents a serious problem for the drinking water suppliers that have to guarantee sound quality for their consumers.
This study for the first time examines the transport behaviour of wastewater-born, organic micro-pollutants, such as organophosphate flame-retardants, polycyclic musks, and the bactericide triclosan, in a karst system. While compounds with a high sorption tendency were effectively retained, significant concentrations of several other compounds were measured at the karst spring along with the increase in bacterial contamination and turbidity. This illustrates that the typically high-flow velocities and low-natural attenuation capacities in karst areas allow many anthropogenic substances to be rapidly transported over huge distances, thus endangering the safety of drinking water. As a consequence, storm water management strategies should be improved to protect these important but exceedingly vulnerable water resources. Combined sewer overflow should not be allowed to infiltrate into karst areas without appropriate pre-treatment. If possible, SWTs should be put at places less vulnerable to contamination, or carefully managed infiltration systems with high sorption capacities or even degradation capacities (biofilters) should be used.
References
Andresen JA, Bester K (2006) Elimination of organophosphate ester flame retardants and plasticizers in drinking water purification. Water Res 40:621–629
Andresen JA, Grundmann A, Bester K (2004) Organophosphorus flame retardants and plasticisers in surface waters. Sci Total Environ 332:155–166
Balk F, Ford RA (1999) Environmental risk assessment for the polycyclic musks AHTN and HHCB in the EU, 1 fate and exposure assessment. Toxicol Lett 111:57–79
Bester K (2003) Triclosan in sewage plants - balances and monitoring data. Water Res 37:3891–3896
Bester K (2004) Retention characteristics and balance assessment for two polycyclic musk fragrances (HHCB and AHTN) in a typical German sewage treatment plant. Chemosphere 57:863–870
Birk S, Geyer T, Liedl R, Sauter M (2005) Process-based interpretation of tracer tests in carbonate aquifers. Ground Water 43(3):381–388
European Community (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption
Field MS, Pinsky PF (2000) A two-region nonequilibrium model for solute transport in solution conduits in karstic aquifers. J Contam Hydrol 44:329–351
Ford DC, Williams PW (2007) Karst hydrogeology and geomorphology. Wiley, Chichester, 562 pp
Geyer T, Birk S, Licha T, Liedl R, Sauter M (2007) Multitracer test approach to characterize reactive transport in karst aquifers. Ground Water 45(1):36–45
Heinz B, Birk S, Liedl R, Geyer T, Straub KL, Bester K, Kappler A (2006) Vulnerability of a karst spring to wastewater infiltration (Gallusquelle, Southwest Germany). Austrian J Earth Sci 99:11–17
Inchem (1998a) United Nations Environment Programme International Labour Organisation, World Health Organisation: international programme on chemical safety, Environmental Health criteria 209, Flame retardents Tris(chloropropyl) phosphate and Tris (chloroethyl) phosphate, Geneva
Inchem (1998b) United nations environment programme international labour organisation, World health organisation: International programme on chemical safety, Environmental Health criteria 218, Tris-(butoxyethyl)phosphate, Geneva
Inchem (1991) United Nations environment programme international labour organisation, World health organisation: international programme on chemical safety, Environmental Health criteria 112, Tri-n-butylphosphate, Geneva
Jenkins DT (1988) Development of storm water management criteria for sensitive karst areas in north-central Florida, USA. In: Proc 2nd Conf on environmental problems in Karst Terranes and their solutions, National Water Well Association, Nashville, TNe, 16–18 November, pp 333–343
Kaçaroğlu F (1999) Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut 113:337–356
Kiderlen H (1963) Expert’s report no. IV/1-943/62, Geologisches Landesamt Baden-Württemberg (unpublished, in German)
Mahler BJ, Personné J-C, Lods GF, Drogue C (2000) Transport of free and particulate-associated bacteria in karst. J Hydrol 238:179–193
Memon BA, Azmeh MM (2001) Failure of an industrial wastewater lagoon in a karst terrain and remedial action. Environ Geol 40:1424–1432
Meyer J, Bester K (2004) Organophosphate flame retardants and plasticisers in wastewater treatment plants. J Environ Monit 6:599–605
Pronk M, Goldscheider N, Zopfi J (2006) Dynamics and interaction of organic carbon, turbidity and bacteria in a karst aquifer system. Hydrogeol J 14(4):473–484
Pronk M, Goldscheider N, Zopfi J (2007) Particle-size distribution as indicator for fecal bacteria contamination of drinking water from karst springs. Environ Sci Technol. doi:10.1021/es071976f
Ryan M, Meiman J (1996) An examination of short-term variations in water quality at a karst spring in Kentucky. Ground Water 34:23–30
Sasaki K, Suzuki T, Takeda M, Uchiyama M (1982) Bioconcentration and excretion of phosphoric acid triesters by killifish. Bull Environ Contam Toxicol 28:752–759
Sauter M (1992) Quantification and forecasting of regional groundwater flow and transport in a karst aquifer (Gallusquelle, Malm, SW Germany). Tübinger Geowissenschaftliche Arbeiten C13, Universität Tübingen, p 150. Available at: http://w210.ub.uni-tuebingen.de/volltexte/2005/2039/
Sauter M (1995) Delineation of a karst aquifer using geological and hydrological data and information on landscape development. Carbonates Evaporites 10(2):129–139
Singer H, Müller S, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36:4998–5004
Taylor R, Cronin A, Pedley S, Barker J, Atkinson T (2004) The implications of groundwater velocity variations on microbial transport and wellhead protection—review of field evidence. FEMS Microbiol Ecol 49:17–26
Thurman EM (1985) Organic geochemistry of natural waters. Nijhoff, Dordrecht, p 497
Vesper DJ, Loop CM, White WB (2001) Contaminant transport in karst aquifers. Theor Appld Karstol 13–14:101–111
Williams PW (1983) The role of the subcutaneous zone in karst hydrology. J Hydrol 61:45–67
Zhou W, Beck BF, Green TS (2003) Evaluation of a peat filtration system for treating highway runoff in a karst setting. Environ Geol 44(2):187–202
Acknowledgments
We are grateful to P. Knaus and co-workers from the water supply company Hermentingen for their assistance in sampling and for providing analytical data and infrastructure. R. Riehle and N.-A. Kummer helped with the analyses of the geochemical parameters. H. Kalmbach provided meteorological data. J. Barth, M. Rolle, and P. Larese-Casanova gave valuable suggestions to improve the manuscript. We further appreciate the comments by the anonymous reviewers. This work was supported by the German Research Foundation (DFG) under grant no. LI 727/10 and by an Emmy Noether fellowship to A. Kappler.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heinz, B., Birk, S., Liedl, R. et al. Water quality deterioration at a karst spring (Gallusquelle, Germany) due to combined sewer overflow: evidence of bacterial and micro-pollutant contamination. Environ Geol 57, 797–808 (2009). https://doi.org/10.1007/s00254-008-1359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1359-0