Abstract
Simultaneous competitive adsorption behavior of Cd, Cu, Pb and Zn onto nine soils with a wide physical–chemical characteristics from Eastern China was measured in batch experiments to assess the mobility and retention of these metals in soils. In the competitive adsorption system, adsorption isotherms for these metals on the soils exhibited significant differences in shape and in the amount adsorbed. As the applied concentration increased, Cu and Pb adsorption increased, while Cd and Zn adsorption decreased. Competition among heavy metals is very strong in acid soils with lower capacity to adsorb metal cations. Distribution coefficients (K dmedium) for each metal and soil were calculated. The highest K dmedium value was found for Pb and followed by Cu. However, low K dmedium values were shown for Zn and Cd. On the basis of the K dmedium values, the selectivity sequence of the metal adsorption is Pb > Cu > Zn > Cd and Pb > Cu > Cd > Zn. The adsorption sequence of nine soils was deduced from the joint distribution coefficients (K dΣmedium). This indicated that acid soils with low pH value had lower adsorption capacity for heavy metals, resulting in much higher risk of heavy metal pollution. The sum of adsorbed heavy metals on the soils could well described using the Langmuir equation. The maximum adsorption capacity (Q m) of soils ranged from 32.57 to 90.09 mmol kg−1. Highly significant positive correlations were found between the K dΣmedium and Q m of the metals and pH value and cation exchange capacity (CEC) of soil, suggesting that soil pH and CEC were key factors controlling the solubility and mobility of the metals in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactions of heavy metals with soils are a key issue in determining the bioavailability, mobility and the ultimate fate of these metals in the environment. Heavy metals that received more attention with regard to accumulation in soil, plant uptake and contamination to groundwater include Cd, Cu, Pb and Zn. In contaminated soils, they commonly exist at the same time and compete with each other for sorption sites. Thus, selective retention and competitive adsorption of these metals by the soils become of major importance in determining their potential bioavailability, toxicity to plants and their leachability of these metals in soils and the ultimate fate of these metals in the environment (Covelo et al. 2004b; Gomes et al. 2001; Jalali and Moharrami 2007; Serrano et al. 2005; Sparks 1995).
The adsorption of heavy metals has been studied on various minerals and soils in single metal system (Gomes et al. 2001; Serrano et al. 2005; Saha et al. 2002; Spark et al. 1995). Numerous studies have also been conducted to understand competitive adsorption of trace elements in pure minerals (Forbes et al. 1976; Saha et al. 2002; Spark et al. 1995), organic compounds (Elliott et al. 1986; Saha et al. 2002) and acid soils (Arias et al. 2006; Fontes and Gomes 2003; Fontes et al. 2000; Gao et al. 1997; Gomes et al. 2001; Naidu et al. 1998; Serrano et al. 2005). These results have shown that the main physical and chemical factors governing the adsorption depends not only on their concentration in soil but also on soil properties, metal properties, and environmental factors (Gurel 2006; McBride et al. 1997; Sastre et al. 2006; Veeresh et al. 2003a). The adsorption characteristics of soils controlling the solubility and bioavailability of heavy metals are pH, redox potential, clay minerals, soil organic matter, Fe and Mn oxides, and calcium carbonate (Agbenin and Olojo 2004; Antoniadis et al. 2007; Appel and Ma 2002; Covelo et al. 2004a). However, little work has been done to model the adsorption of heavy metals onto agricultural soils in multi-element system. Less information is known about the competitive adsorption of heavy metals in agricultural soils and their potential mobility in environment.
The mobility and fate of heavy metals in the soil environment are directly related to their partitioning between soil solid phase and soil solution (Evans 1989). Alloway (1995) stated that the distribution coefficient (K d), which is defined as the ratio of metal concentration in the solid phase to that in the equilibrium solution, is a useful parameter for comparing the sorptive capacities of different soils or materials for any particular ion. Distribution coefficient has previously been used in studies of mobility and retention of trace elements in the soils (Anderson and Christensen 1988; Usman 2008; Veeresh et al. 2003a, b). The consensus reached in these studies, is that Pb, Cu and Cr are more strongly retained compared to Zn, Ni and Cd. Gomes et al. (2001) have shown that heavy metal selectivity sequences varied among soils but the most common sequence was Cr > Pb > Cu > Cd > Zn > Ni, with Cr and Zn exchanging places with Pb and Cd, respectively, for some soils. Metal characteristics such as the atomic weight, electronegativity, ionic radii, hydrolysis constant and softness sequences do not always explain metal bonding selectivity to heterogeneous soil systems (Gomes et al. 2001; McBride 1994; Serrano et al. 2005). The main soil properties that influence the selectivity sequence of heavy metals are pH, organic matter content, the type and amount of clay as well as cation exchange capacity of soil (Adhikari and Singh 2003; Serran et al. 2005; Usman 2008). Given the differences among different soils as regards their adsorbent surfaces, this variability indicates a need for further research to characterize the adsorption of particular adsorbates from mixed solutions onto different soil types.
In the work described here we characterized the adsorption of Cd, Cu, Pb and Zn when added together, to soils of the kind that prevail in Eastern China. The objectives of the present study are to evaluate the competitive adsorption of four heavy metals (Cd, Cu, Pb and Zn) in main soils of Eastern China to establish the selectivity sequences of these metals in these soils, to evaluate the capacity of these soils to adsorb these metals, and to investigate the relationship between soil properties and the adsorption capacity of these metals by soils.
Materials and methods
Soil samples and analytical methods
Nine representative surface soil samples with a wide range of properties were collected from different agricultural areas of Zhejiang Province, Eastern China. These soils were chosen according to their different physical–chemical and mineralogical characteristics to represent the different behavior of heavy metal adsorption. Soil samples were air-dried and ground to pass a 2-mm sieve. Subsamples from each soil were further ground to pass through a 0.15 mm sieve for chemical analysis and adsorption experiments.
Physicochemical properties of soils were analyzed following standard procedures (CSSS 1984). Soil pH was measured with a glass electrode at a 1:5 soil to water ratio. Soil organic matter (OM) was determined by the wet oxidation method. The particle size distribution was determined by the wet sieving and the pipette method. The cation exchange capacity (CEC) of soils was determined using 1 M NH4OAC extraction method. Soil free iron oxides (Fed) were extracted using the dithionite-citrate-bicarbonate (DCB) method and iron was measured by the atomic absorption spectrophotometer. Clay mineralogy of soils was performed using X-ray diffractometer. The analysis results of soils are given in Table 1.
Adsorption experiment of heavy metals
Heavy metal adsorption by each soil type was determined using a batch equilibrium technique. A sum of 1.0 g sample of each type soil was weighed into each of five 50-ml polyethylene centrifuge tubes and equilibrated with 20 ml of mixed solution containing five concentration levels (0.05, 0.1, 0.2, 0.4 and 0.6 mmol L−1) of nitrate salts of Cd, Cu, Pb and Zn in a background of 0.01 M NaNO3. The kinetic experiment indicated that the maximum percent removal of heavy metals was attained after about 4 h and final equilibrium was reached after 6 h in all cases. Soil suspensions were shaken for 6 h at room temperature (equilibrium time was determined by a preliminary experiment) and then centrifuged. The supernatant was collected and filtered (0.45 μm filter paper). Concentrations of heavy metals in the filtrate were determined by using atomic absorption spectrophotometry (AAS). The amount of each of the metals adsorbed by soils were calculated from the difference between the initial concentration of the metal in the solution and the concentration after adsorption reaction. All analyses were performed in duplicate, and the results presented are the means of the two determinations.
Distribution coefficient
The distribution coefficient (K d) was calculated according to the following formula:
where C ads is the amount of metal adsorbed on the soil surface (mg kg−1) and C aq the concentration of metal in the solution (mg L−1) at equilibrium. The distribution coefficients for each metal concentration for the studied soil were calculated. An average K d value (K dmedium) for each metal in studied soils, which calculated based on formula K dmedium = Σ(K d0.05 + K d0.1 + K d0.2 + K d0.4 + K d0.6)/5, was used to comparing the adsorption capacities of different soils for the metals. Joint distribution coefficients (K d∑sp) were also calculated for each soil to establish the preference order of adsorption of the metals by the soil (Usman 2008; Vega et al. 2006):
where CMj.ads and CMj.aq are the concentrations of metal j in the soil (mmol kg−1) and in the solution (mmol L−1), respectively.
Results and discussion
Soil characteristics
The physical and chemical characteristics of the soils are summarized in Table 1, which indicates significant differences in the components and properties of the soils. The soil pH varies between 4.6 (S9) and 7.8 (S2). The organic matter content varies between 9.3 g kg−1 (S9) and 36.2 g kg−1 (S4). The cation exchange capacity varies between 8.06 cmol kg−1 (S3) and 21.53 cmol kg−1 (S6). Free iron oxides content, expressed as Fe2O3, of the studied soils ranges from 8.12 to 44.76 g kg−1. The particle-size distribution of soils exhibits a substantial variation in sand, silt, and clay contents ranging from 116.8 to 361.4 g kg−1 for sand, 386.1 to 560.7 g kg−1 for silt, and 120.4 to 405.6 g kg−1 for clay. The clay mineralogy of studied soils shows that significant differences exist in the types of clay minerals in the soils. The clay minerals of soils 1, 2, 3, 4 and 5 are dominated by illite with a moderate vermiculite and chlorite. Soil 6 is dominated by montmorillonite. The clay minerals of soils 7, 8 and 9 consist predominantly of kaolinite and have small amounts of illite and hematite.
Competitive adsorption of four heavy metals
Figure 1 illustrates the competitive adsorption isotherms for Cd, Cu, Pb and Zn by the studied soils. Adsorption isotherms for these metals by the soils exhibited significant differences in shape and in the amount adsorbed. At the lower applied concentration, the equilibrium concentration (EC) was low for every metal cation, showing that almost all of the metals were adsorbed by the soils. According to ion activity production of M(OH)2, Cu ion may be precipitated as Cu(OH)2 in the soils with high pH values (S1, S2 and S6). For these soils, precipitation mechanism becomes important for the removal of Cu ion. As the applied concentrations increased, Cu and Pb maintained their strong affinity with the soil surfaces while the Cd and Zn were displaced from the soil surfaces. The result showed clearly that competition for adsorption sites affected the heavy metal adsorption behavior by the soils. The competition between metals for exchange sites was enhanced in acid soils (S7–9), with the increase in initial metal concentration resulting in large differences in adsorption of each metal. The observed lower sorption of metals in acid soils could be attributed to the low pH, cation exchange capacity (CEC) and clay type (kaolinite) of low CEC value (Table 1).
The sorption affinity between the metal cations and the soil surfaces can be calculated as the amount of each metal present in the adsorption complex, i.e., the share of a given metal in the total amount adsorbed by the soil expressed as %. Figure 2 presents these values of four metals in the adsorption complex for each soil. At the lowest applied concentration, the heavy metals were adsorbed in nearly similar percentage. Pb, Cu, Cd and Zn occupied on average 27.4, 23.7, 25.7, and 23.2% of the adsorption complex, respectively. The weaker competition may be due to the more available sites for almost all the metal cations in the adsorption complex. As the concentration increased, adsorption proportion of Cu and Pb increased while Zn and Cd decreased. Figure 2 shows that competition is weaker in soils with higher capacity to adsorb cations, since there are available sites for almost all the metal cations in the adsorption complex. Competition among heavy metals is very strong in soils with lower capacity to hold metal cations, where more Zn and Cd are dislocated from the adsorption complex and substituted by Cu and Pb. Figure 3 illustrates as an example the plot of the Soil 2, which shows strong competition, and the plot of the Soil 9, with the weakest competition. The adsorption affinity of metals can be used to evaluate the mobility of Pb, Cu, Cd and Zn. The co-existence of these metals reduces their tendency to be sorbed on the soil solid phases, resulting in a much higher risk of Cd and Zn contamination through leaching and mobility.
Selectivity sequences of heavy metals
The distribution coefficient (K d) is a useful index for comparing the sorptive capacities of different soils for a particular ion under the same experimental conditions (Anderson and Christensen 1988; Alloway 1995; Jalali and Moharrami 2007; Usman 2008). For the majority of adsorption reactions, plots of concentration of metal adsorbed versus equilibrium concentration of metal in solution did not yield a straight line (Fig. 1). Therefore, a K dmedium value was calculated to give one comparable coefficient for each metal and soil (Table 2). Data indicated that there is a great variation in the magnitude of K dmedium values among metals and soil types. Among the four metals, the highest K dmedium value was found for Pb and followed by those of Cu. However, low K dmedium values were pronounced for Zn and Cd. This implies that Cu and Pb, under the competitive condition, are the most strongly sorbed metals by these soils, whereas Zn and Cd are the least sorbed ones. Therefore, these two latter metals (Zn and Cd) may pose more threats to the ground water and plants more than Pb and Cu. Higher affinity for Pb and Cu than Cd and Zn was also reported in many previous studies (Covelo et al. 2004b; Veeresh et al. 2003b; Vega et al. 2006).
The adsorption selectivity sequence of the heavy metals by the studied soils was derived based on K dmedium values and shown in Table 2. According to K dmedium values in Table 2, metal adsorption affinities to the studied soils can be arranged in the following relative adsorption sequences: Pb > Cu > Zn > Cd for soils 1, 2, 3 and 6, and Pb > Cu > Cd > Zn for soils 4, 5, 7, 8 and 9. In general, the results obtained in this study are in agreement with those reported by Elliott et al. (1986), Usman (2008) and Veeresh et al. (2003b), which showed that metals Pb and Cu adsorption is much greater than Cd and Zn adsorption. However, the variation was found in the position of the last two metals (Zn and Cd) in the obtained sequences. Similar result was also reported by Jalali and Moharrami (2007), who measured the adsorption of metal ions on ten calcareous soils from Iran and found three decreasing sequence of adsorption: Cu > Zn > Cd > Ni > Mn, Cu > Ni > Zn > Cd > Mn and Cu > Cd > Zn > Ni > Mn. According to the sequences reported by Schwertmann and Taylor (1989), Zn is always adsorbed to a large extent than Cd for the synthetic samples. Usman (2008) and Veeresh et al. (2003b) reported the similar selectivity of the metal adsorption. This sequence follows approximately the order of the first hydrolysis equilibrium constant: Pb(7.8) > Cu(8.0) > Zn(9.0) > Cd(10.1). However, some studies also showed Zn and Cd exchanging places in the selectivity sequence of adsorption, as indicated by the results of Gomes et al. (2001) for tropical soils and Covelo et al. (2004a) for humic Umbrisols. Basta and Tabatabai (1992) and Bunzl et al. (1976) reported that metal affinity for soils and peat was: Pb > Cu > Cd ≈ Zn.
The joint distribution coefficient (K dΣsp) was calculated as the ratio of the sum of all heavy metals adsorbed on the solid phase to that in the equilibrium solution (Table 3). According to Vega et al. (2006), the distribution coefficient K dΣspmedium is a useful parameter to estimate the high or the low soil capacity for the joint sorption of the applied heavy metals. The results showed that the value of K dΣsp decreased with increasing the concentration of the added metal. Therefore, an adsorption sequence among soils can be deduced according to K dΣspmedium values. It is obvious that the most sorbent soil for the studied heavy metals was soil 1, while the least sorbent one was soil 9. This indicates that the highest adsorption cases occur for the soil that is rich in illite and montmorillonite of high pH value. Another metal removal mechanism for the soils with high pH value can be explained by the formation of M(OH)2 precipitation. Thus, the mechanism of metal retention in the soil appeared to be adsorption and/or precipitation of M(OH)2. The lowest adsorption cases are recorded for the soils that contains kaolinite of low pH and cation exchange capacity values.
Maximum adsorption capacity
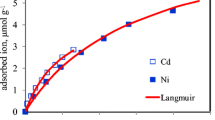
Langmuir isotherms were constructed using the sum of equilibrium concentration and the sum of adsorbed metal cations fo reach applied concentration (Fig. 4). This analysis suggests that the Langmuir isotherm could well describe the sorption of the all studied metals in studied soils. The total adsorbed amount of heavy metals increased with increrasing applied concentration of the metal. Maximum adsorption capacity (Q m), which is estimate of the maximum amount of heavy metals that the soil surfaces can hold, was calculated from the Langmuir equation. The maximum adsorption (Q m) may be a useful in comparing the potential adsorption capacity for the studied soils. The results show that the soils differed markedly in their maximum adsorption capacity for heavy metals, suggesting that soils could have different buffering capacities for these metals. The sequence of adsorption capacity was: S6 > S4 > S2 > S1 > S5 > S3 > S8 > S7 > S9. Acid soils had the lower Q m values. The Q m values of the studied soils varied from 32.57 to 90.09 mmol kg−1 (Table 4). Compared with the reported Q m values of soils, they vary greatly among researchers (Jalali and Moharrami 2007; Usman 2008). Fontes et al. (2003) reported the maximum adsorption capacity of tropical soils ranged from 23.92 to 64.52 mmol kg−1, which is consistent with our results of acid soils. Zhang and Zheng (2007) reported that the Q m values of agricultural soils with a wide range of properties ranged from 15.37 to 30.46 mmol kg−1. These results show that the soils differ markedly in their Q m values. Apparently, the large discrepancies of Q m values in the soils are related to the soil nature. Among these soils, soils having high pH and CEC values adsorbed much higher metals than those having low pH and CEC. Similar observation was also reported by Adhikari and Singh (2003), Serrano et al. (2005), Jalali and Moharrami (2007), and Usman (2008). Their results showed that the adsorption of heavy metals was favoured by high pH, organic matter, CEC, clay and CaCO3 content of soils. Apart from the nature of the soil, factors affecting the Q m values in batch experiments also include type of adsorbate and experiment conditions (e.g., the soil:solution ratio, the period and temperature of contact between soil and solution, solution pH, the type and concentration of background electrolyte, the range of the initial metal concentration used, etc.).
In order to evalute the influence of soil properties on the metal adsorption capacities, the single correlation coefficients (r 2) between the K dΣspmedium or Q m values and the physico-chemical properties of soils were calculated and listed in Table 5. Highly significant positive correlations between the K dΣspmedium or Q m values and pH and cation exchange capacity (CEC) were found, indicating that the K dΣspmedium and Q m were influenced mainly by pH value and cation exchange capacity of soils. The adsorption of metals is generally believed to be directly related to soil pH. In soils with high pH the predominance of the metals is in the hydrolyzed (MOH+) form (Gomes et al. 2001). These forms are more strongly adsorbed than the free metal cations. As soil pH increases, the metals may be precipitated as M(OH)2. Therefore, the influence of the metal hydrolysis on metal adsorption and/or precipitation becomes more important The results indicated that Q m was strongly negatively related (p > 0.01) to DCB-extractable iron oxide content (Table 5) and no significant correlation with organic matter was observed. The lack of a significant effect of organic matter on metal adsorption was also reported by Jalali and Moharrami (2007) and Usman (2008). Usman (2008) reported an important factor regulating the adsorption of heavy metals by soils are the type and the amount of clay, as well as the CEC. The correlation of the heavy metals with the cation exchange capacity was expected. The CEC is directly related to the capacity of the soil to adsorb heavy metals, since these metals occur as cations adsorbed onto the soil exchange complex. The greater the CEC value, the more exchange sites on soil minerals will be available for metal retention. In general, the soil pH and CEC can be considered as most important factors responsible for the adsorption capacity of soils for heavy metals.
Conclusions
The competitive adsorption experiment showed that as the applied concentration increased, the soils adsorbed more Pb and Cu and less Zn and Cd. The percent share of a given metal on the exchange complex was dependent on the level of the applied concentrations. At higher levels, the occupation increased for the strongest competitor Pb and decreased for the weakest competitors Zn and Cd. The selective sequences of the metal adsorption based on the distribution coefficient was Pb > Cu > Cd > Zn and Pb > Cu > Zn > Cd, indicating that Pb and Cu are the most strongly sorbed metals by these soils, whereas Zn and Cd are the least sorbed ones. The total adsorbed amount of these metals on the studied soils was well described by Langmuir equation. The calculated maximum adsorption capacity (Q m) ranged from 32.57 to 90.09 mmol kg−1. The correlation analysis indicated that for the competitive adsorption, the soil properties that were most strongly related to metal adsorption were pH value and cation exchange capacity of soils.
References
Adhikari R, Singh MV (2003) Sorption characteristics of lead and cadmium in some soils of India. Geoderma 114:81–92
Agbenin JO, Olojo LA (2004) Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma 119:257–265
Alloway BJ (Ed.) (1995) Heavy metals in soils. Blackie Academic & Professional, London, pp 11–37
Anderson PR, Christensen TH (1988) Distribution coefficients of Cd, Co, Ni and Zn in soils. J Soil Sci 39:15–22
Antoniadis V, Tsadilas CD, Ashworth DJ (2007) Monometal and competitive adsorption of heavy metals by sewage sludge-amended soil. Chemosphere 68:489–494
Appel C, Ma L (2002) Concentration, pH, and surface charge effects on cadmium and lead adsorption in three tropical soils. J Environ Qual 31:581–589
Arias M, Perez-Novo C, Lopez E, Soto B (2006) Competitive adsorption and desorption of copper and zinc in acid soils. Geoderma 133:151–159
Basta NT, Tabatabai MA (1992) Effect of cropping systems on adsorption of metals by soils: III. Competitive adsorption. Soil Sci 153:331–337
Bunzl K, Schmidt W, Sansoni B (1976) Kinetics of ion exchange in soil organic matter. IV. Adsorption and desorption of Pb, Cu, Cd, Zn, and Ca by peat. J Soil Sci 27:32–41
Chinese Society of Soil Science (CSSS) (editor) (1984) Standard methods of soil and agriochemistry. Science Press, Beijing
Covelo EF, Andrade ML, Vega FA (2004a) Heavy metal adsorption by humic Umbrisols: selectivity sequences and competitive sorption kinetics. J Colloid Interface Sci 280:1–8
Covelo EF, Andrade ML, Vega FA (2004b) Simultaneous adsorption of Cd, Cr, Cu, Ni, Pb and Zn by different soils. J Food Agric Environ 2:244–250
Elliott HA, Liberati MR, Huang CP (1986) Competitive adsorption of heavy metals by soils. J Environ Qual 15:214–219
Evans LJ (1989) Chemistry of metal retention by soils. Environ Sci Technol 23:1046–1056
Fontes MPF, Gomes PC (2003) Simultaneous competitive adsorption of heavy metals by the mineral matrix of tropical soils. Appl Geochem 18:795–804
Fontes MPF, Matos AT, Costa LM, Neves JCL (2000) Competitive adsorption of zinc, cadmium, copper and lead in three highly weathered Brazilian soils. Commun Soil Sci Plant Anal 31:2939–2958
Forbes EA, Posner AM, Quirk JP (1976) The specific adsorption of divalent Cd, Co, Cu, Pb, and Zn on goethite. J Soil Sci 27:154–166
Gao S, Walker WJ, Dahlgren RA, Bold J (1997) Simultaneous sorption of Cd, Cu, Ni, Zn, Pb and Cr on soils treated with sewage sludge supernatant. Water Air Soil Pollut 93:331–345
Gomes PC, Fontes MPF, da Silva AG, Mendonca ES, Netto RA (2001) Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci Soc Am J 65:1115–1121
Gurel A (2006) Adsorption characteristics of heavy metals in soil zones developed on spilite. Environ Geol 51:333–340
Jalali M, Moharrami S (2007) Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma 140:156–163
McBride M, Sauve S, Hendershot W (1997) Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur J Soil Sci 48:337–346
McBride MB (1994) Environmental Chemistry of Soils. Oxford University Press, New York
Naidu R, Summer ME, Harter RD (1998) Sorption of heavy metals in strongly weathered soils: an overview. Environ Geochem Health 20:5–9
Saha UK, Taniguchi S, Sakurai K (2002) Simultaneous adsorption of calcium, zinc, and lead on hydroxyaluminum- and hydroxyaluminosilicate-montmorillonite complexes. Soil Sci Soc Am J 66:117–128
Sastre J, Rauret G., Vedal M (2006) Effect of the cationic composition of sorption solution on the quantification of sorption-desorption parameters of heavy metals in soils. Environ Pollut 140:322–339
Schwertmann U, Taylor RM (1989) Iron oxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. ASA and SSSA, Madison, pp 379–438
Serrano S, Carrido F, Campbell CG, Garcia-Gonzalez MT (2005) Competitive sorption of cadmium and lead in acid soils of central Spain. Geoderma 124:91–104
Soil Survey Staff (2003) Keys to Soil Taxonomy (9th edn). USDA, Natural Resources Conservation Service. Government Printing Office, Washington, D.C
Spark KM, Wells JD, Johnson BB (1995) Characterizing trace metal adsorption on kaolinite. Eur J Soil Sci 46:633– 640
Sparks DL (1995) Environmental Soil Chemistry. Academic Press, New York
Usman ARA (2008) The relative adsorption selectivities of Pb, Cu, Zn, Cd and Ni by soils developed on shale in New Valley, Egypt. Geoderma: doi:10.1016/j.geoderma.2007.12.004
Veeresh H, Tripathy S, Chaudhuri D, Hart BR, Powell MA (2003a) Sorption and distribution of adsorbed metals in three soils of India. Appl Geochem 18:1723–1731
Veeresh H, Tripathy S, Chaudhuri D, Hart BR, Powell MA (2003b) Competitive adsorption behavior of selected heavy metals in three soil types of India amended with fly ash and sewage sludge. Environ Geol 44:363–370
Vega FA, Covelo EF, Andrade ML (2006) Competitive sorption and desorption of heavy metals in mine soils: Influence of mine soil characteristics. J Colloid Interface Sci 298:582–592
Zhang MK, Zheng SA (2007) Competitive adsorption of Cd, Cu, Hg and Pb by agricultural soils of the Changjiang and Zhujiang deltas in China. J Zhejiang Univ Sci A 8:1808–1815
Acknowledgments
This research was supported by the National Basic Research Program of China (973 Program) (2005CB121104) and Science and Technology Department of Zhejiang Province (2006C12027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, S.G., Xu, Q.F. Competitive adsorption of Cd, Cu, Pb and Zn by different soils of Eastern China . Environ Geol 57, 685–693 (2009). https://doi.org/10.1007/s00254-008-1347-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1347-4