Abstract
Based on this preliminary study, existing sand and gravel mining regulations (in Maine, USA) can be inferred to provide some protection to water resources. Sand and gravel deposits are important natural resources that have dual uses: mining for construction material and pumping for drinking water. How the mining of sand and gravel affects aquifers and change aquifer vulnerability to contamination is not well documented. Mining regulations vary greatly by state and local jurisdiction. This study test metrics to measure the effectiveness of mining regulations. The sand and gravel aquifer system studied is covered with former and active gravel pits to nearly 25% of its areal extent. Data from homeowner interviews and field measurements found scant evidence of changes in water quantity. Water quality analyses collected from springs, streams, ponds and wells indicate that the aquifer was vulnerable to contamination by chloride and nitrate. However, water quality changes can not be related directly to mining activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sand and gravel deposits along with their associated aquifers are resources that cover approximately 5% of the state of Maine (Thompson and Borns 1985). These sand and gravel deposits are a legacy of the Laurentide ice sheet that covered the region between 35,000 and 11,000b.p. (Stone and Borns 1986). The study area received deposits consisting of a basal till covered by eskers and fluvial-marine deltas. The eskers and deltas are now important sand and gravel resources. The sand and gravel deposits in the study area extend substantially below the water table (>50m) and are capable of producing high-yielding wells (>3.15L/s) (Neil et al. 2000a, b; Locke at al. 2007).
Presently, there is competition to exploit the deposits: mining for construction material and pumping for drinking water. Sand and gravel mining is necessary for construction materials. The sand and gravel mining industry in the United States has been stable and productive for decades, nearly independent of other construction and economic cycles (Kecojevic et al. 2004). The USGS estimated total USA production in 2005 at 1,260million metric tons (Bolen 2007). This stability does not mean that such activities are uncontroversial. The location of mining operations, regulations, environmental effects, and aesthetic concerns regarding mining are issues of local concern (Drew et al. 2002).
Mining rules vary by state and at smaller civil divisions, but in general, the regulatory trend has been to increase restrictions on the amount of sand and gravel that can be extracted (e.g., Wernstedt and Cummings 1999; Langer 2002). At the present time, the regulation of sand and gravel extraction in the northeastern USA is not uniform from state to state. Regulation and enforcement are highly variable. For example, in the northeastern USA, permitting ranges from none required, to a permit-by-rule process, to formal application and review. Regional regulatory policies are summarized in Table1. This summary presents known permitting structure, size limits, and environmental monitoring requirements. An annotation of state regulations is provided as Appendix I.
The regulatory context is confusing because sand and gravel mining may not be regulated explicitly by local jurisdictions, but mining operations are required to comply with existing rules governing stormwater, groundwater protection, and waste management. Some state regulations may not give special recognition to the use of groundwater from high yielding sand and gravel aquifers, while others have aquifer protection regulations. The degree of coordination between different agencies is not known although the non-alignment of policy goals is certainly a potential source of conflict. Media reports indicate that there is a persistent citizen concern about the operations and management of sand and gravel mining. For example, a search of newspaper archives for sand and gravel mining coverage for the period 1995–2006 resulted in 25,667 citations (ProQuest 2007).
According to the Maine department of environmental protection, there are currently 160 active sand and gravel pits statewide. These pits are 2–105ha (5–260acres) in size. Pit locations are distributed unevenly and some towns may have up to 14 active pits within their borders. In addition, there are an un-estimated number of smaller pits that do not require permits since they are smaller than 2ha (5acres) or were abandoned prior to regulation (circa 1985). Historically, mining in rural areas was rarely in conflict with other uses of natural resources because of a more general acceptance of consumptive use. However, changing demographics and ex-urban development in Maine are bringing more people in contact with such activities as sand and gravel mining (Brookings Institution 2006). This interaction brings to the fore aesthetic issues as well as competition for alternative uses of the same resource.
One competing use is drinking water. There are over 2,000 public water supply wells in Maine and tens of thousands of private drinking water wells (Peckenham et al. 2005). Many of the highest-yielding wells are constructed in sand and gravel aquifers, although wells in fractured rock are most common for private water supplies. In the northeastern USA, shallow aquifers depend upon rain and melting snow to restore water lost to either consumption or discharge, and residence times vary from years to several decades (e.g. Ayotte et al. 2005). Changes at the land surface from mining can affect the quantity and quality of water in aquifers (Welhan 2001). One effect is the modification of recharge area to groundwater by changing the land surface such as forming depressions so that water no longer flows along original pathways. Such changes may increase or decrease rainwater recharge to groundwater. Shorter flowpaths may increase susceptibility to contamination while re-directed flowpaths may deplete total recharge of the aquifer (Worrall and Kolpin 2004).
Another effect of sand and gravel mining is the loss of the protection provided by soil as it filters out pollutants (Rutherford et al. 1992; Kalbitz et al. 2000). Removing the organic layer of soil found on the surface of sand and gravel deposits decreases the soil’s capacity to absorb contaminants and thus clean water as it passes through its pores. This weak retardation property has been made apparent when old gravel pits that became dumps or leach fields contaminated soil and water in the aquifers (e.g. Repert et al. 2006). Current gravel mining regulations are intended to avoid future contamination of groundwater resources, but little is known about how well the rules work in practice. Operations and reclamation of former gravel pits along with inappropriate land-uses at active and former pits can all have an effect on water quality.
The loss of drinking water quality is a human health problem that carries numerous costs to society (Foster and Chilton 2003). Sand and gravel deposits are the primary source of high-yielding wells in the northeastern USA. When the Maine drinking water program completed the source water assessment program (SWAP) in 2004, sand and gravel wells were given special scrutiny because of their high value and vulnerability. Human activities related to land development and increased human presence in the area of such supply wells were viewed as significant risk factors (Peckenham et al. 2005).
In 2002, members of the Lamoine (Maine, USA) conservation commission asked for a study about how sand and gravel mining was affecting their local aquifer. Citizens observed activities associated with development: land being sold; new house lots being established; land clearing; and, sand and gravel trucks leaving the town filled with material from the aquifer. They expressed particular concern about the potential for these activities to harm the town’s public and private water supplies. A project was designed to start collecting data about gravel pits and groundwater. Two intended outcomes of this study were: (1) to provide a context for evaluating how well these natural resources were being protected by state and local regulations; and (2) to provide information to communities to help them manage similar natural resources. Since the sand and gravel deposits (aquifer) studied extended through the towns of Hancock and Ellsworth, these nearby towns were included in the study (Fig.1). Culturally, the region consists of a small urban area (Ellsworth) surrounded by lightly developed rural areas with small villages and large forested tracts.
Methods
Geology
Surficial geological data were compiled from geological maps. Gravel pit and well locations were taken from published reports and state of Maine files (Department of Environmental Protection, Geological Survey, and Drinking Water Program) and field observations. The inventory first addressed public water supplies because there are more people affected per community supply well than with private wells. The evaluation of private wells also used unpublished private well surveys made available by the town of Lamoine.
Well and gravel pit survey
The objective of the well evaluation was to determine how many wells could be affected by gravel mining and to establish a network of monitoring points across the mapped sand and gravel aquifer. Since the boundaries of the aquifer were not precisely located, all private or public wells within 0.4km (0.25miles) of the mapped aquifer boundary were included. Wells were located by overlaying tax maps onto the sand and gravel aquifer maps. Owners were mailed surveys to collect information on: well construction, well age, history of water-related complaints or concerns, and the availability of water quality testing. Once land ownership and land uses were determined, the lots were checked for well locations. Many rural lots were found to be undeveloped, while some developed lots had both older dug wells and newer drilled bedrock wells. The wellhead locations were measured with a Trimble R3 GPS system, with a horizontal resolution of at least 3m for differential positioning. Depth to water level was measured to the nearest 0.01m (0.02feet) using a Solinst model 101 water level meter. Two easily accessible wells were selected as reference wells so that data collected on different dates could be compared relative to the reference wells.
Active and abandoned gravel pits located on the aquifer were also verified in the field. Distinctive landmarks or pit centers were measured using the Trimble GPS system, and pits were photographed. Owners were interviewed to collect historical and current uses of gravel pits. At the time of this study, there were 23 active gravel pits identified with 11 separate pit owners in this sand and gravel-aquifer system.
Water quality
In order to characterize aquifer water quality preliminary testing was directed towards springs. Springs were selected as natural points of water discharge from the aquifer. A total of seven springs were located and sampled. In addition, two seepage ponds located within the sand and gravel deposit (Simmons and Blunts Ponds) were included in this study. Streams were sampled when neither springs, seepage pond, nor wells could be found within a 2km2 block of the sand and gravel deposits. Water samples were collected during the winter when the ground was frozen to make sure that the water collected was ground water and not discharge related to precipitation. Water samples were analyzed for temperature, pH, conductivity, major cations (calcium, magnesium, sodium), major anions (chloride, nitrate, sulfate), dissolved organic carbon, and acid neutralizing capacity.
In order to improve collection of spatially-randomized samples, school children were recruited to test their own well water as a classroom exercise. The school children come from a broad geographical area, including neighboring towns. This sampling provided a means to contrast source locations relative to the sand and gravel aquifer. Teachers and students in grades five and six (Lamoine) and high school (Ellsworth) participated in the study. The students were instructed how to collect and analyze water samples. Actual analyses were performed using test kits in the schools with the assistance of the research team and adult volunteers. Approximately 75 students participated in the experiment in year one (some students had water from community water supplies). Splits of all samples were analyzed in the research laboratory.
Water temperature was measured for the field samples in-situ using a glass thermometer to± 0.5°C. Water samples at each location were collected into one pre-cleaned 500mL HDPE bottle and two 40mL glass VOA vials. The water samples collected were analyzed at the Watershed research laboratory and the Sawyer environmental chemistry research laboratory at the university of Maine.
Cations (calcium, magnesium, and sodium) were quantified using a Perkin-Elmer model 3300XL inductively coupled plasma atomic emission spectrometer (ICP-AES). Anions (chloride, nitrate, and sulfate) were quantified by ion chromatography using a Dionex model ICS-2000. The pH was measured with an Orion ROSS pH electrode. Conductivity was measured using an Orion DuraProbe conductivity cell. Dissolved organic carbon was analyzed using an OI model 1010 analyzer by carbon oxidation and infrared detection. Acid neutralizing capacity was measured using an auto-titrator and the Gran plot method.
Class room analyses were performed using Hach® test kits for chloride, hardness, iron, nitrate, and meters for pH and conductivity. Additional nitrate determinations were made using CHEMets® test kits. Splits of all student samples were analyzed at the university of Maine for the same analytes except iron.
Quality assurance was conducted in the laboratory through the use of duplicated samples (10% of field samples), replicated analyses, spiked samples, and laboratory blanks (10% of samples). Quality control was performed through the use of standard reference material. Performance goals were± 10% for duplicates and± 5% for replicates and standard reference material.
Statistical analyses
The water quality results were tested for an effect due to closeness to gravel pits using two-sample t-tests and the non-parametric Kruskal-Wallace (KW) test using SYSTAT (v.11). These tests were used to determine if any of the chemical analytes were statistically different based upon proximity to the sand and gravel aquifer (and gravel pits). The results were also analyzed for differences by source: wells or springs. Significance for this study was set at≤0.05 (95% level). In addition correlation tests were performed to determine how the chemical parameters varied with respect to each other. The Pearson product–moment correlation coefficient (r) was calculated between all pairs of analytes with subsequent aggregation by proximity to the aquifer and source type.
Results and discussion
Interviews
Forty-one private well owners were interviewed about the quantity and quality of water in their wells. One owner had documented evidence of impact from agricultural activities (pesticides). Several owners had experienced low water levels in dry years, but consistent change in quantity was not mentioned by any owner. Other than comments on water taste (mostly positive) none of the people interviewed had noticed any undesirable change in quality. Well owners have allowed entry to gauge water levels periodically to develop a multi-year record for future analysis. In the study, two homes had ‘salty’ water and one had generic bad-tasting water that they did not drink. None of the well owners had a water problem that they related to mining activities. This may be due to the presence of relative few houses adjacent to mining activities and the conversion of domestic water supply from shallow dug wells to deeper drilled bedrock wells.
Gravel pits
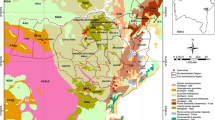
Gravel pits were found in the mapped sand and gravel aquifer that extends from the southern shore of Graham lake in T8 SD, through Ellsworth and Hancock and terminates in the town of Lamoine (Fig.2). On average this sand and gravel deposit was 18km long and up to 2km wide (11miles by 1.25miles) and in excess of 30m thick (100feet) (Weddle et al. 1988). The outline of the sand and gravel deposit was calculated to cover 13±km2 (5±sq miles). At least 34 active and former gravel pits were located, and it is likely that there are additional small abandoned pits hidden in re-forested areas. A total of 18 locations had intensive gravel mining. Eleven owners or operators of active pits were identified. Most of the owners of active pits were able to provide historical information and granted permission to sample pit sites.
The gravel pits observed ranged in size from 0.8 to 34ha (2 to 85acres). Pit size alone is misleading because some pits may lie on adjacent land parcels (based on local tax map deed references) and the pit sizes, although physically contiguous, are recorded separately. The total area covered by gravel pits, active and inactive, was estimated to be 3.4km2 (1.3sq miles). This estimate means that approximately 26% of the aquifer surface has been affected by gravel mining.
Active gravel pits operations included extraction and screening operations and some rock crushing. Inactive pits sometimes contained abandoned vehicles or construction debris. General debris dumping was widespread. Evidence of intentional gravel pit reclamation was limited (<5ha). Seemingly, some pits have not actually been abandoned; they just are being mined at very slow rates. Ponding of surface water was observed in four active pits (one permanent, three seasonal).
Private wells
Approximately 200 parcels were identified as being near or over the aquifer and information was collected for 237 wells or springs by mail survey or students. A total of 103 wells were identified to lie on or near the aquifer and 37 landowners allowed access to document the location and water levels in their wells. Water levels were measured in 55 wells within the study area; some properties had more than one well and some properties shared a common well. Only two properties had water quality testing documentation.
Wells were classified into two groupings: dug wells and drilled wells. Dug wells were typically shallow (<6m) and often were constructed in well-drained soils such as sand or sandy loam. Forty-one wells or springs were less than 15m deep and judged to tap directly the sand and gravel aquifer. Drilled wells were consistently deeper (15–150m) and cased through the soil to open bedrock borings in naturally fractured rock.
Drilled bedrock wells accounted for 60% of the private water supplies while the remainder were dug wells (20%), drilled sand wells (12%), or springs (8%). Well depths ranged from <1 to 244m (<3 to 550feet), 50% of the wells were <30.5m (100feet) deep. Reported well yields ranged from <0.06 to 12.9L/s. (<1 to 205gpm). The mean yield of bedrock wells (0.48L/s; 7.7gpm) was greater than that of the shallow wells (0.31L/s; 4.9gpm) and the difference was significant (t-test, p=0.021) The water levels in dug wells ranged in depth from 0.45 to 3.55m below the surface (1.5–12feet). The water levels in drilled wells range from 11 to> 30m below the surface (36 to > 98feet); deep water levels were beyond the range of the water-level gauge. Since water levels in the bedrock wells were consistently deeper than in the dug wells, the bedrock aquifer was hydraulically distinct from the sand and gravel aquifer. It is believed that some recharge to the fractured rock occurs through the sand and gravel.
Surface and ground waters
Surface waters were sampled from ponds, streams and springs. The objective of this sampling was to develop a regional understanding of water quality. Two seepage ponds lie within the sand and gravel aquifer and have no inlet streams; all of their water comes from precipitation and groundwater. Several streams discharge from the lateral edges of the sand and gravel deposits. The streams are fed by ground water coming from the sand and gravel aquifer. Streams sampled were in the south, central and eastern portions of the study areas. A total of nine springs were located, mapped using GPS, and sampled for laboratory analysis. The springs are considered to be the best indicator of aquifer water quality under ambient conditions. Spring sampling was completed for six springs in Lamoine, two springs in Hancock, and one in Ellsworth. Discharge rates ranged from seeps to approximately 2.5L/s (40gpm).
One spring is also a public water supply, the Cold Spring water company. Flow from this spring was measured at 2.5L/s (40gpm). Overflow discharge rates varied depending upon how much water was being pumped into the distribution system. As part of this project, we sampled four groundwater-monitoring wells that were installed around the source spring. The wells were sited to lie between the spring and potential areas of concern to the south–southeast and northwest.
Water chemistry
In general, the water chemistry indicates that the water draining the sand and gravel aquifer was fairly dilute (low conductivity) and weakly buffered (low ANC) (Table2). The deeper wells, presumably completed in the fractured bedrock aquifer had higher conductivities and greater buffering capacities. Mean values for the two groupings are presented in Table2. Note that some analytes, such as chloride exhibited large variances due to several outliers. No outliers have been excluded from this dataset.
The springs had moderate acidities and low conductivities. The dominate ions in the springs were sodium and chloride. The groundwater well samples had nearly neutral pHs and moderate conductivities and greater ANC compared to the springs. The dominant ions in groundwater were also sodium and chloride with an increased amount of calcium. Several analytes, in all samples, exhibited large variances, particularly: sodium, chloride, and nitrate (sulfate in groundwater wells only). Each of these analytes can be associated with human activities such as: road salt, seawater intrusion caused by over-pumping, and septic system leachate. Additional analysis of the water was performed to determine bromide concentrations to discriminate between seawater that contains bromide from road salt that contains none. In all but one sample, bromide was below detection limits, so road salt is the likely source of the excess sodium and chloride. Chloride concentrations exceeded the US EPA secondary MCL of 250mg/L in two groundwater wells; one exceedance was definitively due to seawater intrusion.
Nitrate concentrations above the US EPA MCL of 10mg/L occurred in four spring and two groundwater well samples. Other than low-bush blueberries there was very little large-scale agriculture in the study area and there were no public sewer systems outside of the Ellsworth municipal district, so excess nitrogen was most likely derived from on-site septic systems or forest disturbance (logging).
One of the goals of this study was to determine if there were differences in water quality for those wells near gravel pits compared to those further away. Since the sand and gravel aquifer has a long history of gravel mining (Fig.2) the sample locations were disaggregated into two groups: (1) location relative to the sand and gravel aquifer; and (2) type of water source. Position was defined as either greater, or less than, 400m (0.25miles) from the mapped limits of the aquifer. The source types were grouped into springs/streams (that drained from the aquifer) and wells (undifferentiated between dug or drilled). The surface water samples for the seepage ponds and streams draining the aquifer were similar to the springs and because of the small number of samples were not separated out. The analytes were compared for significant differences between each pair of groupings. The results of these tests are presented as probabilities for the differences between groups to be significant (Table3).
The water from the springs was slightly acidic (mean pH=6.2) compared to all groundwater wells (mean pH=7.2). This difference was also seen in the acid neutralizing capacity with the springs having a lower value (mean ANC=175μeq/L) than the wells (mean ANC=1009μeq/L). Alkalinity exhibited similar relationships. The differences between springs and wells (sources) was very significant for both ANC/alkalinity (t-test and KW, p<0.001). When the pH in samples were compared, those taken near to the aquifer had significantly lower pH values (t-test and KW, p<0.001).
The concentrations of cations in the water samples varied between springs and wells. Statistical differences for cations that are typically associated with rock weathering were observed to be greater in the wells: calcium (t-test, p<0.05; KW, p=0.023) and magnesium (t-test, p=0.001). Sodium and potassium exhibited no statistical differences between springs and wells. Only one cation, sodium, exhibited a significant difference relative to distance from the aquifer with greater values over the aquifer (KW, p=0.050).
Statistical associations observed for the major anions must be assessed cautiously because of the leverage from a few outliers, such as one case of seawater intrusion. In this setting, chloride and nitrate are linked to anthropogenic sources, or just to seawater intrusion for chloride alone. However, chloride did not exhibit significant differences by source type. Nitrate was significantly higher in the springs (t-test, p=0.055; KW, p=0.032), while sulfate was significantly less in the springs (t-test, p=0.011). When tested by proximity, chloride (KW, p=0.009) and nitrate (t-test, p=0.032) were significantly greater nearer the aquifer, suggesting greater vulnerability.
Chemical correlations
The results of the Pearson product–moment correlation analysis give an indication of how different analytes varied with respect to one another. The results with correlations |r|>0.5 are considered to be indicative of an association (Tables4, 5, 6). Combining all the results, calcium and magnesium exhibited strong correlations with pH. Most of the major ions correlated with conductivity, as would be expected. Sodium and chloride (salt) had a pronounced associations with each other (r=0.881) and with conductivity (r=0.857 and 0.952, respectively). Calcium and magnesium showed a large degree of correlation with each other which suggests that they occur together, probably due to rock weathering. Interestingly, chloride and sulfate also correlated with each other and to a lesser degree with calcium and magnesium. The strong correlation between sodium and chloride reinforces the notion that these analytes are closely related and probably have a common source (i.e. road salt).
When the data were disaggregated by source type, the correlation coefficients changed in magnitude. The changes observed for an analysis of just the spring sources is shown in Table5. The changes are noted as an increase (+), decrease (−), or no change (∼). For most analyte pairs, r-values declined. The primary exception was sulfate, in which r-values increased for most pairings. A possible explanation of this change is that marine aerosols containing sulfate deposited over the aquifer have a more pronounced effect because of the low anion exchange capacity of the sand and gravel and sulfate is more exchangeable than chloride. Changed correlations were observed when the data were grouped by aquifer proximity (springs plus some wells). The values of r declined in most analyte pairs except for sulfate-chloride and cations-pH. This analysis suggests that chloride and sulfate could be used as indicators of vulnerability for the sand and gravel aquifer. Nitrate did not correlate well with any of the other analytes in this analysis and thus may have a unique source.
Conclusion
This study has provided information to address these objectives: (1) to provide a context for evaluating how well these sand and gravel deposits are being protected by state and local regulations and (2) document how mining affects water resources. Based on interviews with well owners and observations of surface water features, there was little evidence of large magnitude changes in surface or groundwater hydrology due to mining. Ponding of surface water was observed in four active pits. Water level measurements and observations made during the field study can now serve as a reference for future comparisons. The absence of significant changes in hydrology was interpreted as evidence that long-term hydrological disruptions were small in total magnitude. In terms of quantity, the existing regulatory structure appeared to be working in Maine USA.
The data collected indicated that water quality has been degraded locally by salt and nitrate and that chemical composition differs by source and proximity to the aquifer. The three types of water analyzed (groundwater from wells, springs, and seepage ponds) have different chemistries that may be associated with land uses. Groundwater near gravel pits had more detections of sodium and chloride (salt). An increase in nitrate in waters near gravel pits was noted, but the number of detections was too small to make a strong cause and effect association. Degradation of water quality also occurs in areas away from gravel pits. Linking all changes in water quality to gravel pit operations is not possible. However, groundwater in the aquifer is more susceptible to contamination from anthropogenic sources. Sodium, chloride, nitrate, and sulfate are relatively inexpensive analytes to trace anthropogenic sources and they can be used to track changes in water quality.
To the limits of our data, degradation of water quality in proximity to sand and gravel mining has occurred, but the magnitudes of the chemical changes were small. Current mining regulations in Maine USA appear to offer some protection to groundwater. Other states in the northeastern USA have standards that offer little to modest protection of groundwater resources as based on a review of mining regulations. The value of periodic groundwater monitoring at active mining sites should be questioned given the vulnerability of sand and gravel aquifers. This is especially true when baseline conditions are undocumented. The use of spatially distributed monitoring (regional rather than site specific) as an alternative approach to regulatory compliance may provide greater certainty and a faster detection of water quality changes. Issues not addressed by the regulations are how land clearing debris is removed and stored (a potential source of nitrate) and the history of salt use and storage in gravel pits.
References
Ayotte J, Argue D, McGarry F (2005) Methyl tert-butyl ether occurrence and related factors in public and private wells in southeast New Hampshire. Environ Sci Technol 39:9–16
Bolen W (2007) 2005 Minerals yearbook: sand and gravel, construction. US. Geological Survey, Washington, DC
Brookings institution (2006) charting Maine’s future. Washington, DC
Drew L, Langer W, Sachs J (2002) Environmentalism and natural aggregate mining. Nat Resour Res 11:19–28
Foster S, Chilton P (2003) Groundwater: the processes and global significance of aquifer degradation. Philos Trans R Soc Lond B 358:1957–1972
Kalbitz K, Solinger S, Park J, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kecojevic V, Nelson T, Schissler A (2004) An analysis of aggregate production in the United States. Miner Energy 19:25–33
Langer W (2002) Managing and protecting aggregate resources. OFR 02–415. U.S. Geological Survey, Denver
Locke D, Neil C, Foley M, Tolman S, Tucker R (2007) Significant sand and gravel aquifers, Salsbury Cove quadrangle, Maine. OFR 07-4-2007. Maine Geological Survey, Augusta
Neil C, Locke D, Tolman S, Tucker R (2000a) Significant sand and gravel aquifers, Ellsworth quadrangle, Maine. OFR 00-200-2000. Maine Geological Survey, Augusta
Neil C, Locke D, Tolman S, Tucker R (2000b) Significant sand and gravel aquifers, Hancock quadrangle, Maine. OFR 00-202-2000. Maine Geological Survey, Augusta
Peckenham J, Schmitt C, McNelly J, Tolman A (2005) Linking water quality to the watershed: developing tools for source water protection. J Am Water Works Assoc 97:62–69
ProQuest (2007) newspaper database, Ann Arbor, Michigan. http://www.il.proquest.com, Cited 20 Dec, 2007
Repert D, Barber L, Hess K, Keefe S, Kent D, Leblanc D, Smith R (2006) Long-term attenuation of carbon and nitrogen within a groundwater plume after removal of the treated wastewater source. Environ Sci Technol 40:1154–1162
Rutherford D, Chiou C, Kile D (1992) Influence of soil organic matter composition on the partition of organic compounds. Environ Sci Technol 26:336–340
Stone B, Borns H (1986) Pleistocene glacial and interglacial stratigraphy of New England, long island, and adjacent Georges bank and gulf of Maine. Quaternary Sci Rev 5:39–52
Thompson W, Borns H (1985) Surficial geological map of Maine. Maine Geological Survey, Augusta
Weddle T, Tolman A, Williams J, Adamik J, Neil C, Steiger J (1988) Hydrogeology and water quality of significant sand aquifers in parts of Hancock, Penobscot, and Washington counties, Maine OF 88-7a. Maine Geological Survey, Augusta
Welhan J (2001) Ground-water interactions near the highway pond gravel pit, Pocatello, Idaho. Staff Report 01-3. Idaho Geological Survey, Moscow
Wernstadt K, Cummings AM (1999) Construction minerals in the Baltimore-Washington metropolitan area: a land management analysis. Discussion Paper 99-13. Resources for the Future, Washington, DC
Acknowledgments
The authors gratefully thank the anonymous reviewers and editors for suggestions that greatly improved this paper. Much support was provided by Carol Korty of the Lamoine conservation commission, Lamoine administrator Stu Marckoon, and the boards of selectmen in Lamoine and Hancock. We thank the teachers, administrators, and students at the Lamoine central school and Ellsworth high school. Assistance was provided by: the Ellsworth city planning office; the helpful staff in each of the town offices; the Frenchman bay conservancy; Hancock county planning office; and the union river watershed coalition plus many community volunteers. Funding for portions of this study was provided by the Island foundation, Maine community foundation, New England grassroots environment fund, and the Maine department of health and humans services-drinking water program.
Author information
Authors and Affiliations
Corresponding author
Appendix I-Annotated sand and gravel regulations for selected states
Appendix I-Annotated sand and gravel regulations for selected states
Connecticut: the water quality standards and criteria (WQS) are part of Connecticut’s clean water program. The WQS set an overall policy for management of Connecticut’s surface and groundwaters in accordance with the directives provided by section 22a-426 of the Connecticut general statutes and section 303 of the Federal Clean Water Act. Groundwater monitoring and protection programs are required, but there are no specifications regarding mining.
Delaware: Gravel and sand mining are not regulated directly in Delaware.
Illinois: sand and gravel mining requires a state or NPDES permit from Illinois EPA, unless allowed to obtain an exemption for small mines (<10acres mined per year) with no discharge that exceed criteria. There are no size limitations for mines. Groundwater protection and groundwater monitoring could be required if needed. The mine excavation is subject to setback zones from potable water supply wells under the Illinois environmental protection act.
Maine: Mining is regulated under 38 MRSA §490-D, performance standards for excavations and 38 MRSA §490-Z, performance standards for quarries. Permits are required for sites>5acres; groundwater protection and monitoring are required.
Massachusetts: Sand and gravel mining is not regulated directly. Associated wetlands issues would fall under wetlands regulations and an air permit might be required for an engine or dust control. Mining is regulated through local bylaws. Massachusetts general laws, Chapter 40, section 21 allows towns to establish by-laws or ordinances...“For prohibiting or regulating the removal of soil, loam, sand or gravel from land not in public use in the whole or in specified districts of the town, and for requiring the erection of a fence or barrier around such area and the finished grading of the same...”.
Michigan: The Department of Environmental Quality (DEQ) administers several state environmental protection statutes that apply to sand and gravel mining. Mining of sand from sand dunes located along the shoreline of lake Michigan are regulated under Part 637, sand dune mining, of the natural resources and environmental protection act, 1994 PA 451, as amended (NREPA). Sand and gravel mining elsewhere is not regulated by a consolidated state law. Several other state laws regulate aspects of a sand and gravel mining operation: (1) part 301, Inland lakes and streams, of the NREPA, requires a permit for excavation or dredging that results in the creation of a lake or a pond; (2) a sand and gravel mining operation below the local water table needs a permit from land and water management division (LWMD) of the DEQ.
Minnesota: Gravel mining operations are under the jurisdiction of local governments. The township, city and/or county in which the operation is located may have specific regulations for development, operation, or reclamation of a pit. State and federal permits may apply that pertain to water quality, water discharge, wetlands, air emissions and mine safety. Environmental review in the form of an environmental assessment worksheet (EAW) is required for operations that will exceed 40acres to a mean depth of 10feet. Local government is responsible for the preparation of an EAW.
New Hampshire: New Hampshire has an alteration of terrain permit program to protect surface waters by controlling soil erosion and managing stormwater runoff from developed areas. A permit is required whenever a project proposes to disturb more than 100,000sq feet of terrain (50,000sq feet if within the protected shoreland). The program applies to both earth moving operations, such as gravel pits, as well as industrial, commercial and residential developments. Permits are issued by DES after a technical review of plans and documentation submitted with an application and the appropriate fee. Applicable rules include section Env-Ws 321.10 monitoring for ground and surface water systems.
New York: The applicable regulations for New York include: (1) process wastewater discharges under the mineral mining and processing point source category as specified in 40 CFR part 436, and (2) the general state and federal water pollution control regulations. Crushed stone, construction sand and gravel, industrial sand, phosphate rock and graphite operations do have specific discharge limits. In the absence of standards in regulation, the NYSDEC can establish “guidance values”. The guidance values are not regulation, but do have public review and are compiled in division of water’s technical and operational guidance series (TOGS) No. 1.1.1. Guidance values can be established more quickly than standards.
Ohio: Sand and gravel operations installed after 1 January 1974 need a permit to install and a permit to operate from Ohio EPA. After installation, the permit to operate is needed for each source for continued operation. Sand and gravel mining is Ohio’s second-largest mining industry.
Pennsylvania: Sand and gravel mining is regulated under Chapter 77 of state regulations. A permit is required, but groundwater protection and monitoring requirements are determined on a site-specific basis. There is no regulatory size limit; however there are some factors which could affect the size permitted, such as distance limitations and permit line setback requirements.
Rhode Island: There are no specific state regulations for sand and gravel mining in Rhode Island—neither permits, groundwater protection nor monitoring. However, sand and gravel mining is subject to the stormwater regulations. Some local governments have instituted local ordinances for sand and gravel mining that incorporate groundwater protection provisions. Applicable rules and regulations can be found in: Chapter 46–12, water pollution; Chapter 46–13.1, groundwater protection; Chapter 23–18.9, refuse disposal; Chapter 23–19.1, hazardous waste management act: Chapter 42–17.1, environmental management; Chapter 42–17.6, administrative penalties for environmental violations; in accordance with Chapter 42–35, administrative procedures, of the Rhode Island general laws of 1956, as amended.
Vermont: Mineral prospecting activities in Vermont streams are regulated under 10 V.S.A. 41, section 1021(h) (1). Gravel removal from rivers is restricted to maximum annual volumes for landowners use and for the maintenance or restoration of stream channel stability. As a commercial activity, gravel mining has proven to be damaging to rivers.
Wisconsin: Primary regulation of mining is at the Wisconsin county level. The county land zoning board has to approve a zoning-use change to allow mining and would issue an operating permit. If the zoning change is approved and the operation permit is issued, there are mine reclamation requirements and a reclamation bond requirement issued by the county zoning office. The statewide mine reclamation requirements are contained in rule NR 135, Wisconsin administrative code. The statewide WPDES permit regulates the discharge of wastewater from a nonmetallic mining operation so surface water and ground water resources are protected. The state program must issue an approval for the mining site to pump more than 70gpm from a groundwater well. If pumping from a nonmetallic mining pit would cause negative consequences to private wells in the local area, the private well owner would have to file suit against the mining operation to recover any damages.
Rights and permissions
About this article
Cite this article
Peckenham, J.M., Thornton, T. & Whalen, B. Sand and gravel mining: effects on ground water resources in Hancock county, Maine, USA. Environ Geol 56, 1103–1114 (2009). https://doi.org/10.1007/s00254-008-1210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-008-1210-7