Abstract
The purpose of this study was to investigate the concentration levels of heavy metals in different species of the main three marine algal divisions from the Gulf of Aden coastal waters, Yemen. The divisions included Chlorophyta—green plants (Halimeda tuna, Rhizoclonium kochiamum, Caldophora koiei, Enteromorpha compressa, and Caulerpa racemosa species), Phaeophyta—brown seaweeds (Padina boryana, Turbinaria elatensis, Sargassum binderi, Cystoseira myrica, and Sargassum boveanum species), and Rhodophyta—red seaweeds (Hypnea cornuta, Champia parvula, Galaxaura marginate, Laurencia paniculata, Gracilaria foliifere, and species). The heavy metals, which included cadmium (Cd), cobalt (Co), copper (Cu), chromium (Cr), Iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), zinc (Zn), and vanadium (V) were measured by Atomic Absorption Spectrophotometer (AAs). The concentrations of heavy metals in all algal species are in the order of Fe >> Cu > Mn > Cr > Zn > Ni > Pb > Cd > V > Co. The results also showed that the uptake of heavy metals by different marine algal divisions was in the order of Chlorophyta > Phaeophyta > Rhodophyta. These heavy metals were several order of magnitude higher than the concentrations of the same metals in seawater. This indicates that marine alga progressively uptake heavy metals from seawater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Gulf of Aden extends from the strait of Bab el-Mandab, the southern entrance to the Red Sea, to the northern part of the Arabian Sea (PERSEGA 1981; Crystal 1990). It separates the Arabian Peninsula from the Somali Peninsula with surface area of ∼220 × 103 km3. The Yemeni shore, which is located along the northern edge of the Gulf, varies in shape according to geological feature of the region. The major features are rocky headlands with series of cuspate beaches between them. The major wadi systems, which are mostly dry river beds in the desert, also influence the coastal shape and environment due to fluvial deposit inputs. There are no major rivers flow into the coastal waters. However, there is seasonal, outflow from the Bana and Hussan wadis (50–70 km east of Aden) that reach the sea from time to time (approximately twice a year) after heavy rain over the mountains (flash flood).
The wind regime and water mass exchange with the Red Sea affect the biogeochemistry of the Gulf of Aden (Morcos 1970; Morcos and Varely 1990; Rushdi et al. 1994). The two seasonal monsoons that occur twice a year affect the hydrographic and ecological structure of the gulf (FAO 1967; Burkill et al. 1993; Cushing 1971, 1973; Pfannkuche and Lochte 2000; van Weering et al. 1997). The southwest monsoon starts late summer (July–September) and generates upwelling system in the Arabian Sea that brings cold nutrient rich water from the deep ocean to the surface (Guraishee 1984; Murty and El-Sabh 1984). This nutrient rich water mass increases the biological productivity in surface water, where chlorophyll a level can reach 1 mg C m−3 h−1, the same level in the Peruvian upwelling region (FAO 1967; Smith 1984). The most obvious coastal ecological impact of the upwelling is the vast growths of sub-tidal and intertidal algal assemblages on the exposed platforms and rocks. The main coastal key habitats include coral reefs, seagrass beds, salt marches, wetlands, mangroves, and nursery areas for fish and shrimps (IUCN 1987b; Rushdi et al. 1994).
There are different types of impacts on the coastal and marine environment of Yemen (Al-Shwafi 2003; Al-Shiwafi et al. 2005; Bawazir and Abu Al-Fatooh 2001; DouAbul and Al-Shiwafi 1998; DouAbul et al. 1997; EPC 1996; Haskoning 1991; IUCN 1987a, b; NAP 2003; Rushdi et al. 1991, 1994). These impacts are mainly caused by human and developmental activities, which introduce pollutants to the marine environment and cause the detraction of some special habitats (Al-Shiwafi et al. 2005; Rushdi et al. 1994). The most widely recognized issue is the oil-related pollution, where considerable attention has been focused (DouAbul and Al-Shiwafi 1998; DouAbul et al. 1997). However, other areas of concern include the impact of growing industrial and domestic effluents, unplanned coastal development as well as various miscellaneous anthropogenic activities such as fishing, hunting, and tourism (EPC 1996; IUCN 1987b; Rushdi et al. 1991, 1994). In fact, the waters off Yemen are now threatened by several kinds of pollution from passing ships and local shore facilities (UNEP 1997).
High levels of heavy metals (e.g., cadmium, cobalt, mercury, copper lead, vanadium, and zinc) in aquatic ecosystems are regarded as serious pollutants, because they can be toxic and incorporated into the food chain (Kishe and Machiwa 2003). EPC (1996), studied heavy metal concentrations in sediments and mollusks and recommended continuous monitoring programs for the Gulf of Aden and Arabian Sea coastal zones to insure that the concentrations of heavy metals remain within the baseline levels recommended by the survey. Thus, the aim of the present study is to investigate the levels of heavy metals (cadmium, cobalt, chromium, copper, iron, manganese, nickel, lead, vanadium, and zinc) in different marine algal groups.
Methodology
Sample collection
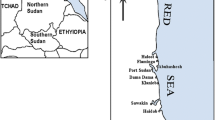
Fifteen species of three marine algal plants were collected from the Yemeni coastal waters of the Gulf of Aden during the winter of 2005. They were obtained from about 20 miles west of the Aden Harbor (Fig. 1) at depths ranging from half to 1 m. The algal groups included Chlorophyta—green plants (Halimeda tuna, Rhizoclonium kochiamum, Caldophora koiei, Enteromorpha compressa, and Caulerpa racemosa species), Phaeophyta—brown seaweeds (Padina boryana, Turbinaria elatensis, Sargassum binderi, Cystoseira myrica, and Sargassum boveanum species), and Rhodophyta—red seaweeds (Hypnea cornuta, Champia parvula, Galaxaura marginate, Laurencia paniculata, and Gracilaria foliifere species). The samples were taken from the algal flesh for the determination of heavy metal concentrations. The samples were washed with sea water at the sampling site and transferred to the laboratory in polyethylene boxes under refrigeration (4°C).
Chemical analysis
After the arrival of the samples to the laboratory, they were rinsed and washed carefully with di-ionized water. Then, they were dried at 60°C (to constant weights), homogenized by grinding each sample in a porcelain pestle and mortar and kept away from metallic materials and dusty conditions to avoid contamination.
Sub-samples (∼1.5 g of each sample) were submitted to acid digestions in a microwave oven by using concentrated 5 ml HNO3 in Teflon-lined vessel. Each digested sample was diluted to 25 ml with double distilled water and analyzed for metals contents (Ho 1987). The analysis of cadmium (Cd), cobalt (Co), copper (Cu), chromium (Cr), Iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), zinc (Zn), and vanadium (V) were performed with a flame atomic absorption spectrophotometer (Perkin-Elmer, Foster City, CA, USA, Model 2380). Procedural blanks were performed between each run. All glasswares, plastic devices and Teflon devices were thoroughly acid washed (Moody and Lindstrom 1977). Reagents of analytical grade were used for blanks and calibration curves.
Results
The analysis results of the three algal group are summarized in Table 1 and are illustrated in Fig. 2. The reported results are mean values of triplicate determinations and are expressed as μg g−1 dry weight (DW). The results showed that Chlorophyta division have the highest concentrations of heavy metals followed by Phaeophyta division and then by Rhodophyta division.
Chlorophyta
The concentrations of various heavy metals in different Chlorophyta species vary (Table 1, Fig. 2a). The concentration of Cd ranged from 0.38 to 2.3 μg g−1 DW with minimum and maximum concentrations in the H. tuna and C. koiei species, respectively. The Co concentration, which ranged from 0.30 to 1.00 μg g−1 DW, was minimum in the H. tuna species and maximum in E. compressa species. The concentration of Cr ranged from 3.40 to 15.30 μg g−1 DW with a minimum concentration in H. tuna species and a maximum concentration in E. compressa species. For Cu, the concentration ranged from 8.31 to 20.50 μg g−1 DW and the minimum concentration was found in H. tuna species and the maximum concentrations were determined in the C. racemosa and E. compressa species. The concentration of Fe ranged from 15.70 to 90.10 μg g−1 DW with minimum and maximum concentrations in the R. kochiamum and C. recemosa species, respectively. The maximum concentrations of Mn, which ranged from 3.50 to 13.70 μg g−1 DW, were found in the E. compressa and C. racemes species, whereas the minimum concentration was observed in the H. tuna species. For Ni, the concentration ranged from 1.20 to 13.30 μg g−1 DW and the minimum concentration was detected in the R. kochiamum species and the maximum concentration was in the E. compressa species. The concentration of Pb ranged from 0.73 to 6.00 μg g−1 DW and the minimum concentrations were in the R. Kochiamum and C. koiei species whereas the maximum concentration was found in E. compressa species. The concentration V, which ranged from 0.30 to 1.20 μg g−1 DW, showed minimum concentrations in the H. tuna and C. racemosa species and maximum concentration in C. koiei species. The Zn concentrations ranged from 2.35 to 8.10 μg g−1 DW and the minimum concentrations were found in the H. tuna, R. kochiamum and C. koiei species and the maximum concentrations were observed in the E. compressa and C. racemosa species.
Phaeophyta
The concentrations of various heavy metals in the samples of different Phaophyta species are shown in Table 1 and Fig. 2b. The concentration of Cd was found to range from 0.35 to 0.76 μg g−1 DW and its minimum concentration was determined in the T. elatensis species and the maximum concentration in the S. binderi species. The concentration of Co ranged from 0.35 to 0.95 μg g−1 DW with a minimum concentration in the T. elatensis species and a maximum concentration in the S. boveamum species. The minimum concentration of Cr, which ranged from 2.20 to 11.00 μg g−1 DW was in the P. boryana species and the maximum concentrations were in the S. boveamum and C. myrica species. For Cu, the concentration ranged from 5.14 to 17.20 μg g−1 DW. The minimum concentration of Cu was observed in the P. boryana species and the maximum concentration was in the of S. boveamum species. The concentration of Fe ranged from 13.81 to 60.2 μg g−1 DW with a minimum concentration in the T. elatensis species and a maximum concentration in the S. boveamum species. The Mn concentration ranged from 2.20 to 7.90 μg g−1 DW and its minimum concentration was determined in the P. boryana species whereas the maximum concentrations were measured in the C. myrica and T. elatensis species. The concentration of Ni ranged from 0.90 to 9.54 μg g−1 DW with a minimum concentration in the T. elatensis species and a maximum concentration in the S. boveamum species. The Pb concentration ranged from 0.70 to 5.30 μg g−1 DW. The minimum concentrations of Pb were determined in T. elatensis and P. boryana species and the maximum concentration was found in the sample of the C. myrica species. The concentration of V ranged from 0.40 to 1.00 μg g−1 DW. The minimum concentration of V was found in the T. elatensis species, whereas the maximum concentrations were determined in the C. myrica and Sargaussum binderi species. The minimum concentration of Zn, which ranged from 2.80 to 9.20 μg g−1 DW, was found in the S. Binderi species and the maximum concentration was in the Cystosoeira myrica species.
Rhodophyta
The concentrations of various heavy metals in different species of Rhodophyta vary as shown in Table 1 and Fig. 2c. The Cd concentration, which ranged from 0.17 to 93 μg g−1 DW, was minimum in the L. paniculata species and maximum in the H. cornuta species. The minimum concentration of Co, which ranged from 0.14 to 0.63 μg g−1 DW, was found in the H. cornuta species and the maximum concentration was in L. paniculata species. The concentration of Cr ranged from 0.70 to 2.35 μg g−1 DW with a minimum concentration in the G. foliifera species, and a maximum in the G. marginate species. For Cu, the concentration ranged from 2.31 to 17.01 μg g−1 DW and its minimum concentration was determined in the H. cornuta species and the maximum concentration was measured in the L. paniculata species. The concentration of Fe ranged from 10.75 to 41.2 μg g−1 DW. The minimum concentration of Fe was measured in the G. marginate species and the maximum concentration was in the G. foliifera species. The concentration of Mn, which ranged from 2.55 to 8.00 μg g−1 DW, showed a minimum concentration in the C. parvula species and a maximum concentration in the G. foliifera species. The Ni concentration ranged from 0.73 to 7.30 μg g−1 DW and its’ minimum concentration was found in the L. paniculata species and its’ maximum concentration was observed in the G. foliifera species. For Pb, the concentration ranged from 0.35 to 2.80 μg g−1 DW with a minimum concentration in the G. marginate species and a maximum in the H. cornuta species. The minimum concentration of V, which ranged from 0.44 to 0.87 μg g−1 DW, was determined in the G. marginate species and the maximum concentration was in the L. paniculata species. In the case of Zn, the concentration ranged from 2.71 to 6.35 μg g−1 DW. The maximum concentration of Zn was found in the L. paniculata species and the maximum concentration was in G. foliifera species.
Discussion
The concentrations of heavy metals in different algal plants vary between different species. Chlorophyta species were found to contain more heavy metals than phaeophyta and Rhodophyta species (Table 1, Figs. 2, 3). The uptake of heavy metals by different marine algal divisions is in the order of Chlorophyta > Phaeophyta > Rhodophyta (Fig. 3). Apparently, the uptake of Fe by these algal divisions is high compared to other heavy metals. The content of heavy metals in various algal species is in the order of Fe >> Cu > Mn > Cr > Zn > Ni > Pb > Cd > V > Co as shown in Fig. 3. This uptake order has been observed also in phytoplankton where the concentrations of heavy metals were found in the order of Fe > Zn > Mn > Cu > Cd > Ag > Pb > (Marin and Knuaer 1973).
It is obvious that the concentrations of heavy metals in marine algal species are several orders of magnitude higher than the concentrations of the same metals in seawater (Table 1; Donat and Dryden 2001; Gerlach 1976; Pytkowciz 1983; Turner et al. 1980; Whitfield 1979). They are lower in concentrations levels relative to their levels in sediments and similar in concentration levels determined in mollusks (Szefer et al. 1999), with the exception for Fe and Cu, which are higher in algal species. This indicates that these algal species are not contaminated and the sources of metals are natural. The presence of high concentrations of Fe and Cu in marine plants can be attributed to the fact that they are important micronutrients for various metabolic functions of the plants (Donat and Dryden 2001). The fact that metal concentration in these aquatic plants are typically several orders of magnitude higher than concentrations of the same metals in the water has led to support some speculation that metal may become progressively concentrated at higher trophic levels in aquatic food chains due to food chain magnification (Clark 1998; Laws 2000). The high level of Fe in all algal groups is consistent with the food chain magnification hypothesis (Laws 2000). The wide range of metal concentrations in different algal species reflects the importance of biochemical factors in affecting the relative tendency of different tissues to concentrate pollutants. Such biochemical or physiological differences may also play a major role in causing certain species to concentrate pollutants to a much higher levels than other organisms, regardless of the relative position of the species in the aquatic food chain (Millward and Turner 2001).
Additionally, the marine plants including algal plants are important in marine biogeochemical cycles not only because they are able to concentrate large quantities of elements (relative to seawater), but also because they can transport them in a variety of ways. These include: detritus sink and decomposition of organic matter in the sediments; direct release through plant tissue-water exchange, and part of aquatic food chain (Millward and Turner 2001).
The concentrations of heavy metals in various Chlorophyta species were found to be relatively high compared those in other two algal plants (i.e., Phaeophyta and Rhodophyta; Fig. 3). This indicates that Chlorophyta group has the tendency to uptake more metals than the other two groups. The uptake of heavy metals is high by C. racemosa followed by H. tuna, C. koiei, E. compressa, and R. kochiamum. However, they all show high levels of Fe and Cu, they are selective with respect to other heavy metals. For instance, for H. tuna, the concentrations are in the order of Fe >> Cu > Ni > Mn > Cr > Pb > Zn > Cd > Co > V and for E. compress they are in order of Fe >> Cu > Cr > Ni > Mn > Zn > Pb > Cd > Co > V.
In marine brown seaweeds (Phaeophyta), the concentration levels of heavy metals are less than in green plants (Chlorophyta). The concentrations are in the order of Fe >> Cu > Cr > Mn ≥ Zn > Ni > Pb > V ≥ Co > Cd. Except for Fe and Cu, different species of Phaeophyta division are selectively accumulate heavy metals (Table 1). The concentration levels of heavy metals in marine red seaweeds (Rhodophyta) are less than the concentrations in green plants (Chlorophyta), and brown seaweeds (Phaeophyta). The heavy metal concentrations are in the order of Fe >> Cu > Mn > Zn > Pb > Cr > V > V > Cd > Co. Also, different species of Rhodophyta division show selectivity toward the accumulation of heavy metals, except for Fe and Cu (Table 1).
Conclusion
It should be apparent that the concentration of metals associated with particulate materials including living species, is at least order of magnitude higher than the concentrations of metals dissolved in the water. It is also true in general that the concentrations of metals may differ greatly between one species to another and between different species in the same group.
In general, one ascribes these concentrations differences in the tendency of metals to bind to the various molecular groups found within the cells of each organism, as well as to the degree of the organisms exposure to the metal as influenced by its metabolic characteristics and its position in the food chain.
Apparently, marine algae may play a significant role in biogeochemical cycles of heavy metals in the coastal zones of Yemen.
References
Al-Shwafi N (2003) Distribution of trace metals in sediment of red sea coat of Yemen. J Fac Educ, Ain Shams University, Cairo, Egypt 28:117–126
Al-Shiwafi N, Rushdi AI, Ba-Issa A (2005) Trace metals in surface seawaters and sediments from various habitats of the Red Sea coast of Yemen. Environ Geol 48:590–598
Bawazir G, Abu Al-Fatooh A (2001) A preliminary study on the present state of the coastal zone in the Aden Governorate. Report submitted to Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden (PERSGA), Integrated Coastal Zone Management Component, C2001/004
Burkill PH, Mantoura RFC, Owens NJP (1993) Biogeochemical cycling in the Northwestern Indian Ocean; a brief overview. Deep Sea Res 40:643–649
Clark RB (1998) Marine pollution, 4th edn. Clarendon Press, Oxford
Crystal D (1990) (ed) The Cambridge encyclopedia. Cambridge University Press, Cambridge, 1334pp
Cushing DH (1971) Upwelling and fish production. Adv Mar Biol 9:255–334
Cushing DH (1973) Production in the Indian Ocean and the transfer from the primary to secondary level. In: Zeitschel B (ed) The biology of the Indian Ocean. Chapman and Hall, London, pp 475–486
DouAbul AA-Z, Al-Shiwafi HA (1998) Dissolved/dispersed hydrocarbons in Arabian region. Mar Pollut Bull 36:844–850
DouAbul AA-Z, Hebba HMA, Fareed KH (1997) Polynuclear aromatic hydrocarbon (PAHs) in fish from the Red Sea coast of Yemen. Hydrobiologia 352:251–262
Donat J, Dryden C (2001) Transition metals and heavy metal speciation. In: Steele J, Thorpe S, Turekian K (eds) Encyclopedia of ocean sciences. Academic, Elsevier Science, New York, pp 3027–3035
EPC (Environmental Protection Council) (1996) Beach pollution study Gulf of Aden/Arabian sea ‘‘chemical analysis’’. EPA-Sana’a University, Yemen, p 48
FAO (1967) The present status of fisheries and assessment of potential resources of the Indian Ocean and adjacent seas. Rome, FAO, COFI: I/1/67/9/1st draft 41pp
Gerlach SA (1976) Marine pollution. Diagnosis and therapy. Springer, Berlin, Heidelberg, New York, p 218
Guraishee GS (1984) Circulation in the north Arabian Sea at Murry Ridge during S.W. monsoon. Deep Sea Res 31:651–664
Haskoning (1991) Proposal for developing a coastal management plan in the Republic of Yemen. Report by Haskoning (Royal Dutch consulting Engineering and Archeticts). Support to Secretariate of the Environmental Protection Council, Yemen, Royal Haskoning, Netherlands, p 53
Ho YB (1987) Metals in 19 international Macro-algae in Hong Kong waters. Mar Pollut 18:564–566
IUCN (International Union for Conservation of Natural Resources) (1987a) Yemen Arab Republic: marine conservation survey, vol 1. Distribution of habitats and species along the YAR coastline. IUCN Red Sea and Gulf of Aden Environmental Program/TMRU York, UK, p 100
IUCN (International Union for Conservation of Natural Resources) (1987b) Yemen Arab Republic: marine conservation survey, vol 2. Distribution of habitats and species along the YAR coastline. IUCN Red Sea and Gulf of Aden Environmental Program/TMRU York, UK, Gland Switzerland
Kishe MA, Machiwa JF (2003) Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environ Int 28:619–625
Laws E (2000) Aquatic pollution. An introductory text, 3rd edn. Wiley, New York, p 369
Marin JH, Knuaer GA (1973) The elemental composition of plankton. Geochimica Cosmochimica Act 37:1639–1653
Millward GE, Turner A (2001) Metal pollution. In: Steele J, Thorpe S, Turekian K (eds) Encyclopedia of ocean sciences. Academic, Elsevier Science, New York, pp 1730–1737
Moody JR, Lindstrom PM (1977) Selection and cleaning of plastic containers for storage of trace element samples. Anal Chem 49:2264–2267
Morcos SM (1970) Physican and chemical oceanography of the Red Sea. In: Barnes H (ed) Oceanogr Mar Biol Ann Rev 8:73–202
Morcos SM, Varely D (1990) Physical and chemical oceanography of the Red Sea. Oceanogr Mar Biol Ann Rev 8:73–202
Murty TS, El-Sabh MI (1984) Cyclones and storm surges in the Arabian Seas: a brief review. Deep Sea Res 31:665–671
NAP (National Action Plan) (2003) Yemen national programme of action for the protection of the marine environment from land-based activities. Republic of Yemen, Ministry of Water and Environment (MWE) and Environment Protection Authority (EPA)
PERSEGA (Red Sea and the Gulf of Aden Development Program) (1981) Draft of the final act on the Jeddah regional conference plenipotentiary on the conservation of the marine environment and coastal area of the Red Sea and the Gulf of Aden, Jeddah
Pfannkuche O, Lochte K (2000) The biogeochemistry of the deep Arabian Sea: overview. Deep Sea Res 47:2615–2628
Pytkowciz RM (1983) Equilibria, nonequilibria and natural waters. Wiley, New York, p 353
Rushdi AI, Ba-Issa AA, Ba-Bagi A (1991) Preliminary investigations of oil pollution along the Red Sea coast of Yemen. In: Proceedings of the seminar on the status of the environment in the Republic of Yemen, Sana’a, 1–5 January 1991
Rushdi AI, Abubakr MM, Hebba HMA (1994) Marine habitats of the Red Sea at AlUrj-Alsalif and Dhubab-Yaktul areas: their ecology, environment and management recommendations preliminary investigations. UN-Faculty of Sciences, Sana’a University, Yemen, p 117
Smith SL (1984) Biological indications of active upwelling in the Northwestern Indian Ocean in 1964 and 1979 and a comparison with Peru and Northwest Africa. Deep Sea Res 31:951–968
Szefer P, Ali AA, Ba-Haroon AA, Rajeh AA, Geldon J, Nabrzyski M (1999) Distribution and relationships of selected trace metals in mollusks and associated sediments from the Gulf of Aden, Yemen. Environ Pollut 106:299–314
Turner DR, Dickson AG, Whitfield M (1980) Water–rock partition coefficients and the composition of natural water—a reassessment. Mar Chem 9:211–218
UNEP (United Nation Environment Program) (1997) Assessment of land-used sources and activities affecting marine sediment in the Red Sea and the Gulf of Aden. UNEP Regional Seas Report Study. No 166
van Weering TCE, Helder W, Schalk P (1997) Netherlands Indian Ocean program 1992–1993. First results and introduction. Deep Sea Res 44:1177–1193
Whitfield M (1979) The mean oceanic residence time (MORT) concept—a rationalization. Mar Chem 8:101–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Shwafi, N.A., Rushdi, A.I. Heavy metal concentrations in marine green, brown, and red seaweeds from coastal waters of Yemen, the Gulf of Aden. Environ Geol 55, 653–660 (2008). https://doi.org/10.1007/s00254-007-1015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1015-0