Abstract
Heavy metal levels in surface sediments from Tamaki Estuary demonstrate significant up estuary increases in Cu, Pb, Zn, Cd and mud concentrations. Increased metal levels towards the head of the estuary are linked to local catchment sources reflecting the historical development, industrialisation and urbanisation of catchment areas surrounding the upper estuary. The relatively narrow constriction in the middle estuary (Panmure area), makes it susceptible to accumulation of upper estuary pollutants, since the constriction reduces circulation and extends the time required for fine waterborne sediments in the upper estuary to exchange with fresh coastal water. As a result fine fraction sediments trapped in the upper estuary facilitate capture and retention of pollutants at the head of the estuary. The increase in sandy mud poor sediments towards the mouth of the estuary is associated with generally low metal concentrations. The estuary’s geomorphic shape with a mid estuary constriction, sediment texture and mineralogy and catchment history are significant factors in understanding the overall spatial distribution of contaminants in the estuary. Bulk concentration values for Cu, Pb, Zn, and Cd in all the studied surface samples occur below ANZECC ISQG-H toxicity values. Cd and Cu concentrations are also below the ISQG-L toxicity levels for these elements. However, Pb and Zn concentrations do exceed the ISQG-L values in some of the surface bulk samples in the upper estuary proximal to long established sources of catchment pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When heavy metals are released into the marine environment, they are soon transferred to the sediment phase by adsorption on the surface of suspended particulate matter and eventually settle as sediment (Hatji et al. 2002). This process is common in intertidal sediment flats in estuaries. Heavy metals in surface water systems can be derived from natural or anthropogenic sources. Today, in many coastal areas anthropogenic inputs of heavy metals significantly exceed natural inputs. Excessive metal levels in surface water may pose a health risk to the biotic environment both above and below the sediment water interface. Sediment grain size is a significant factor in influencing the concentration of heavy metals in estuarine sediments with metal concentrations significantly enriched in fine grained sediments. Typically, heavy metal adsorption increases with decreasing grain size of the sediment. This reflects the tendency for heavy metals to be preferentially adsorbed on the large surface area of fine grained sediments rich in clay minerals (Horowitz 1991; Green et al. 2001). As a result clay rich muddy sediments play an important role in the entrapment and deposition of chemical contaminants through surface adsorption. Furthermore, industrial wastes released into estuaries also tend to be fine grained particles and they are transported and deposited along with naturally occurring fine grained sediments. This leads to the proposal that fine grained estuarine sediments act as sinks for pollutants (Bell et al. 1997; Birch 2000) released into surrounding catchments and directly into estuaries through industrial and stormwater outfalls (Summerhayes et al. 1985). In contrast, coarse grained quartz and shell rich sediments tend to dilute trace metal concentrations in bulk sediments (Deely 1991).
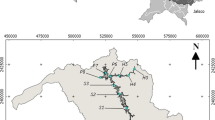
Tamaki Estuary is the third largest water body in Auckland, New Zealand (Fig. 1). The estuary is situated on the eastern side of Auckland City and the catchment comprises about 11,500 ha of diverse land use: extensive suburban housing, light to medium industry, marinas, barge and ferry facilities, power plants, major arterial traffic routes and some areas still under farming. The estuary water covers an additional 1,600 ha and is intensively used for a wide range of recreational and commercial activities (Abrahim and Parker 2002). Tamaki Estuary catchment is divided approximately into the following land use types: residential 37%, industrial 27%, and rural 17% with the reminder in use for mixed purposes (commercial, park and motorway). Tamaki Estuary is low mesotidal with a neap tide range of 1.9 m and a spring tide range of 2.7 m. The main Tamaki Estuary channel meanders through broad intertidal flats locally flanked by very low cliffs (Abrahim 2005). Tamaki Estuary is reasonably sheltered from all winds other than from the northerly and southerly quarters, and for which fetch distances reach 3–4 km. Auckland estuaries are classified as inshore waters by Green and Hume (2002) in which waves are primarily generated by local winds. Tidal current velocities reach 0.85 m/s in the channel during the ebb tide in the vicinity of Buckland Beach in the lower estuary and increase to c 1.0 m/s at spring flood tides in the Panmure area (KRTA 1989).
The most abundant benthic biota are Austrovenus stutchburyi (common cockle), Dosina zelandica, Arthritica bifurca and Nucula (nut shell), Zeacumantus lutulentus horn shell, Micrelenchus tenebrosus and Maoricolpus roseus species. Furthermore, trough shells (Mactra ovata) which reached high densities 20 years ago (Grange 1982) have now almost disappeared with little sign of juvenile recruitment (ACC 2001). These molluscs indicate marine environments ranging from intertidal to subtidal conditions.
Various studies (ARC 1990, 1992, 1999) have indicated that Tamaki Estuary sediments have been impacted by heavy metal pollution. Studies on Tamaki Estuary cores, taken from both the lower and the upper estuary, have clearly demonstrated very significant anthropogenic heavy metal enrichments in surface layers compared to sediments at depth (Abrahim 2005). The surface sediment enrichments are three to four times the metal levels found at the base of the cores. The present study on surface sediments is focused on the spatial distribution, origin and potential toxic effects of heavy metals in the estuary. The observed surface sediment metal concentrations and distributions can be related to historical anthropogenic pollutant inputs, variation in grain size and mineralogy and estuary morphology.
Based on radiocarbon dating, marine transgression in the Tamaki estuary started at least 7500 years B.P. (Abrahim 2005). The main Tamaki Estuary channel is relatively narrow with broad tidal flats forming the borders to the central channel. The middle estuary narrows significantly in the Mt Wellington-Panmure area and this constriction isolates water circulation in the upper estuary thereby increasing the water residence time and facilitating the deposition of fine fraction sediments (Fig. 1). As a result in the upper reaches of Tamaki Estuary the shallow intertidal flats are typically composed of clay rich muddy sediments. In contrast, in the relatively wide areas towards the lower estuary, the intertidal flats become progressively coarser grained sands rich in quartz and shell material (Abrahim 2005).
The sediments in the Tamaki Estuary have been subject to a range of pollution processes and events occurring at various times over the last 150 years, particularly since the advent of European settlement in the Tamaki catchment (Abrahim and Parker 2002). Detailed mapping of surface sediment heavy metals in estuarine environments such as Panmure Basin show heavy metal enriched areas that are linked to specific outfall point sources (Parker et al. 2002)
Heavy metal concentrations in 36 bulk surface samples were analysed to investigate spatial concentration trends along the length of the estuary and for comparison with the sediment quality guidelines proposed by Long et al. (1995) and ANZECC (2000). In the surface samples the <63 μm fine fraction was also separated for analysis.
Materials and methods
Sample collections
Surface samples were obtained from 36 sites distributed over the Tamaki Estuary low tide flats by scooping the top 1–2 cm of sediment with a plastic scoop. The sediments were packed in plastic bags and refrigerated until the time of chemical analysis. All samples were subsampled and subjected to both grain size analysis and geochemical analysis. Location of samples is shown in Fig. 1.
Grain size analysis
Subsamples for grain size analysis were fractionated into greater or less than 63 μm by wet sieving in order to establish the fine fraction proportion (%mud) (Vasconcelos et al. 1995).
Geochemical analysis of sediments
Chemical analysis for sediment heavy metals (Cd, Cu, Pb, Zn) was performed on bulk and fine fraction (<63 μm) samples by acid extraction. The extraction method used in this study was adapted from Stewart (1989) and is based on a 4HNO3 acid digestion. In the present study, 1 gm of freeze-dried sediment was boiled gently in 20 ml of 4HNO3 acid for 45 min in a covered 100 ml Pyrex beaker. The resulting solution was cooled and filtered through an acid washed Whatman 540 filter paper into a 50 ml volumetric flask. The solution was analysed by atomic absorption (AA) spectroscopy for lead, zinc and copper and by graphite furnace atomic absorption (GFAA) for cadmium.
Accuracy of the metal analyses was checked against certified reference material (MAG-1), which is a polluted, marine sediment standard prepared by the United States Geological Survey (Abrahim and Parker 2002). The precision of the analytical procedure was determined by subsampling one of the sediment samples eight times and then doing a complete digestion and analysis for each subsample. The results were used to calculate the absolute and relative standard deviations for the analysed elements (Abrahim 2005).
Distribution of surface sediment heavy metal concentrations
A total of 36 surface samples from Tamaki Estuary have been assigned to seven zones depending on their geographic position (Fig. 1). Grain size analysis reveals a predominance of the fine mud fraction in the upper reaches of the estuary, while the lower reaches of the estuary are noted for coarser sediments with a significant decrease in the mud fraction. Since the morphological subdivisions of the estuary (Abrahim 2005) has a specific bearing on the sediment particle-size distribution, evaluation of the spatial distribution of heavy metals should take into consideration the hydrodynamic influence of the estuary’s morphology and geography (Fletcher et al. 1994; Ladd et al. 1998). The long and narrow shape of the main estuary channel, and the relatively narrow constriction present in the Panmure area, means that tidal flushing is inefficient in moving pollutants down to the mouth and out of the estuary.
Heavy metal analysis of surface sediment samples was undertaken on both the bulk and fine fraction (<63 μm) and the results for seven Tamaki Estuary zones are presented in Table 1. Increasing heavy metal concentrations tend to be associated with finer grained sediments and this can be seen in the high metal levels found in zones with high mud contents (Figs. 2, 3).
Scatter plots of bulk sediment heavy metal concentration versus mud content in surficial sediments in the different zones of Tamaki Estuary. For zone locations see Fig. 1
Mean heavy metal concentration in surficial sediments in each zone for bulk and fine fraction <63 μm sediments. For zone locations see Fig. 1
The concentrations of Cu, Pb, Zn, and Cd in the bulk sediment are clearly linked to the textural characteristic of the sediments. Heavy metal concentrations in bulk surface sediments are much lower and variations are less pronounced in samples which contain low mud contents (Fig. 3). Surface sediment become progressively coarser down the length of the estuary and in zones A and B the mud concentration has dropped to 7–14% with concomitant low metal concentrations. The decrease in heavy metal concentration in bulk samples enriched in the coarse fraction reflects the increase in coarse detrital minerals such as quartz, feldspar and carbonates. These phases are noted for low heavy metal contents and act as diluting agents in the mud. In contrast the high metal concentrations in Zone G in the upper estuary are associated with significantly elevated mud content (83%).
Compared to the bulk samples, the fine fraction concentrations for the four heavy metals show enrichment ratios ranging from 1.40 to 1.85 (calculated from the average data in Table 1). This fine fraction enrichment can be explained in term of the increase in the surface area and metal adsorption capacity in the sequence sand < silt < clay (Deely and Fergusson 1994; Abrahim 2005).
The spatial distribution patterns for heavy metals of potentially anthropogenic origin (Cu, Pb, Zn and Cd) in the surface sediments are broadly similar with the highest concentrations for all the elements being particularly associated with the increasing fine fraction found towards the upper reaches of the estuary (Fig. 3). Similar heavy metal enrichments in estuarine headwaters have been reported by Birch (1996) and Birch and Taylor (1999) in their studies of the Port Jackson Estuary in Australia. Spatial association of contaminants with industrial areas and yacht anchorages, and temporal enrichments associated with the intensive urbanisation and development of the catchment since 1945, indicate that these pollutants are related to anthropogenic activities (Abrahim and Parker 2002).
The most important finding of the surface sediment work was that the analysed heavy metals in both the bulk and fine fractions show the highest concentrations (with the exception of fine fraction Cu) in the Otara and Otahuhu creeks in zones G and F (Fig. 3). The origin of these upper estuary enrichments are linked to the following factors: (a) Otara and Otahuhu were the earliest areas within the Tamaki catchment to be developed [first urbanisation and industrialisation dates from 1880s (Cumberland 1966)]; (b) many industrial and commercial sites into Otara catchment cause pollution as a result of poor and sometimes illegal handling and release of solid and liquid wastes (ARC 2000); (c) Otara has three landfills which may not meet modern standards for leachate contaminant; and (d) the southern Auckland motorway and another main urban road cross the upper zones of the estuary.
Cu is anomalous in that this element does not follow the general trend of lower concentration in the lower estuary. The relatively high enrichments for copper observed in zone B and zone C are found in proximity to the large numbers of boats moored at the Half Moon Bay and Panmure anchorages as compared to the relatively few boats moored in the upper estuary. Boat yards associated with Half Moon Bay marina and ferry terminal are also important features of the area. The use of Cu antifouling paints on marine vessels in boat yards is considered to be the source for these lower estuary Cu enrichments (ARC 1995). Cd is also anomalous in that zone C is significantly enriched compared to the adjacent B and D zones. This enrichment may reflect a local pollutant or downstream movements of Cd released from the adjacent industrial area in Mt Wellington.
Sediment toxicity and sediment quality guidelines (SQGs)
Over the last two decades a considerable amount of research effort has been put into investigating sediment toxic threshold levels (Long and Morgan 1990; MacDonald et al. 1992; Long et al. 1995; Smith et al. 1996; ANZECC 2000). As a result there are now a number of international guidelines relating to toxic concentrations as determined by field and laboratory data. The work of Long et al. (1995) on SQG provides useful tools for screening sediment chemical data to identify pollutants of concern and prioritise problem sites (Birch and Taylor 2002). Long et al. (1995) defines ranges of chemical concentrations found in bulk estuarine sediments based on toxicity levels estimated from experimental laboratory studies and field observations and measurements. The results of this work provide estimates of two contaminant levels that are likely to produce adverse biological effects in 10 and 50% of biota populations.
Using this approach, Long et al. (1995) classified the toxicity of contaminants into ERL and ERM concentrations. The abbreviation ERL refers to Effect Range Low, which indicates that below this level, a chemical element or compound is considered to be of low or minimal concern, since adverse effects on organisms are infrequently observed (i.e. in <10% of biota) when the concentration falls below the ERL value. ERM refers to an Effect Range Median concentration. At and above this level harmful effects are likely to be observed in 50% or more of the biota population and this concentration is considered to be toxic and of significant concern. Concentrations between the ERL and ERM values represent an intermediate range within which effects occur in 10–50% of biota populations.
In Australia and New Zealand the introduction of SQGs has been facilitated by the work of the Australian and New Zealand Environment and Conservation Commission (ANZECC 2000). The current sediment quality guidelines for Cd, Cu, Zn, and Pb are presented in Table 2.
The ANZECC (2000) guidelines are generally based on the work by Long et al. (1995). However the ANZECC (2000) interim sediment quality guideline-low (ISQG-L) proposes that higher concentrations than the Long et al. (1995) ERL values be used as “acceptable” background levels for Cd, Cu, Pb and Zn in sediment assessment. Sediments below the ANZECC (2000) ISQG-L concentration will generally be acceptable and no further investigation of a site due for development or modification will need to be undertaken. Note that in the case of Cu the ANZECC (2000) ISQG-L concentration (Table 2) is nearly twice the Cu ERL proposed by Long et al. (1995).
With regard to more heavily polluted sediments, the interim sediment ANZECC (2000) ISQG-H concentrations are essentially equivalent to the ERM values from the American guidelines proposed by Long et al. (1995).
Application of SQGs to Tamaki Estuary
In the present study the sediment heavy metal concentrations measured in bulk surface samples are compared with the ANZECC (2000) interim SQGs (ISQG-L and ISQG-H) as shown in Fig. 4. The dashed vertical lines distinguish screening criteria for the ISQG-L and ISQG-H toxicity levels. Figure 4 shows that all the samples are well below the ISQG-H levels. However Pb and Zn in some of the surface samples exceed the ISQG-L line while Cu and Cd values all occur below the ISQG-L line. Table 3 shows that 35% of the samples for Pb exceed ISQG-L but none exceed the ISQG-H value. For Zn only 14% of the samples exceed ISQG-L and none exceed ISQG-H concentrations.
Frequency distribution of heavy metal concentration in bulk surface Samples of Tamaki Estuary compared to sediments quality guidelines (ANZECC 2000). The horizontal axes refer to concentrations in μg/g of Cu, Zn, Pb and Cd. The vertical axes indicate the number of analyses within concentration classes (the class size is in units of 10 μg/g). F = Frequency
It is clear, that despite relatively high upper core metal enrichments in the fine fraction (Abrahim 2005), that the Tamaki Estuary bulk surface sediments are nevertheless still generally well below the SQG-H concentrations where substantial adverse effects on benthic biota could be expected.
Comparison of Tamaki Estuary sediment contamination with other New Zealand estuaries is complicated by the fact that some studies (e.g. Glasby et al. 1988) have focused on fine fraction contamination and this prevents the application of SQG since this approach requires data on bulk sediment metal levels. However a number of bulk sediment studies on Manukau and Waitemata Harbours in the Auckland region (Williamson et al. 1995, 1999; Mills et al. 2000) indicate significant metal contamination with maximum concentrations (Cu: 107 μg/g, Pb: 121 μg/g, Zn: 316 μg/g) which are in excess of the Tamaki Estuary results listed in Table 1. However like Tamaki Estuary, these maximum concentrations are above ISQG-L but below ISQG-H levels (Table 2).
Comparison between Port Jackson estuary (Sydney, Australia) and Auckland estuaries (Parker et al. 2002) demonstrates much higher levels of metal contamination in Port Jackson estuary where metal levels reach more than 800 μg/g for Cu, 900 μg/g for Pb and 1,000 μg/g for Zn in several strongly contaminated inshore areas (Birch and Taylor 1999; Birch 2000). These areas are far above ISQG-H concentrations for Cu, Pb and Zn. In addition elevated metal levels in Port Jackson are relatively widespread (Birch and Taylor 2002) whereas surveys of Auckland estuarine contamination (this work, Williamson et al. 1995, 1999; Tang 1999; Mills et al. 2000; Parker et al. 2002; Abrahim 2005) generally indicate, with some exceptions, relatively localised areas of comparatively low level metal contamination.
The data from Port Jackson is significant in indicating possible future trends in sediment contamination that areas of elevated metal accumulation, such as found in the upper Tamaki Estuary, may lead to in the absence of environmental regulation. The Port Jackson data underlines the importance of proactive measures to manage and mitigate pollution in the catchments surrounding Tamaki Estuary and other estuaries in the Auckland region.
Conclusions
The concentration of heavy metals in Tamaki Estuary surface sediments can be related to pollutant inputs and grain size variation. Since high levels of heavy metals occur in the sediment fine fraction (<63 μm) the increasing mud content in the tidal flats of the upper estuary plays a significant role in the accumulation of heavy metals in these areas at the head of the estuary. High concentrations of heavy metals in the upper estuary in the Otara and Otahuhu Creeks correlate strongly with the increasing proportion of mud in these sediments and are associated with historic and present day sources of local pollution at the head of the estuary (Abrahim and Parker 2002). Although the lower estuary sediments are more sandy, and generally have lower metal levels, nevertheless high concentrations of Cu are observed in the surface sediments near Half Moon Bay. This Cu enrichment is considered to be linked to the use of Cu based antifouling paints and the discharge onto nearby intertidal mudflats of both stormwater run off and wastewater washing from boat-yards situated in the Half Moon Bay ferry terminal area and nearby marina.
The generally mud-rich nature of the upper estuary sediments, the constricted down estuary sediment movement, the relatively small amount of fresh water inflow into the estuary (particularly in summer) and the up stream location of the long established urban and industrialised areas of Otara and Otahuhu are all features that have resulted in elevated concentrations of heavy metals in the upper estuary.
The surface layers of the estuary represent the sediments deposited during the current sea level highstand. After the sea level stabilised and attained its present level about 6500 year BP, Tamaki Estuary continued to infill the natural incised valley. These natural infill sediments are mainly composed of sand and mud deposits. Accelerated anthropogenic infilling during the last 150 years has led to a considerable rise in sedimentation rates. As a result of (1) the constricted nature of the middle estuary (i.e. Panmure area), (2) reduced tidal flushing and increased water residence time, (3) narrow shape of upper estuary (i.e. limited accommodation), and (4) catchment processes linked to urbanisation and industrialisation, the fine fraction sediments trapped in the upper estuary have become more prone to capturing and retaining pollutants.
A geomorphic understanding of the shape of the estuary, the grain size distribution of the sediments, land use of the catchments area, and the presence of a mid-estuary constriction to sediment movement along with historical locations of anthropogenic activity are all significant factors in understanding the broad scale spatial contamination affecting the intertidal sediments in Tamaki Estuary. In addition, point source pollution (e.g. Cu in antifouling paint used in boatyards and marinas) has resulted in localised areas of Cu enrichment in the lower estuary.
Although there is clear evidence of fine fraction pollutant enrichment in surface layers of the estuary (Abrahim 2005), bulk concentration values for Cu, Pb, Zn, and Cd in all the studied surface samples occur well below the ISQG-H toxicity values given by ANZECC (2000). Cd and Cu concentrations are also below the ISQG-L toxicity levels for these elements. However, Pb and Zn concentrations do exceed the ISQG-L values in some of the surface bulk samples in the upper estuary proximal to long established sources of catchment pollution.
References
Abrahim G (2005) Holocene sediments of Tamaki Estuary: characterisation and impact of recent human activity on an Urban Estuary in Auckland, New Zealand. PhD Thesis, University of Auckland, Auckland, New Zealand, 361 p
Abrahim G, Parker R (2002) Heavy metal contaminants in Tamaki Estuary: impact of city development and growth, Auckland, New Zealand. Environ Geol 42:883–890
ACC (2001) Assessment of environmental effects, Auckland City drainage system resource consents. Auckland City Council, Metrowater 282 p
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council, October (http://www.ea.gov.au/water/quality/nwqms/volume1.html)
ARC (1990) Bathing beaches water quality surveys, 1990–1991, Technical Publication, Auckland Regional Water Board No. 88
ARC (1992) Tamaki Catchment and Estuary: Review of water quality, Auckland Regional Council Technical Publication No.20, Auckland, New Zealand 88 p
ARC (1995) The characteristics and fate of copper-based anti-fouling paint residues in the marine environment around Auckland, prepared for Auckland Regional Council by NIWA, Environment Division 58 p
ARC (1999) Tamaki Estuary water quality survey 1985–1997: review report, Auckland Regional Council Technical Publication, No. 105, 21 p
ARC (2000) A target catchment study: The upper Otara Creek. Auckland Regional Council Technical Publication, No. 79, 37 p
Bell FG, Lindsay P, Hytiris N (1997) Contaminated ground and contaminated estuary sediment illustrated by two case histories. Environ Geol 32:191–202
Birch GF (1996) Sediment-bound metallic contaminants in Sydney’s Estuaries and adjacent offshore, Australia. Estuaries Coast Shelf Sci 42:31–44
Birch GF (2000) Marine pollution in Australia, with special emphasis on central New South Wales estuaries and adjacent continental margin (Review). Int J Environ Pollut 13:573–607
Birch GF, Taylor SE (1999) Source of Heavy metals in sediments of the Port Jackson Estuary, Australia. Sci Total Environ 227:123–138
Birch GF, Taylor SE (2002) Assessment of possible sediment toxicity of contaminated sediments in Port Jackson, Sydney, Australia. Hydrobiologia 472:19–27
Cumberland T (1966) Man’s changing role in the Otara-East Tamaki environment. Unpublished thesis, Department of Geography, The University of Auckland, Auckland, New Zealand 58 p
Deely JM (1991) Sediment and heavy metal distribution in the Avon-Heathcote Estuary, Christchurch, New Zealand. Unpublished PhD thesis, Department of Geology, University of Canterbury, Christchurch, 262 p
Deely JM, Fergusson JE (1994) Heavy metal and organic matter concentration and distributions in dated sediments of a small estuary adjacent to a small urban area. Sci Total Environ 153:97–111
Fletcher CA, Bubb JM, Lester JN (1994) magnitude and distribution of anthropogenic contaminants in salt marsh sediments of the Essex Coast, UK: II. Selected metals and metalloids. Sci Total Environ 155:47–59
Glasby GP, Stoffers P, Walter P, Davis KR, Renner RM (1988) Heavy-metal pollution in Manukau and Waitemata Harbours, New Zealand. N Z J Marine Freshw Res 22:595–611
Grange KR (1982) Macrobenthic communities at possible combined cycle power station sites in Manukau and Waitemata Harbours, Auckland. New Zealand Oceanographic Institute, Oceanographic summary 19:20
Green G, Williamson B, Bull D, Oldman J, MacDonald I, Mills G (2001) Prediction of contaminant accumulation in Auckland estuaries. NIWA Client Report: ARC01252, NIWA, Hamilton, New Zealand, 78 p
Green MO, Hume TM (2002) A strategy for determining wave climate in the Auckland Region. Technical Publication No. 154, Auckland Regional Council, Auckland, New Zealand. 26 p
Hatji V, Birch GF, Hill DM (2002) Spatial and temporal variability of particulate trace metals in Port Jackson Estuary, Australia. Estuaries Coast Shelf Sci 53:63–77
Horowitz AJ (1991) A primer on sediment-trace chemistry. Lewis Publishers, Chelsea, 136 p
KRTA Limited (1989) Tamaki River Planning study, prepared for the Waitemata Harbour Maritime Planning Authority, 168 p
Ladd SC, Marcus WA, Cherry S (1998) Differences in trace metal concentrations among fluvial morphologic units and implications for sampling. Environ Geol 36:259–270
Long ER, Morgan LG (1990) The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trend Program. NOAA Tech Memo. NOS OMA 52 US National Oceanic and Atmospheric Administration. Seattle, Washington, 175 p
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
MacDonald DD, Smith SL, Wong MP, Mudroch P (1992) The Development of Canadian Marine Environmental Quality Guidelines. Ecosystem Science and Evolution Directorate, Conservation and protection, Marine Environmental Quality Series No. 1, Canada, Ottawa, Ontario, 32 p
Mills GN, Pickmere SE, Olsen GM and Williamson RB (2000) Marine sediment monitoring programme: 1999 results. Auckland Regional Council Technical Publication 135, Auckland
Parker R, Abrahim G, Gregory M, Tang J, Nichol S, Birch G, Taylor S (2002) Heavy metal contamination in Auckland estuarine sediments: a comparison with sydney Harbour and implications for biodiversity and sustainable development of Auckland City. Proceedings of the Sustainable Auckland Congress, Auckland New Zealand 112–119
Smith SL, MacDonald DD, Keenleyside KA, Gaudet CL (1996) The development and implementation of Canadian sediment quality guidelines. In: Munawar M, Dave G (eds) Development and progress in sediment quality assessment: rationale challenges, techniques and strategies. Ecovision World monograph series. SPB Academic Publication, Amsterdam, pp 233–249
Stewart C (1989) Spatial and temporal trends in trace metal deposition in Canterbury, New Zealand. Unpublished PhD thesis, Department of Chemistry, University of Canterbury, Christchurch, New Zealand, 155 p
Summerhayes CP, Ellis JP, Stoffers P (1985) Estuaries as sinks for sediment and industrial waste-a case history from the Massachusetts coast. Contributions to Sedimentology, 14, 47 p
Tang J (1999) Environmental Geology Study on Panmure Basin, Auckland, New Zealand. Unpublished MSc thesis, University of Auckland, Auckland
Vasconcelos FP, Piron-Frenet M, Perthuisot JP, Ben Haj S, Alliot A (1995) Trace–metal dynamics in tidal estuaries (Bay of Pen Be, Brittany, France). J Coast Res 11(3):763–775
Williamson RB, Mol-Krijnen J, Van Dam LF (1995) Trace metal partitioning in bioturbated, contaminated, surficial sediments from Mangere Inlet, New Zealand. N Z J Marine Freshw Res 29:117–130
Williamson RB, Costley KJ, Mills GN and Pickmere SE (1999) Marine sediment monitoring programme design and 1998 results. Auckland Regional Council Technical Publication 107, Auckland
Acknowledgments
The authors wish to thank Murray Gregory and Fadhil Sadooni for their assistance in the field and for helpful comments at various stages in the work. In addition, we wish to acknowledge and thank Carol Stewart, John Wilmhurst, Vincent Lane and Noel Renner for help and assistance with geochemical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abrahim, G.M.S., Parker, R.J. & Nichol, S.L. Distribution and assessment of sediment toxicity in Tamaki Estuary, Auckland, New Zealand. Environ Geol 52, 1315–1323 (2007). https://doi.org/10.1007/s00254-006-0570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0570-0