Abstract
Groundwater flow fields in aquifers are often determined by water level data measured in monitoring wells. The flow field can be further refined by mass balance simulations, especially when groundwater level data is limited. The mass balance simulation is based on the principle of mass conservation and relies on water quality data in the same aquifer. The approach is applied to the Luohe aquifer in the Binchang area, China. The water-rock interactions and the hydrogeochemical evolution were studied along four typical flow paths. The study indicates that groundwater in the Luohe formation flows from the southern border to the interior of the Ordos Basin. The southern border, approximately 1,400 km2, is a recharge zone, where the Luohe formation outcrops. The total dissolved solids of the groundwater in the southern boarder are less than 1 g/l, and the hydrochemistry type is HCO3–Na. This new finding refines the flow field of the water-bearing formation, and an additional 1,400 km2 is included in the water resource planning of the area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Binchang area is on the border of Shaanxi and Gansu Provinces. Because of lack of groundwater data, the flow field has been poorly defined. Some recharge zones have been mistakenly considered as discharge zones. To better define the flow field, water quality data was collected in this study. The mass balance simulation, also known as “reverse hydrogeochemical reaction path simulation” was used to study the hydrogeochemical evolution in the main water-bearing formation of the area, i.e., the Luohe formation. As a result, the groundwater flow filed in the Luohe formation was refined and even corrected, which leads to findings of additional potable water resources in the study area.
Geologic settings
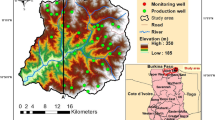
The study area, also referred to as Binchang area, includes Bin and Changwu counties of Shannxi Province (Fig. 1), where the water resource is severely scarce. The amount of water available for supply is limited, and a significant portion of the groundwater and surface water is of poor quality. The concentrations of total dissolved solids (TDS) can be high, which makes the water unsuitable for either drinking or irrigation purpose. The shortage of water, especially high quality water for drinking, seriously restricts the improvement of people’s standards of living in this area.
Geographically, the Binchang area is in south edge of the Ordos Basin, with an area of 2,300 km2. A surface stream, the Jing River, enters the study site from the eastern border and flows into the Changwu County. After taking in water from the Malian River, the Jing River turns toward south and takes in water from the Hei River 20 km downstream. The Jing River flows southeast out of the site at the Bin County seat, and then flows to the city Xi’an.
In terms of geologic structure, the study site is on the Tianhuan syncline, which travels through the basin from north to south. The syncline is asymmetrical. The eastern limb is wide and gently dipping, whereas the western limb is narrow and steeply dipping. The Tianhuan syncline includes the Lower Paleozoic formations with the Cretaceous Luohe formation as its core (Hou and Zhang 2004). The thickness is up to 800–1,000 m along the axis, which crosses east of the Changwu County seat.
The thickness of the Luohe formation is over 400 m. It is a set of detrital rock formations with a complex lithology under continental environment. The Luohe formation is multilayered, comprising of sandstone, siltstone, and mudstone. The unique geological structure leads to a multilayered, confined and relatively closed groundwater aquifer system in this formation. Groundwater in each aquifer within the two limbs of the syncline flows laterally, resulting in an accordant junction.
The Luohe formation has the most extensive distribution in the study area and has the greatest potential providing potable groundwater. It has been correctly considered to be the most important aquifer. The groundwater quality in the Luohe formation is very complex, and both fresh water and saltwater exist. The fresh water has TDS less than 0.5 g/l, and the saltwater has TDS greater than 5.385 g/l. The hydrogeochemical type includes both Cl–Na and HCO3–Ca. The hydrochemistry shows a clear lateral transition from the recharge area to the discharge area.
The Luohe formation is overlain by an overburden that is 800 m thick in the river valley and more than 1,400 m inside the watershed. Boring through the Luohe formation has been challenging. There are only a few hydrogeological boreholes in which the water level data is available. As a result, the flow field produced from the limited data points is not accurate. The inaccuracies in delineating discharge and recharge zones will influence the identification of potable groundwater.
Fortunately, many water quality data were collected in the last 20 years. The mass balance simulation is used to study the hydrogeochemical evolution and to evaluate the recharge, runoff, and discharge conditions in the Luohe formation.
The current understanding of the groundwater flow in the Luohe formation is based on the 2004 flow field map of the Ordos Basin, in which the entire Binchang area was considered to be discharge zone (Li et al. 2005, 2006). In 2005, ten exploratory boreholes were drilled to study the water quality in the southern border. The new data and the mass balance simulation significantly improved knowledge in the hydrogeochemical evolution of the area. The recharge, runoff, and discharge conditions in the Luohe formation were accordingly reevaluated.
Mass balance simulation
Mass balance simulation, also known as hydrogeochemical simulation, is often used to confirm hydrogeochemical reactions in water-rock systems. It relates the geochemical components at one point to those at another in the same system. When the geochemical components are known at two points, the reaction processes can be determined so that the hydrogeochemical evolution is evaluated. In principal, the hydrochemical components at downgradient point equal to the hydrochemical components at upgradient point plus variations of the components along the flow path where the interaction of groundwater and rock, evaporation, and mixing of different waters occur. Theoretically, mass balance simulation is similar to mass balance reaction model (Plummer 1983,1993), which can be described as:
The reactant phase is the component coming into the groundwater, and the product phase is the component leaving the groundwater. The reactant or product phase can be gas, mineral or ion exchange between solid and liquid. Mineral facies is a general designation of them.
Mathematically, the relation can be expressed by the mass balance equation and electron conservation equation.
where a n is the mole concentration of the nth mineral phase coming into or leaving the solution, b n,k is the chemistry computation coefficient of k element in nth mineral facies, Δ is the difference between upgradient point and downgradient point, m T,k is the overall mole concentration of k element in the solution, u n is the efficiency valence state of the nth mineral facies, ΔRS is the oxidation–reduction state of solution.
Δm T,k and ΔRS can be obtained by hydrochemistry assay and the component distributing calculation, and b n,k , u n is related to the possible reactant facies determined by reactions.
Because the number of possible mineral facies is sometimes fewer than the number of the elements considered, the results are not unique. Under some circumstances, the calculations cannot be converged. Uncertainty in the results makes it important to validate the model using measurable concentrations. The verification can be achieved by using the existing geological and hydrogeological data including the characteristics of the mineral in the formations, the sediments and the isotopic analysis.
Mass balance simulation of groundwater in Luohe formation
Selection of simulation paths
Four flow paths are selected to study the recharge, runoff, and discharge conditions in the Luohe formation and they are shown in Fig. 2 as:
-
Simulation path (1): W1→W2
-
Simulation path (2): W2→W3
-
Simulation path (3): W3→W4
-
Simulation path (4): W5→W4.
The arrow indicates the flow direction. The four flow paths are arranged from south to north along the Jing River valley. Such an arrangement ensures that the connection between the recharge and discharge zones is simulated.
The first step of simulation is to calculate the saturation index (SI) and analyze the balance between water and mineral to quantity and determine the dissolved minerals and deposited minerals. The inverse simulation is performed after the hydrochemistry changes on the simulation path are qualitatively analyzed.
Hydrochemistry evolution on flow path (1)
Simulation flow path (1) is from W1 to W2 (Fig. 2). It is at the northern end of the four paths. Table 1 shows the characteristics along the path. The ion concentrations and TDS increase from W1 to W2. The TDS increase from 3.300 to 3.514 g/l. The hydrogeochemical type changes from SO4–Na to SO4·Cl–Na. These data indicate that the cation absorption becomes weak from W1 to W2. The absorption ratio of Na decreases from 20.05 to 12.85. The tendency of sedimentation is shown by the SI of calcite and dolomite that are bigger than 0 in both starting and ending points. The tendency of dissolution is shown by the SI of gypsum and halite that are less than 0 in both starting and ending points.
Hydrochemistry evolution on flow path (2)
Simulation flow path (2) is from W2 to W3 (Fig. 2). It is south of simulation path (1). Path (2) is east of and next to the Jing River. Path (2) points toward the south.
As shown in Table 1, the ion concentrations and TDS increase from W2 to W3, except HCO −3 and Mg2+. The TDS increases from 3.514 to 5.126 g/l. The absorption ratio of Na increases from 12.85 to 18.32 and the SI of gypsum and halite are less than 0 at both starting and ending points of the path. Dissolution and the cation absorption of gypsum and halite occur from W2 to W3.
Hydrochemistry evolution on flow path (3)
Simulation flow path (3) is from W3 to W4 (Fig. 2). It is south of path (2). Path (3) crosses the Jing River and points to the south.
As shown in Table 1, the ion concentration and TDS increase from W3 to W4, except Ca2+, Mg2+, and SO 2−4 . The TDS increases from 5.126 to 5.385 g/l. The absorption ratio of Na increases from 18.32 to 31.23 g/l. The SI of gypsum and halite are less than 0 at both starting and ending points. These characteristics indicate that dissolution and stronger cation absorption on gypsum and halite occur from W2 to W3.
Hydrochemistry evolution on flow path (4)
Simulation flow path (4) is from W4 to W5 (Fig. 2). It is at the southern end of the four paths. It locates at the west side of the Jing River and points to the northeast, the basin.
As shown in Table 1, the ion concentration and TDS increase quickly from W4 to W5, except HCO −3 . The TDS increases from 0.441 to 5.385 g/l. The absorption ratio of Na increases from 3.16 to 31.23 g/l. The SI of gypsum and halite are less than 0 at both starting and ending points. The hydrogeochemical type changes from HCO3–Na to SO4·Cl–Na. These characteristics indicate that dissolution and stronger cation exchange absorption on gypsum and halite occur from W5 to W4.
Possible mineral facies and bounded variable
Firstly, the possible mineral facies were selected to determine the hydrogeochemical reaction along the simulation paths. The synthesis methods used to select the possible mineral facies include:
-
1.
Contrasting chemical constitution at the starting point from the constituent at the ending point.
-
2.
Analyzing hydrochemistry evolution along the simulation paths.
-
3.
Calculating mineral saturation condition.
-
4.
Considering the mineral components of the aquifer, chemical constituents of minerals, pore structure, eluviate condition and its product.
Calcite, dolomite, plagioclase, illite, potash feldspar, muscovite are major minerals at the study site. Because the Cl− of groundwater results from dissolution of chlorate, halite is also considered as possible mineral facies. Because CO2 may participate in reactions at locations where the Luohe formation buries shallow or outcrops, CO2 is also considered as possible mineral facies. Therefore, the possible mineral facies for hydrogeochemical simulation include dolomite, calcite, plagioclase, gypsum, NaCl, illite, potash feldspar, muscovite, CO2, and cation exchange.
Bounded variables are the chemical elements considered in the mass balance reaction model. The possible mineral facies that dissolve or deposit are determined by the change of element concentration at the starting and ending points. Besides NaCl, other mineral sources of Cl are also considered. Six elements are selected as bounded variables and they are K, Na, Ca, Mg, C, and S.
Water-rock reciprocity model
Based on the possible mineral facies and bounded variables, the following water-rock reciprocity model are created.
-
[CaCO3] + [CaMg(CO3)2] + [CaSO4·2H2O] + [Ca(ex)] + [Na0.62Ca0.38Al1.38Si2.62O8] = ΔMT-Ca
-
[CaMg(CO3)2] + [K0.6Mg0.25Al2.3Si3.5O10(OH)2] = ΔMT-Mg
-
[NaCl] + [Na(ex)] + [Na0.62Ca0.38Al1.38Si2.62O8] = ΔMT-Na
-
[KAlSi3O8] + [KAl3Si3O10(OH)2] + [K0.6Mg0.25Al2.3Si3.5O10(OH)2] = ΔMT-k
-
[CaSO4·2H2O] = ΔMT-S
-
[CaCO3] + [CaMg(CO3)2] + [CO2] = ΔMT-C
Results of water-rock reciprocity simulation
The water-rock simulation includes the following three steps:
-
Determine the existing form of groundwater component.
-
Calculate the concentration of component dissolved in existing forms.
-
Calculate SI of each mineral.
The aim is to provide the total concentration of all elements at the starting and ending points and identify the SI that determines the dissolution or deposition of the possible mineral facies for the mass balance reaction model. The software PHREEQC (Parkhurst 1999) is used to calculate the precipitation capacity and dissolving quantity of component dissolved in different existing forms and each possible mineral facies.
There are ten unknown variables in response to the ten possible mineral facies selected above. There are six equations in response to the six major elements. Therefore, the equation matrix consists of ten unknown variables and six equations on every simulation path. Because the unknown variables are more than the number of equations, the equation matrix has more than one result. Several mass balance reaction models are possible in interpreting the data. To make the solutions more unique, some mineral facies are specified in the equation matrix. For example, plagioclase and potash feldspar are hyperthermia genesis minerals, and they dissolves only during hydrogeochemical reaction path simulating.
Through mass balance reaction models calculation, a set of reaction models are obtained. And then separately discuss the simulating result on each path.
Simulation results on flow path (1)
Table 2 presents the simulation results for flow path (1) from two models. The reactions taking place along this path include:
-
Incongruent dissolution reaction of calcite and dolomite.
-
Deposition of illite.
-
Ca–Na cation exchange reaction.
-
Dissolution of other mineral.
In model 1, the quantity of calcite and illite precipitated is 6.372 and 0.352 mmol/l, respectively. The quantity of Ca–Na cation exchange is 4.465 mmol/l. The quantity of dolomite, halite, muscovite, and plagioclase dissolved is 3.278, 8.75, 0.113, and 0.34 mmol/l, respectively.
In model 2, the quantity of calcite precipitated is 6.059 mmol/l. The quantity of Ca–Na cation exchange is 4.389 mmol/l. The quantity of dolomite, halite, and CO2 dissolved is 3.17, 8.808, and 0.469 mmol/l, respectively. Gypsum, potash feldspar, muscovite, plagioclase, and illite drop out of the reaction.
The simulations indicate that the leaching is quite strong during the course of groundwater flow on flow path (1) in the Luohe formation. The ultimate drainage of the aquifer is south of W2.
Simulating results on flow path (2)
Table 2 presents the simulation results for flow path (2) from three simulation models. The result shows that the tendencies of dissolution or deposition of the minerals in the reaction are consistent in the three models, except plagioclase in model 1, dolomite in model 2, and CO2 in model 3. The quantity of gypsum dissolved is the same (10.36 mmol/l) in the three models. The dissolution quantity of halite ranges from 3.605 to 3.685 mmol/l in the three models. The quantity of Ca–Na cation exchange is 3.843–7.825 mmol/l. In model 1, calcite and dolomite have incongruence dissolution reactions.
The results of simulation indicate that the leaching is quite strong during the course of groundwater flow. The ultimate drainage of the Luohe formation is south of W3.
Simulating results on flow path (3)
Table 2 presents the simulation results for flow path (3) from three models. The results show that the tendencies of dissolution or deposition of the mineral in the reactions are consistent in the three models, except plagioclase in model 1, dolomite in model 2, and CO2 in model 3. The quantity of gypsum dissolved is approximately the same (6.382–6.383 mmol/l) in the three models. The dissolution quantity of halite is the same in three models and it is 18.15 mmol/l. The quantity of Ca–Na cation exchange ranges from 12.202 to 3.258 mmol/l. The greatest difference among three models is for feldspar. The feldspar in model 1 does not take part in the reaction; while the feldspar in models 2 and 3 has a dissolution tendency. The dissolution quantity in model 1 and model 2 is 3.402 and 3.058 mmol/l, respectively. In model 1, calcite and dolomite have incongruence dissolution reactions.
The results of simulation indicate that the dissolution and deposition are approximately equal over the course of groundwater flow in the Luohe formation. The deposition of gypsum suggests that the ultimate discharge zone of the Luohe formation is in the vicinity of W4.
Simulating results on flow path (4)
Table 2 presents the simulation results for flow path (4) from two models. The results show that some reactions take place; including incongruent dissolution reaction of calcite and dolomite, deposition of illite, Ca–Na cation exchange reaction, and dissolution of other minerals. The quantity of calcite and illite deposited is 8.349 and 9.749 mmol/l, respectively. The quantity of Ca–Na cation exchange, in which Ca is absorbed, is 12.8 mmol/l. The quantity of gypsum, halite, potash feldspar, muscovite, and plagioclase dissolved is19.22, 39.12, 4.967, 5.16, and 1.424 mmol/l, respectively. The characteristics of the hydrochemistry evolution show that the ion concentration and TDS increase quickly from W4 to W5, except HCO −3 . The hydrogeochemical type changes from HCO3–Na to SO4·Cl–Na. The simulation results indicate that the Luohe formation near the southern border accepts precipitation or unconfined water. The groundwater moves from W5 to W4, i.e., from south to north. The leaching is quite strong during the course of flow. It is estimated that the ultimate discharge zone of the Luohe formation is in the vicinity of W4. The groundwaters from south and north are mixed along a section of flow path (4). We believe that the groundwater in the Luohe formation at W4 comes from north, not from W5 in the south. W4 and W5 do not belong to the same flow path.
Groundwater flow in the Luohe formation by simulations
Integration of the simulation results on four flow paths indicates that the groundwater on flow paths (1), (2), and (3) always moves from north to south, and the groundwater on path (4) moves from south to north. This suggests that flow paths (1), (2), and (3) are at the discharge zone and the region in south of W4 is a recharge zone. The region in the north of W4, where Changwu County, Bin County, and Nin County join, is the discharge zone. The area in the south of W4, where the Luohe formation outcrops or is shallowly buried, accepts precipitation or infiltration from the overlying aquifer. The new data and interpretations refine or correct the flow field map of the Luohe formation at the study site. The revised map is shown in Fig. 3, where a recharge zone is added on the southern border. In this added recharge zone, the hydrogeochemical type is HCO3–Na, and the TDS < 1 g/l. The total area is approximately 1,400 km2 and is therefore considered as an area that can deliver potable groundwater.
Conclusions
-
1.
Both leaching and cation exchanges occur in the Luohe formation in the Binchang area. The main water-rock interaction is dissolution of gypsum, halite, potash feldspar, muscovite, and plagioclase, which leads to high TDS.
-
2.
At the southern border, the groundwater in the Luohe formation moves from south to north and to the interior of the Ordos Basin. The map of groundwater flow field is modified according to the new information.
-
3.
The Luohe formation outcrops or is shallowly buried on the southern border. It can accept precipitation or water from the overlying aquifer. It is a recharge zone, with an area of 1,400 km2. The TDS of the groundwater is less than 1 g/l, and the hydrochemistry type is HCO3–Na.
References
Hou G, Zhang M (2004) Groundwater resources and their sustainable utilization in the Ordos Basin. Shaanxi Science and Technology Publisher, Xi’an, pp 149–165
Li Y, Feng J, Wang W, Li J, Wang J, Xu Z, Li Y (2005) The groundwater system analysis of Cretaceous system of Ordos basin. Northwest Geol 37(2):90–96
Li Y, Wan W, Wu Y, Qu H, Hou G (2006) Application of hydrochemical signatures to delineating potable groundwater resources in Ordos Basin, China. Environ Geol 49(3):430–436
Parkhurst DL, Appelo CAJ (1999) User guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: US geological survey water-resources investigations report 99-4259, 310 p
Plummer LN, Parkhurst DL, Thorstenson DC (1983) Development of reaction models for ground-water systems. Geochim Cosmochim Acta 47:665–686
Plummer LN (1993) Regional groundwater quality edited by William M. Alley U.S. Geological Survey, pp 199–255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Wang, J., Wu, Y. et al. Mass balance simulation and its application to refining flow field in Binchang area, China. Environ Geol 52, 739–745 (2007). https://doi.org/10.1007/s00254-006-0511-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0511-y