Abstract
In this paper, Shuwaymiyah palygorskite in the Sultanate of Oman has been characterized mineralogically by X-ray diffraction (XRD) and electron microscopy, chemically by oxide compositions, structural formulae, and cation exchange capacity (CEC), and physically by specific surface area and adsorption isotherms. Batch adsorption studies were performed to evaluate the adsorption performance of methylene blue (MB) basic dye on the local clay mineral. The quantitative XRD analysis indicates that the purity of some selected samples of palygorskite clay is very high (about 70% of the clay minerals are palygorskite and 30% kaolinite). The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images clearly support this conclusion. The adsorption equilibrium revealed that Shuwaymiyah palygorskite clay can uptake up to 51 mg of MB per 1 g mass of clay. MB adsorption is best fitted by Langmuir isotherm, and a pseudo-second-order kinetic model can be efficiently used to predict the kinetic of adsorption of MB by the palygorskite. The results obtained from these laboratory-scale adsorption tests indicate the promising adsorption capability of the Omani palygorskite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Palygorskite (or attapulgite) is a hydrated magnesium aluminum silicate mineral characterized by an elongated microfibrous morphology, moderate surface charge, moderate cation exchange capacity (CEC), high specific surface area, and high adsorption capacity (Murray 2000). Galan (1996) reviewed the basic mineral characteristics of palygorskite, as well as its wide range of applications. Although palygorskite is relatively rare, it exists in the semi-arid country, Sultanate of Oman, in huge reserves and high purities. Palygorskite has been recorded at many locations in Dhofar, the southern part of Oman, in the Hasik Member of the Umm er Radhuma formation, in the Andur Member of the Dammam formation and in the Nakhlait member of the Ashawq formation (Minerals Division 2002). However, it has always been a problem locating a place with commercially appealing thickness of palygorskite beds along with a low overburden thickness. In 1995, two sites (Shuwaymiya and Tawi Attair) were selected by the Department of Geological Survey and Exploration for reserve estimation, taking into consideration exposure, overburden, accessibility and vegetation. The exploration confirmed that palygorskite exists in the Sultanate in huge reserves: the Shuwaymiyah deposit contains 300–400 million tons, and the Tawi Attair deposit contains 200,000 to 1 million tons (Minerals Division 2002). Presently, palygorskite is not being mined or used in Oman (Minerals Division 2002).
The long-term objective of the study is to evaluate the potential of using the local clay mineral palygorskite as a sorbent in the treatment of groundwater polluted with hydrocarbons/heavy metals, which is widespread in the country (Bajjali 1996; El-Zawahry 2001; Scheu 1993). The application of clay minerals in various processes of environmental engineering is gaining increasing interest worldwide (Haydn 1991). For example, bentonite and palygorskite are used as sorbents for the treatment of contaminated water (Chang et al. 1991; Zunan et al. 1995; Pillon 2001; Wang et al. 1998; Pamukcu and Hijazi 1992), as slurry trench cut-off walls for environmental pollution control (Gurbe 1992), and as a liner in properly designed waste landfill (Broderik and Daniel 1990).
In this paper, the Shuwaymiyah palygorskite is characterized and its potential adsorption capacity for methylene blue (MB) basic dye is investigated. The paper is organized as follows. First, an overview of the geologic settings of the Shuwaymiyah deposit in Dhofar is provided. Second, the basic mineralogical, chemical, and physical characteristics of the Shuwaymiyah palygorskite are described. Third, the kinetic and adsorption capacities of the clay for MB dyes as an indication of the adsorption potential of Shuwaymiyah palygorskite are discussed.
Clay deposits and geological setting
The Shuwaymiyah palygorskite deposit is found in the Shuwaymiyah and Thadbut topographic sheets, which occur about 3.5 km NW of Shuwaymiyah village on the sea shore. The Shuwaymiyah area constitutes a closed coastal plain about 30 km long and 4–5 km wide. The plain is bounded to the south by the sea, and the north by two sharp, successive scarps; the Dammam Formation and the Aydim Formation. To the east and west the scarp rises directly from the shore. The area is drained by numerous, south-flowing wadis, the biggest of which is Wadi Shuwaymiyah. These wadis dissect the plain into numerous, very low-lying hills. The geological map of the Shuwaymiyah area (Fig. 1) shows the distribution of the Andhur Member in Shuwaymiyah area, which more or less represents the distribution of palygorskite at the foot of the Shuwaymiyah scarpment. The Andhur Member forms a gentle slope along which a substantial amount of palygorskite can be easily scarped without having to remove much of the overburden. In addition to the occurrence of palygorskite along the foot of the scarp, a good part of the Quaternary deposits on both sides of Wadi Shuwaymiyah cover low-lying hills representing the lower part of the Andhur Member and containing a lot of easily quarryable palygorskite deposits.
In 1995, the Department of Geological Survey and Exploration in the former Directorate General of Petroleum and Minerals selected two sites (site 1 and site 2) for reserve estimation, taking into consideration exposure, overburden, accessibility, and vegetation. The lithologic section shown in Fig. 1b was logged at site 2. The lithologic structure of site 1 is very similar. It is clear from the figure that the Andhur Member is composed of several clay layers (mainly palygorskite) varying in thickness with interbedded layers of chalky, marly limestone. At site 1, the palygorskite horizons range in thickness from 0.4 to 3.5 m with an average of 1.1 m. The site is estimated to contain about 321 million tons of palygorskite and 264.6 million tons of interbedded limestone over an area of 6.7 km2. At site 2, the limestone beds range in thickness from 0.2 to 0.6 m, with a total thickness of 3 m. The palygorskite beds range in thickness between 0.2 and 2 m. Their total thickness is 7.1 m. Site 2 contains about 33.6 million tons of palygorskite and 18.7 million tons of interbedded limestone over an area of 2.4 km2 (Minerals Division 2002).
Experimental procedures
The raw clay was dried and mechanically crushed and sieved to pass the 200 sieve (75 μm). For mineralogical analysis, some samples were further washed to remove carbonates, soluble salts, and organic matter. Then, the clay fine fraction was separated by centrifuge. These processes of clay washing and separation were implemented following the detailed procedure described in Al-Rawas et al. (2001). Oriented clay samples on glass slides were prepared for phase identification by X-ray diffraction (XRD). The X-ray diffractometer used in this analysis was Philips PW1710 automated powder diffractometer, with a generator settings of 40 kV, 40 mA, and nickel-filtered CuKα (λ = 1.54056 Å) radiation over a 2θ range of 2.5–70° at a scanning rate of 2°/min. Ethylene glycol and heat treatments were used to provide additional information essential for the identification of clay minerals. Clay phase identification has been examined in six forms: as an oriented clay sample (untreated), and as an oriented clay sample treated with ethylene glycol, as an oriented clay sample heated to 350, 600, 700, and 800°C for 2 h. Size and morphology of particles were determined using Jeol JSM-560 V 20 kV scanning electron microscope (SEM) and Joel JEM-1230 120 kV transmission electron microscope (TEM). Oxide content in the palygorskite samples was determined using wet chemical analysis. The structural formulae for palygorskite were calculated on the basis of 21 oxygen atoms per unit cell. The cation exchange capacity (CEC) was determined by the ammonium acetate exchange. The specific surface area (SSA) of the clay mineral was determined using the N2-BET with the Quantachrome Autosorb Automated Gas Sorption Instrument. These two properties have also been determined by the MB adsorption method developed by Hang and Brindley (1970), which is described in detail in the following section.
Sorption experiments were performed using the standard batch technique. Initially, kinetic experiments were performed to study the effect of contact time on the adsorption processes. In the kinetic experiment, a fixed mass of palygorskite (30 mg) and a fixed concentration of dye (100 mg/l) were added in 40-ml borosilicate glass bottles with Teflon-lined caps. The mixture total volume was set to 20 ml. Several identical bottles were placed on a specially designed end-over-end shaker with a fixed speed motor. At specified times, bottles were removed from the shaker and centrifuged at 3,000 rpm for 30 min. Then, the dye concentration in the supernatant was measured using a UV-visible spectrophotometer (Perkin-Elmer) at a wavelength of 665 nm. For the adsorption isotherms, one set of several 40-ml glass bottles containing constant palygorskite clay mass (30 mg) and variable initial concentrations of dye (5–100 mg/l) was prepared. A second set of glass bottles containing the dye with a constant initial concentration (100 mg/l), but with a variable palygorskite clay mass (10–90 mg), was simultaneously prepared. The volume of the mixture was also set to 20 ml. Both sets, along with blank bottles (containing MB without palygorskite clay), were placed on the shaker overnight (18 h) at room temperature (25°C) to reach equilibrium. Then, the bottles were centrifuged at 3,000 rpm for 30 min. The equilibrium concentration of MB was measured using the UV-visible spectrophotometer at the previously mentioned wavelengths. In all cases, dye solutions were prepared using distilled water. All experiments were performed in duplicate. The amount of dye adsorbed on the surface of the palygorskite, q e (mg/g), was obtained as follows:
where C I and C e (mg/l) are the initial and final or equilibrium contractions of MB respectively, V (ml) is the solution volume and M is the palygorskite clay mass (mg).
Results and discussion
X-ray diffraction
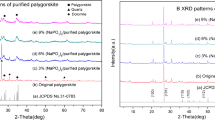
The X-ray powder diffractogram of the Shuaimiah palygorskite (Fig. 2) shows the presence of two main sets of phases: palygorskite and kaolinite. The d values of palygorskite are 10.5, 6.3, 5.5, 4.5, 4.2, 3.7, and 3.2 Å, whereas the d values of the kaolinite are 7.1 and 3.6 Å. The figure also clearly shows that both minerals are unaffected by glycolation. When subjected to heat treatment at 350°C, the intensity of the main reflection decreases for both minerals. At 600°C heat treatment, the d values of 10.5 Å for palygorskite and 7.1 Å for kaolinite completely disappeared.
The constant mineral standards method (Rohe and Olson 1979) was used to quantify the percentages of palygorskite and kaolinite minerals in Shuwaymiyah palygorskite clay. Different percentages of clay were added to constant standard minerals of equal weights. All the prepared samples were treated with ethylene glycol. The peak area ratio was calculated from the XRD of the standard mixes with the clay. The mineral peak area ratio against the percentages of clay is linearly best fitted, as shown in Fig. 3, and the percentage of clay minerals was calculated from the slope of the fitted line. The estimated percentage of palygorskite in Shuwaymiyah palygorskite is within the range of 67–72%.
Electron microscopy
Electron microscopy is an effective tool for observation of the morphology, size, and texture of the individual particles of clay minerals, particularly palygorskite (Paquet et al. 1987; Chahi et al. 1993). Selected SEM images of Shuwaymiyah palygorskite are shown in Fig. 4 and TEM images in Fig. 5. The micrographs shown on the right-hand side are always obtained at a higher magnification than the corresponding micrographs on the left-hand side. The micrographs clearly reflect the abundance of palygorskite fibers in Shuwaymiyah palygorskite. Individual palygorskite fibers are 0.2–0.5 μm long (Fig. 5c, d). However, these individual fibers often form long palygorskite bundles that are 5–10 μm long (Fig. 5a, b). As seen from the SEM images, the palygorskite fibers may exist as individual, well-separated fibers (Fig. 4a, b) or as elongated bundles of many fibers (Fig. 4c, d), or sometimes circular aggregates of clay fibers (Fig. 4e). Due to such variations in the existence of palygorskite fibers, the pore space in the formation consists of tiny pores between the individual fibers and larger pores between the bundles of fibers (Fig. 4c, d).
Chemical compositions
Chemical analyses were performed on 25 palygorskite samples obtained from the Shuwaymiyah reserve. Some of the samples were collected and chemically analyzed by the Mineral Division (2002) of the Ministry of Commerce and Economy. The major element oxide composition of the samples are reported in Table 1. As seen in the table, the main constituent of the clay is SiO2 (32–56%). The next three major oxides are Al2O3 (7–14%), MgO (3.6–11%), and Fe2O3 (3.6–8%). Interlayer exchangeable cations are mainly Ca and Na with some K. It was noticed that the Ca oxide showed considerable variation from a very low amount (0.1%) in some samples to a relatively high amount (13%) in other samples. Cross plots of the data (not included here) revealed that there is no apparent correlation between Si and Mg. However, there is a slight negative correlation between Si and Ca. There is also no correlation between Mg and Ca. In general, chemical compositions of the Shuwaymyah palygorskite samples confirm the fact that the 25 collected samples are considerably variable. The Si/Mg ratios range from 2 to 15%, whereas the Mg/Ca ratios range from 0.5 to 36%.
This large variability in chemical composition indicates the impurity of the collected samples, and hence affects the accuracy of the obtained chemical formula for the Shuwaymiyah palygorskite. At present, the most accepted form of the structural formulae represents palygorskite as intermediate between di- and trioctahedral phyllosilicate (Bailey 1980, Paquet et al. 1987), with 21 oxygens for a half, dehydrated unit cell, with the formula: (Si8−x R 3+ x ) (Mg5−y−z R 3+ y □ z ) O20 (OH)2 (OH2)4 R2+ (x−y−z)/2 4H2O. Here, all the Fe present was assumed to be Fe3+. On average, 4 out of each 5 octahedral positions are occupied. The mean structural formula obtained for Shuwaymiyah palygorskite was (Si7.04Al0.95) O20 (Al1.07Fe0.61Mg1.86□1.46) (OH)2(OH2)4 (Ca0.42K0.15Na0.27). This structure formula is compared in Table 2 with the structure formulae of other palygorskite reserves analyzed and reported by Garcia-Romero et al. (2004).
Compared with all other palygorskite, Shuwaymiyah palygorskite has a considerable substitution of tetrahedral Al for Si (0.2–1.71). The average Al in the tetrahedral layers of Shuwaymiah palygorskite is the largest seen in the literature compared with other sources of this clay. Mg, Al, and Fe are the dominant cations in the octahedral site, accompanied by minor amounts of Ti. The values of Mg atoms per half unit cell range from 0.8 to 2.5. The sum of octahedral cations per half unit cell ranges from 2.48 to 4.25; the mean value of the number of octahedral cations is 3.61. This mean value is lower than all reported values of the sum of octahedral cations (see Table 2). According to Newman and Brown (1987), the sum of octahedral cations in palygorskite lies between 3.76 and 4.64, with a mean value of 4.00, which is also higher than the mean Shuwaymiyah Mg value.
The ratio between divalent and trivalent octahedral cations (Mg/Al + Fe3+) in Shuwaymiyah palygorskite is 1.44. Galan and Carretero (1999) affirmed that palygorskite contains mainly Mg, Al, and Fe with an Mg/Al + Fe3+ ratio close to 1. Garcia-Romero et al. (2004) reported that the Mg/Al + Fe3+ ratio for Attapulgus (USA), Bercimuel (Spain), and Torrejon (Spain) are 1.35, 1.01, and 1.22 respectively. The values are very similar to those found in Shuwaymiyah samples (mean 1.44). Figure 6 shows the relationship between the Si/Mg and Al + Fe3+/Mg and the Al + Fe3+ and Mg cations of Shuwaymiyah palygorskite samples along with the corresponding reported values extracted from the Garcia-Romero et al. (2004) paper. The figure clearly indicates that the samples obtained from Shuwaymiyah palygorskite have very variable chemical compositions and hence more detailed purified samples should be considered to obtain more precise chemical formulae.
Relationship between a Si/Mg and (Al + Fe3+)/Mg and b Al + Fe and Mg cations of Shuwaymiyah palygorskite samples (closed markers) along with some literature values (open markers) obtained from the Garcia-Romero et al. (2004) paper
Physico-chemical properties
The measured SSA and CEC of Shuwaymiyah palygorskite are reported in Table 3. The SSA was measured using the N2-BET method and the MB adsorption method described in the following sections. Similarly, the CECs are calculated by ammonium acetate exchange and MB adsorbtion. All samples were crushed to the same fine fraction level. MB adsorption is largely used as an efficient procedure for determining CEC and SSA (Rytow et al. 1991). The difference between the determined values of SSA obtained by the two methods is expected since it is known that the MB adsorption method allows the total surface area of clay mineral to be measured, whereas the Brunauer-Emmett-Teller (BET) technique measures the external SSA only. However, the CEC obtained by MB adsorption is slightly less than the corresponding value obtained by ammonium acetate extraction, reflecting the fact that MB was not able to replace all the cations on the clay surfaces. The SSA and CEC of Shuwaymiyah clay were also compared in Table 3 against the corresponding values obtained for a pure palygorskite sample bought from Engelhard Corporation, NJ, USA, and also the literature values of the palygorskite in the Hawthorne formation in Florida. It is very clear that the Shuwayminah palygorskite has almost the same quality as the pure palygorskite standard bought from Engelhard. The Shuwaymiyah palygorskite has almost the same CEC as the Hawthorne palygorskite, but slightly less in the case of specific surface area.
Adsorption kinetics
To study the rate-determining step for the adsorption of MB onto the surface of palygorskite, two kinetic models (Wu et al. 2001; Ho and McKay 2000) were employed: pseudo-first-order and pseudo-second-order models. The simple Lagergren pseudo-first-order model assumes that the rate of change of solute uptake over time is directly proportional to the difference in the amount of solute adsorbed at the time of equilibrium, q e [mg/g], and the amount of solute adsorbed at any time, q(t) [mg/g]:
where k 1 [1/min] is the rate constant of pseudo-first-order model. When integrating over time and linearizing the model, the following equation is obtained:
The pseudo-second-order equation based on adsorption equilibrium capacity is expressed in the form:
where k 2 [g/mg min] is the constant rate of the pseudo-second-order model. With a similar manner to that performed for the previous model, the linearized form of the variation of clay uptake over time is:
Figure 7 summarizes the results obtained from the kinetic MB experiments. Figure 7a plots the palygorskite clay uptake of MB at various contact times. One can immediately observe the fast decrease in residual dye concentration on a short time scale (just a few minutes), implying the strong electrostatic interaction between the negatively charged surface and the basic dye cations. The estimated equilibrium time for MB adsorption is 60 min.
The validity of the two kinetic models was checked using linear plots of ln[q e−q(t)] versus t (Fig. 7b) for the pseudo-first-order and t/q(t) versus t (Fig. 7c) for the pseudo-second-order model. Even though the pseudo-first-order model has provided a good fit (R 2 = 0.90) in the early time stages, the experimental data have shown considerable deviation at later times. However, the pseudo-second-order model has shown an almost perfect fit (R 2 = 0.9999) over the whole range of time considered. The constant rate of the pseudo-first-order model is k 1 = 0.035 (1/min), whereas the corresponding constant rate for the pseudo-second-order model is k 2 = 0.031 (g/mg min). This perfect fit of the pseudo-second-order kinetic model suggests that the model can be efficiently used to predict the kinetics of adsorption of MB by palygorskite. Since this model is based on the adsorption capacity, it will help to predict the adsorption behavior over the whole range of concentrations and supporting chemisorption (chemical reaction) as a rate controlling mechanism. Chemisorption usually indicates that the adsorption process is irreversible. Views of a similar kind have been put forward by other colleagues (Ho and McKay 2000; Wu et al. 2001; Vinod and Anirudhan 2003) who had studied basic dyes adsorption.
Adsorption isotherms
When the system is in the state of equilibrium distribution of dye between the adsorbent and the dye solution, it is important to establish the adsorption isotherms and the capacity of the adsorbent for the dyestuff. Figure 8a shows the experiential curves of equilibrium clay uptake (q e) and equilibrium concentration (C e) for adsorption of MB by the palygorskite clay. The dye shows strong affinity for palygorskite clay. Reading the obtained isotherm, the clay uptake increased sharply when the solution concentration was increased from 0 to about 5 mg/l to reach an adsorption capacity of 40 mg/g. This sharp increase in adsorption is followed by a gradual increase in the adsorption capacity in the regions from 5–20 mg/l beyond which the adsorption is leveled to a maximum value of 51 mg/g.
The adsorption isotherms that are usually used for describing dye adsorption are the Langmuir and the Freundlich. The linear transformation of the Langmuir isotherm is:
The isotherm incorporates two constants, one (q max) establishing the maximum adsorption capacity or density and the other (b) establishing the affinity of the adsorbent for the adsorbate. Using a least square linear fit of C e /q e versus C e, the two unknown parameters can be determined from the slope of the line (1/q max) and the intercept [1/(bq max)]. In the other hand, the Freundlich isotherm is linearly transformed to:
Like the Langmuir isotherm, a Freundlich isotherm is fully defined by two fitting parameters: K f, which describes the adsorption density, and 1/n, which indicates how dramatically the binding strength changes as the adsorption density changes (intensity of adsorption). A linear fit of ln(q e) versus C e would give ln(K f) (intercept) and 1/n (slope). The value of n is usually greater than 1 and must reach some limit when the surface is fully covered.
The goodness of fit of the Langmuir model to the MB experimental data is shown using the linear plot in Fig. 8b, whereas the goodness of fit of the Freundlich model is shown in Fig. 8c. The parameters of the fitted models with their regression coefficient are given in Table 4. Visual inspection and value of regression coefficient yield the conclusion that MB adsorption is best fitted by the Langmuir isotherm and poorly fitted by the Freundlich isotherm as summarized in Fig. 8a. This is in full agreement with the prevailing chemisorption mechanism concluded from the kinetic study, and suggests that the sorbed layer has a thickness of only one molecule and that all surface sites have an equal sorption affinity for MB.
Conclusion
A very important clay mineral in the Sultanate of Oman has been mineralogically, chemically, and physically characterized. The adsorbtion capacity of the clay with regard to basic dyes has also been investigated. The quantitative mineralogical investigation on Shuwaymiyah palygorskite clearly indicates its high purity with palygorskite minerals that exceeds 70%. SEM and TEM images clearly indicate the existence of palygorskite as a dominant mineral in Shuwaymiyah clay. The average Al in the tetrahedral layers of Shuwaymiah palygorskite is the largest seen in the literature; however, the average sum of octahedral cations per half unit cell is lower than all reported values in the literature. Shuwaymiyah palygorskite was used as a sorbent for the removal of MB basic dye from water. The adsorption equilibrium revealed that the clay can uptake up to 51 mg of MB per 1 g mass of clay. MB adsorption is best fitted by the Langmuir isotherm. A pseudo-second-order kinetic model can be efficiently used to predict the kinetic of adsorption of MB by the palygorskite. All these investigations suggest that Shuwaymiyah palygorskite is a very promising, naturally occurring adsorbent that could substitute in the Gulf region the use of highly expensive activated carbon.
References
Al-Rawas AA, Sutherland H, Hago AW, Yousif AA, Al-Shihi M, Al-Shihi B (2001) A comparative quantitative study of an Omani soil using X-ray diffraction technique. J Geotechnical Geol Eng 19:69–84
Bailey W (1980) Structure of layer silicate. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification. Monograph 5. Mineralogical Society, London. pp 1–123
Bajjali W (1996) Groundwater contamination by hydrocarbon in Nizwa, Zukait, Jaalan Bani Bu Hasan, and Barka areas. Technical report, Ministry of Water Resources, Oman
Broderick GP, Daniel DE (1990) Stabilizing compacted clay against chemical attack. J Geotechnical Eng 116(10):1549–1567
Chahi A, Duplay J, Lucas J (1993) Analysis of palygorskite and associated clays from the Jbel Rhassoul (Morocco): chemical characteristics and origin of formation. Clays Clay Miner 41(4):401–411
Chang SH, Ryan ME, Gupta RK (1991) Competitive adsorption of water-soluble polymers on attapulgite clay. J Appl Polym Sci 43(7):1293–1299
El-Zawahry A, Shahalam AM, Taha R, Al-Busaidi T (2001) An assessment of solid waste and landfills in Muscat area, Oman. Sci Technol SQU 6:1–11
Galan E (1996) Properties and applications of palygorskite–sepiolite clays. Clay Miner 31:443–453
Galan E, Carretero I (1999) A new approach to compositional limits for sepiolite and palygorskite. Clays Clay Miner 47:399–409
Garcia-Romero E, Sauarez Barrios M, Bustillo Revuelta MA (2004) Characteristics of a Mg-palygorskite in Miocene rocks, Madrid basin (Spain). Clays Clay Miner 52(4):484–494
Gurbe WE (1992) Slurry trench cut-off walls for environmental pollution control. In: ASTM Special Technical Publication, Symposium on slurry walls: design, construction, and quality control, Atlantic City, NJ, USA. Sponsor: ASTM, pp 67–77
Hang PT, Brindley GW (1970) Methylene blue adsorption by clay minerals: determination of surface areas and cation exchange capacities (clay-organic X VIII). Clays Clay Miner 18:203–212
Haydn MH (1991) Some applications of selected clay minerals. In: Applied clay science, 24th Annual Meeting of the Clay Minerals Society, 18–22 October 1987, NM, USA, pp 379–395
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Minerals Division (2002) Shuwaymiya and Tawi Attair Attapulgite. Technical report, Ministry of Commerce and Economy, Oman
Murray HH (2000) Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci 17:207–221
Newman AC, Brown G (1987) Palygorskite and sepiolite. In: Newman AC (ed) Chemistry of clays and clay minerals. Monograph 6, Mineralogical Society, London
Pamukcu S, Hijazi H (1992) Improvement of fuel oil contaminated soils by additives. In: Geotechnical Special Publication. In: Proceedings of the 1992 ASCE Specialty Conference on Grouting, Soil improvement and Geosynthetics, 25–28 February, 1992, New Orleans, LA, USA, pp 1285–1297
Paquet H, Duplay J, Valleron M, Millot G (1987) Octahedral compositions of individual particles in smectite-palygorskite and smectite-sepiolite assemblages. In: Proceedings of the 8th International Clay Conference, Denver. The Clay Minerals Society, Bloomington, pp 73–77
Pillon LZ (2001) Effect of attapulgite clay on the composition of jet fuels. Petroleum Sci Technol 19(7–8):875–884
Rohe RV, Olson CG (1979) Estimate of clay–mineral content: additions of proportions of soil clay to constant standard. Clays Clay Miner 27(5):322–326
Rytow R, Serban C, Nir S, Margulies L (1991) Use of methylene blue and crystal violet for determination of exchangeable cations in montmorillonite. Clays Clay Miner 39(5):551–555
Scheu M (1993) Assessment study on municipal solid waste management in Muscat. Technical report. Kalwasswerp Engineering, Wetter
Vinod VP, Anirudhan TS (2003) Adsorption behaviour of basic dyes on the humic acid immobilized pillared clay. Water Air Soil Pollut 150:193–217
Wang L, Huang Z, Sun X, Liu X, Jin J (1998) Treating dyeing wastewater with modified absorbent of attapulgite. J Nanjing Univ Sci Technol 22(3):240–243
Wu F, Tseng R, Juang R (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35(3):613–618
Zunan Q, Yi Z, Yuqiao F (1995) Removal of oil from concentrated wastewater by attapulgite and coagulant. Water Qual Res J Can 30(1):89–99
Acknowledgement
This study has been supported by Sultan Qaboos University Internal Grant IG/ENG/CAED/04/02. The support of the Directorate of Mineral Exploration in the Ministry of Commerce and Industry (Salim Al-Busaidi and Khalid Al-Toobi) is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Futaisi, A., Jamrah, A., Al-Rawas, A. et al. Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ Geol 51, 1317–1327 (2007). https://doi.org/10.1007/s00254-006-0430-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0430-y