Abstract
The maximum concentration of the majority of the trace metals in the leachates from shake and column test of lignite fly ash (LFA) was within the prescribed limits; however, total dissolved solids, total hardness, cations and anions (except K+), being above the prescribed limits, may lead to the increase in the hardness and salinity in the soil on the disposal of LFA. Present generation of huge amount of fly ash from thermal power plants (TPPs) is a big challenge concerning contamination of soil, crop produce and surface and ground water bodies due to the presence of some of the toxic trace metals in it. The leaching behavior of alkaline LFA (pH, 10.94), from TPP of Neyveli Lignite Corporation (NLC), India, was investigated by shake and column tests using water and sodium acetate buffer. The leaching of trace metals from LFA was governed by their concentrations, association with the ash particles, leaching duration and pH of the leachate (most influencing parameter). The leaching of metals followed the order: buffer column > aqueous column > aqueous shake > buffer shake test.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meeting energy requirements from coal and lignite via combustion in thermal power plants (TPPs) will indubitably continue to predominate the world energy scenario until alternative sources of energy are fully developed and commercially exploited. Though the emission of fly ash from TPPs to the atmosphere can be controlled appreciably by new techniques, ash disposal in slurry form in ash ponds is not free from environmental concerns. At present about 110 million tons of fly ash per annum is being generated from 85 TPPs in India. With present utilization of about 38.4%, mainly in civil construction, building materials and some in agro-forestry, the presence of trace metals in ash has become a subject of much concern (contamination of soil, surface and ground water bodies), inhibiting more complete utilization on a sustainable basis. In the case of Indian fly ashes it may be more worrying, as they are derived from high ash (mineral matter), and low calorific value coals. Moreover, enrichment associated with combustion may concentrate elements by factors of four to ten times (Fernandez-Turiel et al. 1994).
Ash may contain As, B, Be, Ca, Cd, Cr, Fe, Hg, Mg, Mo, Na, Ni, Pb, Ra, Se, Th, U, V, Zn, etc. either on the surfaces of ash particles or in the aluminosilicate matrix phase or in both, as a consequence of volatilization and condensation during combustion (Henry and Knapp 1980; Hansen et al. 1984; Smith 1987). Elements having their surface association may release more rapidly than those contained in glass and crystalline fraction (Ramesh and Kozinski 2001). Generally, glass phase and magnetic fractions in combustion residues contain most of the potentially toxic elements (EA-3236 1993; Zevenbergen et al. 1994; Kukier et al. 2003). Elements like Pb, Cd, Cr, As and Hg may cause detrimental effects on human health and aquatic life (Pande and Hasan 1983). Fly ash in contact with water may experience solid phase dissolution, with changes in pH and release of toxic trace metals (Fulekar 1993). The principal processes affecting the leaching process are dissolution of primary solids, precipitation of secondary solids, as well as sorption and hydrolysis reactions etc. (Jankowski et al. 2006). Free lime in the fly ash may have higher reactivity, leading to several problems, including leaching of trace metals to the ground water (Sloss 1996) and cations and anions in ash may increase the hardness, salinity, alkalinity and proportion of dissolved solids in soils (Fulekar and Dave 1989).
The US EPA has already expressed concern about some waste disposal/landfills (EPA 1984). In fact, many countries are carrying out leaching tests for assessing the nature and magnitude of pollutants in the leachate from coal ash piles, and also to develop appropriate environmental control technologies. Despite the advances in testing procedures, uncertainties in simulation of interactions in real environmental conditions make reliable long-term prognosis of environmental behavior of waste deposits difficult (Van der Sloot et al. 1993). In truth, there is no globally accepted standard leaching test (Drakonaki et al. 1998). A number of leaching tests such as the US EPA-TCLP, German DIN 38414 S4, and other batch and column leaching tests or field studies have been attempted over the years, where liquid to solid (L/S) ratio, pH (Henery and Knapp 1980; Van der Sloot 1995; Karuppiah and Gupta 1997; Ram et al. 1999, 2000), type of leachant, stirring conditions, duration of leaching, temperature (Henery and Knapp 1980; Karuppiah and Gupta 1997; Khanra 1998; Claudia et al. 1999) and particle size distribution (Biermann, and Ondow 1980) are inferred as significant variables in influencing the results. Based on these variables, a range of long-term environmental assessments can be devised (Lee and Kahn 1997).
The traditional tests including USEPA-TCLP are being questioned in the context of coal ash management and making it difficult to choose the best (Hassett et al. 2005). It is not easy to simulate field conditions; however, useful information can be obtained through laboratory experiments (Swaine 1994). Column testing is meant to simulate percolation of the leaching agent through an ash dump. It provides information on the initial rather than the total concentration in the percolate from a deposit, and likely to simulate field leaching on a time scale of several years (Smith 1990) as it is approximately more close to the conditions, particle size distribution and pore structure, leachant flow and solute transport found in the field (Zacharia and Streile 1990). The test is normally terminated when a liquid/solid (L/S) ratio of approximately 10 is reached, the reason being the unduly long time required to reach a higher L/S ratio. An L/S ratio of 3, 5, or 10 is considered equivalent to the leaching that may take place within 15, 25, or 50 years, respectively (Van der Sloot et al. 1984). Normally, a dilution factor of 10:1 is allowed for leachates as being more representative of the true environmental risk. This has been generally adopted factor in considering the likely level of attenuation able to be achieved in situ ground water flow effects (Lindon 2001). Shake tests, on the other hand, give information on the total quantity of an element leachable from an ash residue. Long-term leaching tests are preferable to short-term tests as the findings with the latter may mislead the decision implementation; however, short-term test is also suggested to be considered in a regulatory testing scheme (Hassett et al. 2005). The geochemical reactions between coal combustion solid waste and infiltration water may be useful for assessing the leaching behavior of inorganic wastes from fly ash (Murarka et al. 1992). For alkaline/acidic wastes, a sodium acetate buffer solution (pH, 5) is used as the buffering can provide a more stable pH condition even with the presence of alkaline and/or acidic materials (Chong et al. 1990). As such, a combination of both column and shake tests in the laboratory with aqueous and buffer extracts for long- and short term may possibly enable the user to assess the potential toxicities associated with LFA.
Around 0.7 million tons lignite fly ash (LFA) per annum is being generated from two TPPs (I & II) of Neyveli Lignite Corporation (NLC), India. Less than 15% of this is being utilized, and the remaining is disposed of in an ash pond. In view of the different characteristics relative to coal fly ash (http://www.tfhrc.gov/hnr20/recycle/waste/), and the limited information available on leaching characteristics of Indian fly ash (Mukherjee and Zevenhoven 2006; Ram et al. 2000; Praharaj et al. 2002; Saikia et al. 2006), the present study was undertaken in simulating aggressive conditions to assess the toxicity that may take place in field conditions using both column and shake tests with water and sodium acetate buffer. The aggressive conditions include application of these test methods for longer duration, sodium acetate buffer (pH, 4.9), and shaking (in case of shake test) (USEPA 1987; Egemen and Yurteri 1996). The obtained results were evaluated in the light of national and international standards prescribed for drinking water, industrial effluents, leachates and land fill drainage.
Materials and methods
Collection and characterization of LFA
A 10 kg sample of LFA was collected from the hopper of TPP-II, NLC. Chemical analysis of the LFA was carried out following (IS 1969) standard procedures. The particle size distribution was determined using a laser-based particle size analyzer (Fritsch Gmbh, Germany). An X-ray diffractogram of the original LFA, lignite feed and water–air treated LFA was obtained using a D8 ADVANCE XRD system (Bruker AXS, Germany) with parallel beam geometry with Cu-Kα radiation at 40 KV/40 mA.
Experimental set-up
The shake tests were performed using a rotary shaker at 100 rpm, with a L/S ratio of 10:1, using 30 g of air-dried LFA in 300 ml of either water or buffer solution, over varying periods of leaching (1 day, and at weekly intervals up to 4 weeks). Each medium was tested in five separate sets in triplicate, using conical flasks of 500 ml capacity. After shaking for the pre-determined period, the suspension was filtered and analyzed.
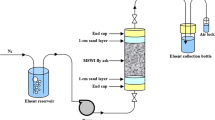
The column set-up consisted of a vertical glass tube of 73 cm length with internal diameter 12 cm, the top of which was open (loosely covered with plastic sheet) and the bottom closed. A small amount of glass wool was packed at the bottom of the column to prevent fine particles loss during leaching. The distance of the inlet and outlet from the each of the corresponding ends of the column was 6 cm. A stopcock was placed at a distance of 6 cm from the bottom of the column to facilitate withdrawal of air and liquid samples when required. The bottom-to-top flow of water/buffer was chosen to prevent channelization and to ensure complete saturation. The water was of high purity (milli-Q quality) with pH 7 and conductivity 0.1 μS/cm. The buffer was sodium acetate (pH 4.9). A flow rate of 0.1 ml/min was maintained, equivalent to L/S ratios of 0.35, 2.5, 5.0, 10 at the end of 1 day, and at week intervals of 1, 2, 3, and 4, respectively. The effluent was collected from the outlet on the top of the column. Because of its high calcium content, the LFA was observed to undergo an exothermic reaction resulting in hard lump formation exothermally during the very first column test with water. To avoid this, the LFA was moistened uniformly and air-dried. This process of water–air drying was repeated two to three times, and finally the ash was powdered by simple hand mixing/crushing. This water–air drying treatment transforms the highly reactive lime to portlandite, calcite and some gypsum (Vassilev et al. 2005). Four hundreds of processed LFA samples were tested in two separate columns. Water and buffer from the reservoir were allowed to pass through the bottom inlet of the column in separate experiments, and the leachate was collected after 1 day and at 1, 2, 3, 4-week intervals which corresponds to a cumulative L/S ratio of 0.35:1, 2.5:1, 5:1, 7.5:1 and 10:1, respectively.
The leachate samples from each leaching experiment were separately preserved in accordance with prescribed methods (Trivedi and Raj 1992), where leachates were filtered in 0.45 μm cellulose membrane. pH, conductivity, total dissolved solids (TDS), total hardness (TH), cations and anions were determined in the filtered solution immediately and for the analysis of trace metals the filtrate was acidified (pH < 2) with HNO3 and preserved at 4°C to prevent any additional reaction.
Characterization of leachates
The leachates were analyzed for pH, conductivity, TDS, TH (as CaCO3), cations and anions, following standard methods (IS 1964). The trace metal contents were determined using a liquid ion chromatography system (HPLC, Waters). The determination of Cu, Zn, Mn, Fe, Pb, Ni, Cd and Co was made using a C18 column; sodium with octane sulphonate, tartaric acid, and acetonitrile as eluent; PAR (post-column reagent) and UV detector (520 nm). An IC Pak-A HR column, borate/gluconate eluent and UV detector (365 nm) were used for determination of Cr (as CrO 2−4 ). Wherever necessary the elements were also analyzed by AAS (Shimadzu, Japan, Model AAS-680) using graphite furnace. The standard sample of the trace elements used in the study was ICP multi-element standard solution IV (Cat. No.1.11355.0100), procured from Merck. The determination of Se and Mo in the LFA and leachate samples was performed using energy dispersive X-ray Fluorescence; As and Hg were determined by Unicom SP-2900 atomic absorption spectrometer using the hydride cold vapor generation method. The detection limits for the various elements are included in Table 1.
Statistical analysis
A correlation matrix study was performed using the SPSS computer program (SPSS, Inc, Chicago, IL, USA) to assess the relation between the various leaching characteristics of the LFA (df = 4, p < 0.05). Principal component analysis (PCA) of the whole data was performed using SPSS package. Principal components (PCs) that receive high eigen values were assumed to best represent the variation in the system. Therefore, only the PCs with eigen value ≥ 1 were examined.
Results and discussion
Characteristics of LFA
From Table 2, it is evident that the LFA consists of 34.24% sand (20–200 μm), 61.81% silt (2–20 μm) and 3.95% clay (< 2 μm) size particles. This distribution of particle size shows the predominance (65.76%) of finer particle (size < 20 μm) in LFA, which may contain relatively higher concentration of some of the elements (Davidson et al. 1974; Swaine 1977) than coarser particles on their volatilization followed by condensation on fly ash particles during combustion (Smith et al. 1979). The bulk density (BD), porosity, and conductivity are 0.90 Mg/m3, 63.34% and 4.25 dS/m, respectively.
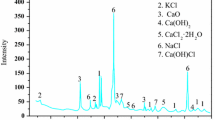
The properties of coal fly ash depend on the physicochemical properties of coal, coal burning process and other factors. Among the major mineral species, SiO2 (40.77%), CaO (17.62%), Fe2O3 (14.26%), Al2O3 (11.04%), SO3-retained sulphur (10.82%) and MgO (4.14%) are most abundant and P2O5 (0.10%) and TiO2 (0.06%) are in minor proportions. The pH (10.94) of the LFA suspension at L/S of 1:2.5, depending on the balance between the concentrations of Ca, Mg, K on one hand and the proportion of potentially acid-generating SO3 on the other (Van der Sloot 1995), Killingley et al. (2000), indicates an alkaline nature (CaCO3 equivalent total alkalinity 4,500 mg/kg). X-ray diffraction analysis of lignite feed, LFA and LFA undergone water–air drying treatment (Fig. 1) shows the presence (in decreasing order) of quartz, gypsum and siderite in lignite; quartz, anhydrite and hematite in LFA; quartz, anhydrite, hematite, mullite and calcite in water–air dried LFA. Besides, the LFA may contain large amount of amorphous aluminosilicates (glass) and some poorly crystalline phases which do not interact with X-rays The presence of hematite in the LFA indicates its formation at lower temperature (400–700°C) from iron-bearing minerals i.e., siderite present in the feed lignite (as evinced from XRD, Fig. 1) for the combustion (Mitchell and Gluscoter 1976; Ram et al. 1996). The absence of magnetite (Fe3O4) in the LFA is also an indicator of lower combustion temperatures, as the formation of magnetite takes place at a temperature of ∼1,390°C (Shuey 1975; Ram et al. 1995). The loss on ignition (LOI) value (4.2%) observed is probably not the true measure of unburnt carbon, where the liberation of some components like CO2, H2O and OH groups during ignition in sufficient air (800°C) is also possible (Vassilev et al. 2005). Though over estimation of unburnt carbon in fly ash derived from coal having carbonates (siderite in our case) has been inferred (Dunn-Rankin and Kerstein 1987; Robert and Jeff 1995), yet fly ash sample with higher hematite than magnetite is likely to contain higher unburnt carbon (Chaddha and Seera 1983; Sandelin and Backman 2001). As such, the absence of magnetite in LFA is the indirect indicator of the formation of hematite along with unburnt carbon in appreciable amount during shorter burning span at low temperature.
The presence of some other unidentified mineral species in LFA in very minor proportions is also possible. Quartz may for example react with other minerals to form various aluminosilicate phases, which along with calcium carbonate (or portlandite) and carbonates of Mg and Fe may possibly be among the unidentified mineral species (Khanra and Mallick 1998; Mitchel and Gluscoter 1976). Coal/lignite power plants usually operate with a slight excess of air (about 20%) over the required quantity for complete combustion. Flue gas, generally comprising H2O, O2, CO2, SO2, SO3 after leaving the combustion zone (Sandelin and Backman 2001) may have reacted with some of the oxides of Ca, Mg and Fe (in the ash) at lower temperatures (∼1,000°C) to form their sulphates and carbonates, which condense on fly ash particles (Querol et al. 1995; Weintraub 1961). The formation of sulphates in the coal ash containing higher amount of Ca and Mg on reaction with SO3 in flue gas when temperature drops are also reported (Grand and Weymouth 1962). The chemical analysis (Table 2) showing the substantial amounts of calcium oxide (17.62%), magnesium oxide (4.14%), iron oxide (Fe2O3, 14.26%) and sulphur-retained (SO3, 10.82%) is the indirect indicator of the proposed hypothesis. The formation of gypsum, calcite, ferrous carbonate and other amorphous materials during combustion of coal has been observed by other researchers (Swaine 1994; Vassilev et al. 2005; Baurer and Natush 1981; Saferinoglu et al. 2003). Furthermore, the formation of tertiary carbonates in the LFA undergone water–air drying treatments prior to leaching test is possible by the reaction between the oxides of Ca–Mg–Fe and CO2, released from air and H2O during water–air drying treatment, (Fig. 1) (Vassilev et al. 2005).
In view of the foregoing discussions, the principal component of LFA comprising quartz, anhydrite and hematite along with minor phases of carbonates and aluminosilicates in weathering environment may form clay minerals (Bradely et al. 1999) and elements may possibly be released into solution and contaminate ground water (Spears et al. 1994).
Trace metals (Table 2) such as Fe, Cu, Ni, Co, Pb, Mn, Zn, Cd, Cr, Se, As, Hg and Mo are present in the LFA in the range 12–156 mg/kg depending on the volatilization–condensation mechanism (Smith et al. 1979; Pires and Tiexeria 1992; Tiexeira et al. 1992) and decomposition of carbonates during combustion (Furuya et al. 1987; Sanchez et al. 1994). The other trace metals such as Se, As and Mo are in very low concentrations (< 3.22 mg/kg), and Hg is below detection limit (BDL). Even so, the presence of many potentially toxic metals especially As, Cd, Pb, Cr, Hg and Se, in the LFA, considered as potential contaminants in ash disposal (Bernhard et al. 1986; Turner 1981), indicate a need to examine the ash leachability.
Leaching of LFA by shake tests
The results on leaching tests performed are included in Table 3.
Aqueous leaching
From Fig. 2, it is observed that when the ash is shaken with water having an initial pH of 7, the pH of the leachate increases to 11.55 after 1 day of leaching, followed by progressive decrease to 8.04 after the fourth week. The pH value especially at the initial stages of leaching is beyond the prescribed limits for drinking water and industrial effluents/leachates, Table 4 (IS 1983; DWT 2001). The conductivity of the leachates also decreased, from an initial value of 4.17 dS/m after 1 day to 2.81 dS/m after the fourth week of leaching. The conductivity values are directly related to the release of products present in LFA (Brown and Bland 1997) particularly free lime (leachable calcium), and leachable sulphate (Moreno et al. 2005). TDS decreased progressively from 3,330 to 2,620 mg/l during the same period of leaching, and again is much higher than the prescribed limit (500 mg/l) for drinking water (IS 1983). TDS in water extract of fly ash may vary widely from hundred to ten thousands milligrams per liter (Iyer 2002). Similarly TH also decreased from 1,400 to 670 mg/l. The decrease in conductivity, TDS and TH of the leachates is probably due to the formation of insoluble precipitates from some of the dissolved salts, and their adsorption on to the surface of LFA particles. The concentration of Ca in solution decreased from 385 to 166 mg/l, but other elements, such as Mg and K, show an increasing trend, from 0.23 to 2.0 mg/l and 27.8–48.2 mg/l, respectively. Cl- in solution increased from 14.2 to 20.9 mg/l, and SO 2−4 decreased from 2,455 to 1,700 mg/l with increased leaching time (Fig. 2). This observation of higher concentration of Ca2+ and SO 2−4 is in agreement with the findings of Lindon (2001) who assumed that calcium and sulphate constituted the majority of water-soluble components, where sodium (not measured by us), potassium and magnesium may be present in small amount. Lowering of pH of the leachate with increasing leaching duration is possibly attributable to relatively higher decrease in concentration of Ca than increase in concentration of Mg and K. (Querol et al. 2000, 2001). The substantial release of sulphate at initial stage of leaching at higher pH reveals that chemical form of sulphur leached is not acidic sulphate but probably neutral sulphate (Iwashita et al. 2005). This observation strengthens Ca as principal component in controlling the pH among the cations by releasing OH ions on hydrolysis (Elseewi et al. 1980; Mattigod et al. 1990). The pH and the, concentrations of TDS, Ca2+, Cl−, SO 2− 4 are well above the limits prescribed for both drinking water and industrial effluent (IS 1974, 1983).
From the foregoing discussions, it is observed that there is no regular trend in the characteristics of the aqueous leachate from the shake test. The conductivity, TH and concentration of TDS, Ca2+, SO42− all decrease, and the concentration of Mg2+, K+, Cl− increase with the decrease in pH over time. The change in pH value of the leachate is a result of the balance between the availability of Ca, Mg, K ions in solution with the progress of time, leaching and their fixation on the surfaces of the ash particles or in the aluminosilicate matrix of the coal ash (Pande and Hasan 1983; Hansen et al. 1984). Apart from this, precipitation/adsorption and dissolution/desorption may also play an important role.
Of the various trace metals present in the LFA (Table 2), only Pb, Cd, Zn, Cr has shown their presence in the aqueous leachate (Fig. 2). The concentration of these elements varies from 0.002 to 0.018 mg/l over one day to four weeks of leaching; Zn varies from 0.035 to 0.076 mg/l. The elements like Pb, Cd, Zn, etc in coal fly ash have been observed to have minimum solubility at higher pH (Shim et al. 2005). Other elements, such as Fe, Ni, Cu, and Mn, were observed in the leachates only in the third and fourth week. This is probably attributable to the fact that at higher pH, any leached Fe, Ni, Cu, and Mn from fly ash are precipitated as their insoluble hydroxides and their appearance in later stages is due to lowering of pH to 8.39 and 8.04 in the third and fourth weeks of leaching, respectively. The concentrations of these elements in the leachates after the third and fourth weeks are 0.35 and 0.38 mg/l for Fe; 0.045 and 0.055 mg/l for Ni; 0.006 and 0.008 mg/l for Cu; 0.082 and 0.085 mg/l for Mn. In another possibility, at high pH, dominant constituents like SiO2, Al2O3, CaO and SO 2−4 present in LFA may form various secondary minerals like calcium silicate hydrate gel, calcium alumino silicate hydrate and ettringite (3CaO.Al2O3.3CaSO4.32H2O), which may decrease the leaching of the elements either by decreasing the porosity between the fly ash particles or by incorporating these elements in their structures (Stumm 1992; Ecke et al. 2002). The maximum values for most of these trace metals are not only much less than the corresponding content in LFA, but also well within the permissible limits for drinking water and industrial effluent/leachates/land fill drainage (IS 1983, 1974; DWT 2001; CEC 1991). It is worth mentioning that many of the potentially toxic trace elements, such as As, Pb, Cd, Cr, Zn, are commonly associated with the nonmagnetic fraction of fly ash in glass phase, and are distributed on the surface of the ash particles with their higher dissolution characteristics (Smith et al. 1979; Meserole et al. 1979; Natusch et al. 1974). Cu, Mn, Co, Ni, etc. are distributed in the magnetic fraction, with poor dissolution characteristics, and Fe occurs in both phases of fly ash (Murarka et al. 1992; Ram et al. 1996; Smith et al. 1979). Probably for this reason, Pb, Cd, Cr, Zn undergo dissolution (Fig. 2) in the initial stages of leaching. The leaching of the ions and trace metals present in the LFA therefore depends on their distribution pattern (either on the surface or in the matrix of ash particles or abiding by both), where the pH of the leachate and leaching duration play an important role. The dependence of leaching of Cd, Cr, Cu, Pb, Ni, Zn from fly ash on pH is in agreement with the findings of other workers (Iyer 2002; Theis and Wirrth 1977). The concentrations of Se, As, Hg and Mo are BDL in the leachates, probably because of their very minor contents (< 3.22 mg/l) in the LFA.
Buffer leaching
The leachates from the shake test with the buffer solution show gradual decrease in pH from 10.79 to 10.46 over the period from 1 day to 4 weeks (Fig. 3). The initial pH (10.79) of the leachate after 1 day of leaching is less than that of the aqueous leachate (11.55) after the same period, which decreased to 10.46 with the progress of leaching up to fourth week. This reflects the use of the sodium acetate buffer (pH, 4.9). The conductivity is very high, i.e., 12.50 dS/m after first week of leaching, as compared to 4.17 dS/m from aqueous leaching, which is again attributable to the buffer used. Conductivity progressively decreases to 10.64 dS/m after the fourth week of leaching. The leachates obtained at the initial stage of leaching (i.e., first week) show higher concentrations of Ca2+, Mg2+, Cl− (1,011, 1.57, 39.8 mg/l, respectively) and lower values for SO 2−4 (2,281 mg/l) (Fig. 3) as compared to the aqueous shake test (Fig. 2). A decrease in the concentration of Ca2+, Mg2+, Cl−, SO 2−4 also takes place with the progress of leaching, the minimum (i.e., 358, 0.95, 18.5, 2,087 mg/l, respectively) occurring after fourth week of leaching. As with the aqueous shake test, K+ increases in almost the same range with the progress of time up to the fourth week of leaching. As such, the observed values of pH, Ca2+ and SO 2− 4 in the leachates are far above, and Mg2+ is well below the limits prescribed for drinking water and industrial effluents/leachates (IS 1983, 1974; DWT 2001). No regular trend was seen in the concentrations of cations and anions during either shake test (aqueous or buffer); all the ions except K+ decrease in concentration in the buffer leachates, but in the aqueous leachates only the concentrations of Ca2+ and SO 2− 4 decrease and those of Mg2+, K +, Cl- increase with the progress of time.
Among the trace metals originally present in the LFA (Table 2), only Pb, Cd, Zn, Cr occur, at very minor concentrations, in the leachates obtained over the different intervals, having their range 0.002–0.006 mg/l (Fig. 4). However, Zn is in the range 0.044–0.058 mg/l. The other elements, such as Fe, Ni, Cu and Mn, which were detectable only in the leachates after third and fourth week of the aqueous shake test (discussed above), are here BDL in all the leachates. This is ascribable to high pH of the leachate in the range from 10.79 to 10.46 (Rajasekhar 1996) where any leached element is probably precipitated as insoluble hydroxides or due to the formation of secondary complexes leading to less porosity and incorporation of these metals in their structures (Stumm 1992; Ecke et al. 2002). The concentration of Se, As, Hg and Mo is BDL. The difference in the behavior of Fe, Ni and Mn between aqueous and buffer shake tests indicates that though the association of the elements with fly ash particles is an important factor in the leachability, it is the pH which plays the crucial role. The concentrations of these trace metals in the leachates are well within the limits prescribed for drinking water and industrial effluents/leachates/land fill drainage (IS 1974, 1983; DWT 2001; CEC 1991).
Leaching of LFA by column tests
Aqueous leaching
Figure 4 shows that the pH of the leachate increases from neutral to 8.63 after 1 day of leaching, followed by a progressive decrease up to 7.61 after the fourth week. These values of pH in the leachates are close to the prescribed limits for drinking water and industrial effluents (IS 1974, 1983; DWT 2001). The conductivity of the aqueous leachate decreases from an initial value of 8.17 dS/m after 1 day of leaching to 0.47 dS/m after the fourth week. Such a drastic reduction indicates that, during the aqueous column test, the dissolved salts were higher in the initial stages than in the corresponding leachates from the aqueous shake test, and vice versa after the fourth week of leaching (Figs. 4, 2). This is substantiated by the TDS, which were 9,400 mg/l in the leachate after 1 day and 432 mg/l after the fourth week (Fig. 4). The TH decreased from 1,220 to 280 mg/l (Fig. 4) as against 1,400 to 670 mg/l in the aqueous shake test (Fig. 2). The observed decrease in conductivity, TDS and TH of the leachates with time is again probably due to the precipitation of some of the dissolved salts and their adsorption on the surface of the LFA particles. Apart from this, exhaustion of soluble fraction through leaching is another likely mechanism that contributes to decline in such values. The values for TDS especially at the initial stages of leaching in the leachates are again too high to be considered within 500 mg/l, the limit prescribed for drinking water (IS 1983).
The concentration of Ca2+ in the column test leahate decreases from 266.7 to 75 mg/l, and Mg2+ and K+ from 3.7 to 0.4 mg/l and 12.8 to 1.3 mg/l, respectively (Fig. 4). The observed decrease in the pH of the leachates from aqueous column test (8.63–7.61) also indicates less availability of these ions as compared to the aqueous shake test (pH, 11.55–8.04) and buffer shake test (pH, 10.79–10.46). The concentrations of Cl− and SO42− over the complete span of leaching decreased from 46.9 to 11.4 mg/l and 6,027 to 207 mg/l, respectively (Fig. 4). Here the initial values of these anions are relatively higher than that of aqueous shake and buffer shake test but with less values after fourth week of leaching. Most of these parameters, except the concentration of Mg2+, are either above or close to the regulatory levels for both drinking water and industrial effluents (IS 1974, 1983).
In contrast to the disappearance of Fe, Ni, Cu, Mn in the leachates from aqueous shake test particularly up to the second week of leaching (Fig. 2) and in all the leachates from buffer shake test (Fig. 3), all the elements present in the LFA occur in measurable concentrations in the leachate from the column test (Fig. 4). The concentrations of Pb and Cd vary in the range 0.003–0.009 mg/l, Zn from 0.009 to 0.15 mg/l, Cr from 0.029 to 0.045 mg/l, but Fe, Ni, Cu and Mn, which were BDL in the aqueous and buffer shake test (discussed above), were detected in the aqueous column leachate from the very first day. This is followed by progressive increase with the gradual decrease in pH (8.63–7.61) as the leaching duration extended up to the fourth week. In particular, Fe varies from 0.15 to 0.24 mg/l; Ni from 0.035 to 0.064 mg/l; Cu from 0.008 to 0.055 mg/l; Zn from 0.009 to 0.015 mg/l; Mn from 0.003 to 0.008 mg/l. It is pertinent to mention here that the leaching of trace metals depends on their availability in the leachate and their sorption capacity, where the former increases and the latter decreases with decrease in pH of the supernatant liquid (Henry and Knapp 1980). The alkaline nature of the supernatant liquid from the LFA controls the solubility of these metals. The concentrations of Se, As, Hg and Mo in the leachate are BDL. The concentration of these detected trace elements are below the tolerance limits [IS 1974, 1983; DWT 2001; CEC 1991).
Buffer leaching
Figure 5 shows that the leachates after the buffer column test undergo an appreciable decrease in pH from 8.05 to 5.43 from 1 day to 4 weeks. The observed pH range is less than the ranges of the other three tests. The conductivity is higher (12.18 dS/m) after the first week of leaching, as compared to corresponding aqueous shake and column tests (4.17 and 8.17 dS/m), but less than that in the buffer shake test (conductivity l2.50 dS/m). Conductivity also decreases with the leaching duration.
The leachates have relative higher contents of Ca2+, Mg2+ and K+ contents (Fig. 5) than those after corresponding times in the aqueous column test (Fig. 4). Also the concentration range of Ca2+is relatively lower than aqueous shake and buffer shake test, but the range in the case of Mg2+ and K+ is higher. As with the aqueous column, these concentrations also decrease with leaching duration. This trend of decrease is also applicable to Cl- and SO 2−4 (Fig. 5), although they have relatively lower concentrations particularly at initial stage of leaching and higher values at later stages than the equivalent values in aqueous column leaching. The concentrations of these cations and anions are above the prescribed limits for drinking water and industrial effluents/leachates (IS 1974, 1983; DWT 2001 ). Unlike the shake test, there is a regular trend in the conductivity and the concentrations of cations and anions in the leachates from the column test; these parameters decrease with decrease in pH and leaching duration. The effect is also much more pronounced with the buffer column than with the aqueous column test.
As with aqueous column leaching (Fig. 4), all the elements were detected in the leachates from the buffer column test (Fig. 5). The concentrations of Pb and Cd vary in the range 0.004–0.251 mg/l, but Fe, Ni, Cu, Zn, Mn and Cr show gradual increase with decrease in pH from 8.05 to 5.43 and progress of leaching duration. In particular, the variation is from 0.18 to 0.86 mg/l for Fe, 0.049–0.24 mg/l for Ni, 0.021–0.12 mg/l for Cu, 0.011–0.12 mg/l for Zn, 0.006–0.078 mg/l for Mn, 0.032–0.13 mg/l for Cr. As such, the leaching of trace metals from buffer column test is the maximum among all the tests performed. This is obviously due to the lower pH of the leachate. The more acidic environment increases the leaching behavior of most trace elements in comparison with more alkaline media (Vassilev et al. 2005). A significant increase in the extractability of trace elements from coal combustion ash at pH < 6 has also been reported (Rajasekhar 1996). The concentration of Se, As, Hg and Mo is BDL. The maximum concentration of most of these trace metals is also well within the regulatory levels as per standards for industrial effluents/land fill drainage (IS 1974, 1983; CEC 1991).
Over all, the concentration of leached trace metals is relatively less in shake test than column test. The progressive and relatively higher increase in the concentration of trace metals during column test (open system) with decrease in pH and increase in leaching duration than shake test (closed system), even at higher L/S ratio (10:1) in the case of shake test than column test (cumulative L/S ratio of 0.35:1–10:1), is probably ascribable to the more precipitation/adsorption taking place in closed system than open system (Querol et al. 2001). As such the L/S ratio in the case of column test is in a linear relationship with the concentration of trace metals, whereas in the case of shake test it is not applicable because of fixed L/S ratio.
Correlation analysis
The correlation analysis showed a significant negative correlation of pH with most of the trace metals (r = −0.92 to −0.99), thereby evincing that pH could be a controlling factor on the leaching characteristics of the LFA. Statistically pH is the most influential parameter, which is in agreement with the findings of other workers (Kim and Lee 1997). The relationships between cations/anions and trace metals appear complex and difficult to explain individually. More understanding could be enabled by PCA. PCA was applied to the whole set of data. The component plots are presented in Fig. 6. The three principal components (PC1 = 51.74%; PC2 = 35.55%; PC3 = 6.62%) explained 93.92% of the total variation. The loadings were larger for pH, EC, Ca, Mg, SO4, Cl, TDS and Ni in first component; for K, Pb, Zn, Fe and Mn in second component; for Cr in third component. The component plots presented in Fig. 6 revealed that the data set can be classified into few groups viz. pH contributors: pH, Ca, Mg and TH; conductivity contributors: conductivity, SO4, Cl, TDS; Elements Group (Group I: K, Fe, Mn, Zn, Cd, Pb; Group II: Cu; Group III: Ni; Group IV: Cr). This group formation reveals that the members of pH and conductivity contributors as well as Group I elements behaved similarly with different leaching procedures. From Fig. 6 it is clear that the leaching procedures greatly affected the leaching behavior of Cu, Cr and Ni. The data on Table 3 shows the distinctly different behavior of Cu, Ni and Cr, where Cu and Ni are towards BDL (up to 0.006 mg/l for Cu) with respect to shake test and were much pronounced in column test (Cu: 0.008–0.12 mg/l (mean 0.0561 mg/l); Ni: 0.035–0.24 (0.1009) mg/l). Traces of Cu and Ni appeared only at the third and fourth week of aqueous shake test reveal the higher tenacity of these metals with the solid phase and in fact these elements are of the magnetic phase of fly ash. In case of Cr also the leaching intensity of column test is about 10 times higher than shake test (Table 3). Thus, in general, the shake and column tests differ in terms of the leachability of the magnetic phase elements, where the column test shows more effectiveness than the shake test. The leaching of elements of non-magnetic phase, having their association on the surface of the fly ash particle is almost similar in all the tests.
Conclusions
In general, pH is correlated with the availability of cations and anions, among which Ca2+ plays its important role due to its higher concentration. In the shake test, no regular trend was observed in the characteristics of the leachates (conductivity, TH, TDS, Ca2+, Mg2+, K+, SO 2−4 and Cl-), whereas such a trend is pronounced in the column test, where the parameters decrease with leaching duration and with the decrease in pH. The leaching of trace metals is controlled by their concentrations in the LFA, their association with the ash particles, the pH of the leachate and the leaching duration. It is inversely proportional to the pH of the leachate and directly proportional to the leaching duration. Statistically pH is the most influential parameter. Generally, the shake and column test differs in terms of the leachability of the trace metals present in magnetic phase, where the later test is more effective, and in case of non-magnetic phase, it is more or less the same as per PCA. The leaching of these metals is at a maximum in the buffer column test, and follows the order: buffer column > aqueous column > aqueous shake > buffer shake test. Overall, the concentrations of TDS, TH, anions and cations are well above the permissible limits for drinking water/industrial effluents/leachates, but the concentrations of most of the trace metals are within the prescribed limit. The higher concentration of TDS, cations and anions may lead to the increase in the hardness, salinity and proportion of dissolved solids in the soil on disposal. Though the release of trace metals is within the permissible limit and not alarming, these elements despite their lower concentration in the leachates can cause ground water pollution in the course of longer period due to their accumulation.
References
Bauer CF, Natush DFS (1981) Identification and quantitation of carbonate compounds in coal fly ash. Environ Sci Technol 15:783–7880
Bernhard M, Brinkman FE, Sadler PJ (1986) The importance of chemical speciation in environmental processes, Dohlem Kon Feremen. Springer, Berlin Heidelberg New York
Biermann AH, Ondow JM (1980) Application of surface depositions models to size-fractinated coal fly ash. Atmos Environ 14:289
Bradley J, Shyam A, Reeuwijk LB, Zevenberges (1999) Clay formation during weathering of alkaline coal fly ash. In: Proceedings of the international ash utilization symposium and the world of coal ash conference, Lexington
Brown TH, Bland Wheeldn JM (1997) Pressurized fluidized bed combusion ash: 2. soil and mine spoil amendment use options. Fuel 76:741–748
Chaddha G, Seera MSJ (1983) Magnetic components and particle size distribution of coal fly ash. J Phys D Appl Phys 16:1767–1776
Chong SL, Peart J, Ormsby WC (1990) Griffith MS Leaching test studies using extraction procedure toxicity test and toxicity characteristic leaching procedure. Public Roads 54:241
Claudia B, Stefania B, Morabite R (1999) Int J Environ Anal Chem 75:19
Council of the European Communities (1991) Proposal for a Council Directive on the landfill. Off J Eur Commun 34(C 190):1
Davidson RL, Natusch DFS, Wallace JR, Evans CA (1974) Trace elements in fly ash-dependence of concentration on particle size. Environ Sci Technol 8:1107–1113
Drakonaki S, Diamadopoulos E, Vamvouka D, Lahaniatis M (1998) Leaching behavior of lignite fly ash. J Environ Sci Health A 33(2):237–248
Drinking Water Threshold Values in U.K. (2001) (Derived from Guidance Note, CI RCI Note 59/83). In: Sears LKA (ed) Properties and use of coal fly ash: a valuable industrial by-product. Thomas Telford Publishing Thomas Telford Ltd., London, pp 30–62
Dunn-Rankin D, Kerstein A (1987) Numerical simulation of particle size distribution evolution during pulverized coal combustion. Combust Flame 69:193–209
EA-3236 (1993) Physico-chemical characteristics of utility solid waste. Electric Power Research Institute, Palo Alto
EPA (1984) Ground water protection strategy. USEPA Office of Ground-Water Protection, Washington
Ecke H, Menad N, Lagerkvist Mater AJ (2002) Cycles Waste Manage 4:117
Egemen E, Yurteri C (1996) Regulatory leaching tests for fly ash: a case study regulatory leaching tests for fly ash: a case study. Waste Manage Res 14:43–50
Elseewi AA, Page AL, Grimm SR (1980) Chemical characterization of fly ash aqueous system. J Environ Qual 9:224
Fernandez-Turiel JL, de Carvalho W, Cabanas M, Querol X, Lopez-Soler A (1994) Mobility of heavy metals from coal fly ash. Environ Geol 23: 264–270
Fulekar MH (1993) The pH effects on leachability of fly-ash heavy metals: laboratory experiment. Indian J Environ Prot 13:185–192
Fulekar MH, Dave JM (1989) Leaching of fly ash constituients along stream bed flow to Yamuna river, New Delhi, India. Indian J Environ Prot 9:773
Furuya K, Miyajima Y, Chiba T, Kikuchi T (1987) Environ Technol 21:898
Grand K, Weymouth JH (1962) The nature of inorganic deposits formed during the use of Victorian Brown Coal in large industrial boilers. J Inst Fuel 35:154–160
Hansen LD, Silberman D, Fisher D, Fisher GL, Etaough DJ (1984) Chemical speciation of elements in stack-collected, respirable-size, coal fly ash. Environ Sci Technol 18:181–186
Hassett DJ, Debra F, Pflughoeft-Hassett L, Heebink V (2005) Leaching of CCBs: observations from over 25 years of research. Fuel 84:1378–1383
Henery WM, Knapp KT (1980) Compound forms of fossil fuel fly ash emissions. Environ Sci Technol 4:450–456
IS (1964) Indian Standard for Water Analysis. IS: 3025, 1964
IS (1969) Indian Standards for Ash Analysis. IS: 1350 (Part I), 1969
IS (1974) Indian Standards for Industrial Effluents. IS: 2490, 1974
IS (1983) Indian Standards for Drinking Water. IS: 10500, 1983
Iwashita A, Sakaguehi Y, Nakajima T, Takanashi H, Ohki A, Kambara S (2005) Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 84:479–485
Iyer RS (2002) The surface chemistry of leaching coal fly ash, J Hazard Mater B93:321–329
Jankowski J, Ward CR, Frech D, Groves S (2006) Mobility of trace elements from selected Australian fly ashes and its potential impacts on aquatic ecosystems. Fuel 85:243–256
Karuppiah M, Gupta G (1997) Toxicity of metals in coal combustion ash leachates. J Hazard Mater 56:53–58
Khanra S, Mallick D (1998) Studies on the phase mineralogy and leaching characteristics of coal fly ash. Water Air Soil Pollut 10:251–275
Killingley J, McEvoy S, Dokumeu C, Stanber J, Dale L (2000) Trace element leaching from fly ash from Australian power stations End of grant report, Australian coal association research program, project C8051, CSIRO division of energy technology, pp 98
Kim JB, Lee WK (1997) Leaching characteristic of heavy metals from ashes discharged from MSWI. J KSEE 19:481–490
Kukier U, Ishak CF, Summer ME, Muller WP (2003) Composition and element solubility of magnetic and non-magnetic fly ash fractions. Environ Pollut 123:255–266
Lee S, Kahn JJ (1997) Environ Health A33:649
Lindon KAS (2001) The properties and use of coal fly ash: a valuable industrial product. In: Lindon KAS (ed) Fly ash and the environment, ISBN: 07277 30150. Thomas Telford Publishing, Thomas Telford Ltd., London
Mattigod SV, Eary LE, Dhanpal R (1990) Geochemical factors controlling the mobilisation of inorganic constituents from fossil fuel combustion residues 1: review of major elements. J Environ Qual 191:188–201
Meserole FB, Schnitzgebel K, Magee Mann RM (1979) Trace elements emission from coal filed power plants. J Environ Pollut 101:620–624
Mitchell RS, Gluscoter HJ (1976) Mineralogy of ash of some American coals: variations with temperature and source. Fuel 55:90–96
Moreno N, Querol X, Andres JM, Stanton Towler M, Nugteren H, Janssen-Jurkovicova M, Jone R (2005) Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 84:1351–1363
Mukherjee AB, Zevenhoven R (2006) Mercury in coal ash and its fate in the Indian sub-continent: a synoptic review. Sci Total Environ (In press)
Murarka L, Rai PD, Ainsworth CC (1992) ASTM specification technical publishing STP 1075 (Waste test quality assurance vol 3.) Chem Abstr 17:219027
Natusch DFS, Wallace JR, Ewans Jr CA (1974) Toxic trace elements: preferential concentration is repairable particles. Science 183:202–204
Pande SP, Hasan MJZ (1983) Inst Eng India 63:53
Pires M, Tiexeira EC (1992) Geochemical distribution of trace elements in Leao coal, Brazil. Fuel 71:1093–1096
Praharaj T, Powell MA, Hart BR, Tripaty S (2002) Leachability of elements from sub-bituminous coal fly ash from India. Environ Int 27:609–615
Querol X, Fernandez-Turiel JL, Lopez-Soler A (1995) Trace elements in coal and their behavior during combustion in a large power station. Fuel 74:331–343
Querol X, Umana JC, Alastuey A, Bertrana C, Lopez-Soler A, Plana F (2000) Energy Source 22:733–750
Querol X, Umana JC, Alastuey A, Ayora C, Lopez S, Plana F (2001) Extraction of soluble major and trace elements from fly ash in open and closed leaching systems. Fuel 80:801–813
Rajasekhar C (1996) Retention and permeability characteristics of clays and clays-fly ash systems subjected to flow of contaminants. Ph.D. thesis, Indian Institute of Science
Ram LC, Tripathi PSM, Mishra SP (1995) Moessbauer spectroscopic characterization and studies on their transformations of iron-bearing minerals during combustion of coals: correlation with fouling and slagging. Fuel Process Technol 42:47–60
Ram LC, Tripathi PSM, Mishra SP (1996) In: Proceedings of the international symposium on coal science technology industry business environment, Dhanbad, pp 38
Ram LC, Srivastava NK, Das MC, Singh G (1999) Leaching behavior of fly ash under simulated conditions vis-à-vis quality of the leachate. In: Ram LC et al (eds) Proceedings of the national seminar on bulk utilization of fly ash in agriculture and for value-added products. Dhanbad (India), ISBN 81–7525-184-0
Ram LC, Srivastava NK, Singh G (2000) Prediction of leaching behavior of TPP ash under simulated condition by column studies. In: Proceedings of the international conference on fly ash disposal and utilization (CBIP), New Delhi technical session-IV-3, pp 16
Ramesh A, Kozinski JA (2001) Investigations of ash topography/morphology and their relationship with heavy metals leachability. Environ Pollut 111:255–262
Robert CB, Jeff D (1995) Systematic errors in the use of loss-on-ignition to measure unburned carbon in fly ash. Fuel 74:570–574
Saikia N, Kato S, Kojima T (2006) Composition and leaching behavior of combustion residues. Fuel 85:264–271
Sanchez J, Tiexeira EC, Fernandes I, Pestana MHD, Machado R (1994) Geochim Brasil 8:1
Sandelin K, Backman R (2001) Trace elements in two pulverized coal-fired power stations. Environ Sci Technol 35:826–834
Seferinoglu M, Paul M, Sendstorm A, Koker A, Toprak S, Paul J (2003) Acid leaching of coal and coal-ashes. Fuel 82:1721–1724
Shim YS, Rhee SW, Lee WK (2005) Comparison of heavy metals from bottom and fly ashes in Korea and Japan. Waste Manage 25:473–480
Shuey RT (1975) Semi-conducting ore minerals. Elsevier Science, New York
Sloss L (1996) Residues from advanced coal-use technologies. IEA Coal Research, London
Smith RD, Campbell JA, Nelson KK (1979) Concentration dependence upon particle size of volatilizer elements in fly ash. Environ Sci Technol 13:553–558
Smith IM (1987) Trace elements from coal combustion:emissions, chap 2, Source of trace elements, IEACR/01 IEA Coal Research, London
Smith IM (1990) Management of AFBS Residues. IEACR/21, IEA Coal Research, London
Spears DA, Tarazona MRM, Lee SS (1994) Pyrite in U.K. Coal: its environmental significance. Fuel 73:1051–1055
Stumm W (1992) Chemistry of the solid-water interface. Wiley, New York
Swaine DJ (1977) Trace elements in coal. In: Hemphill DD (ed) Trace substances in environmental Health-XI. University of Missouri, Columbia pp 107–116
Swaine DJ (1994) Trace elements in coal and their dispersal during combustion. Fuel Process Technol 39:121–137
Theis TL, Wirth JL (1977) Sorptive behavior of trace metals on fly ash in aqueous systems. Environ Sci Technol 11:1096–1100
Tiexeira M, Samama ECJ, Brun A (1992) Environ Technol 13:995
Trivedi PR, Raj G (1992) Environmental water and soil analysis, Akashdeep Publishing House, New Delhi
Turner RR (1981) Oxidative state of arsenic in coal ash leachate. Environ Sci Technol 15:1062–1066
USEPA (United States Environmental Protection Agency) (1987) Characterization of municipal waste combustor ashes and leachates from municipal solid waste landfills and co-disposal sites, I-VI, 530-SW-87–028A-E. Washington
Van der Sloot HA (1995) Development in evaluating environmental impact from utilisation of bulk inert using lab leaching tests and verification. In: International symposium on bulk industrial waste an opportunity for use, Leeds (UK), September 1995, Report No. ECN-RX-(5-056), P34, Nov 1995
Van der Sloot HA, Piepers O, Kok AA (1984) Standard leaching test for combustion residues. In: Petten ECN (ed) Bureau for energy research projects, Netherlands Energy Research Foundation. The Netherlands, p 34
Van der Sloot HA, Heelmar TH, Aalberts G, Wahlstrom FAM (1993) In: Petten ECN (ed) Proposed leaching test for granular solid wastes. ECN-C-93–012, Ground water. Netherlands Energy Research Foundation, The Netherlands
Vassilev SV, Vassileva CG, Karayigit AI, Bulut Y, Alastuey A, Querol X (2005) Phase mineral and chemical composition of fractions separated from composite fly ashes at the Soma Power Station, Turkey. Int J Coal Geol 61:65–85
Weintraub M, Goldbery S, Orning AA (1961) A study of sulfur reactions in furnace deposits. Trans ASME J Eng Power Ser A 83:444–450
Zacharia JS (1990) Use of batch and column methodologies to assess utility wastes leaching and surface chemical attenuation. In: Report to EPRI, Palo Alto, CA, EN 73/3, pp 3–5, B1–B14
Zevenbergen C, Vander WT, Bradley JP, van der Broeck P, Orbons AJ, Van Recuwijk LP (1994) Hazard Waste Hazard Mater 11:371
Acknowledgments
The authors thank the authorities of NLC, India, for providing financial assistance. They are also thankful to Dr. S. C. Roy, Director, CFRI, for constant encouragement, providing necessary facilities for carrying out this work and for permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram, L.C., Srivastava, N.K., Tripathi, R.C. et al. Leaching behavior of lignite fly ash with shake and column tests. Environ Geol 51, 1119–1132 (2007). https://doi.org/10.1007/s00254-006-0403-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0403-1