Abstract
Mine tailings may be remediated using metal tolerant microorganisms as they may solve the limiting conditions for healthy development of plants (i.e., low organic mater content and poor physical conditions). The aim of this study was to investigate the consequences of microbial colonization on the chemical speciation of trace metals. Surface samples from the Valenciana mine tailings (Guanajuato, Mexico) were used for long-term bioassays (BA), which consisted in the promotion of microorganisms, development on tailings material under stable laboratory conditions (humidity, temperature, and light exposure). A five-step sequential extraction method (exchangeable, carbonate/specifically adsorbed, Fe–Mn oxides, organic matter (OM)/sulfide, and residual fractions) was performed before and after BA. Extraction solutions and leachates were analyzed by inductively coupled plasma-mass spectrometry. OM content, cationic exchange capacity, and pH values were also assessed before and after BA. The results indicate that trace elements are generally present in nonresidual fractions, mainly in the Fe–Mn oxides fraction. The concentration of total Zn, As, Se, Pb, and exchangeable Cu and Pb is above the recommendable limits for soils. Despite the high bioavailability of the former elements, biofilms successfully colonized the tailing samples during the BA. Cyanobacteria and green algae, heterotrophic fungi, aerobic bacteria, and anaerobic bacteria composed the developed biofilms. Chemical controls of trace elements could be attributed to absorption onto inorganic complexes (carbonates, metal oxides), while biofilm occurrence seems to enhance complexation and immobilization of Cr, Ni, Cu, Zn, As, and Pb. The biofilm developed does not increase the bioavailable forms and the leaching of the trace elements, but significantly improves the OM contents (natural fertilization). The results suggest that biofilms are useful during the first steps of the mine tailings remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The economic extraction of metals from ore deposits usually generates large amounts of noneconomic by-products known as mine tailings (mine waste material). The actual worldwide increase of metals demand in industrial societies has caused the intensification of ore exploitation. Most of these ore deposits are low grade, with the concomitant generation of high amounts of mine waste material. It is estimated that 95–99% of the material extracted from the mines is disposed as mine tailings worldwide (Allan 1995).
Guanajuato mines, at central Mexico, have been exploited for over 450 years (since 1548) for their gold and silver-rich epithermal veins. Guanajuato Mining District is still an important silver and gold producer with nearly between 13 and 8% national production of gold and silver, respectively (García-Meza 1999). Estimates indicate that more than 33,000 ton of silver and some 170 ton of gold had been extracted in Guanajuato, resulting in more than 20 million tons of tailings (Carrillo-Chávez et al. 2003).
Chemical analysis of different mine tailings from Guanajuato showed that 29 trace elements were present, more than half are in milligram per kilogram range concentration, including Cr, Cu, Zn, As, Se, Cd, Pb, and Hg (Carrillo-Chavez et al. 2003; García-Meza et al. 2004). The environmental risk associated with these heavy elements is the potential natural leaching (after the summer heavy rains) or aeolian dispersion during the dry season (winter and spring). Therefore, tailings material represents a source of heavy metals potentially hazardous to people and the surrounding ecosystem.
Mine tailings reclamation programs aim to stabilize the deposits through promoting plant growth (Ernst 1988) that inhibits metal dispersal. However, soil acidity, high metal toxicity, low content of nutrients, and poor physical structure of the tailings may limit the plants establishment (Ye et al. 2002). The development of microorganisms forming biofilms in the presence of a variety of heavy metals has been well documented in aquatic and terrestrial environments (Admiraal et al. 1999), and also on the surface of old mine tailings (Anagnostidis and Roussomoustakaki 1988; García-Meza 1999).
It is well known that metals are present in soils in different chemical forms, which influence their reactivity, and therefore, their mobility and bioavailability (Morgan and Stumm 1995). That is why it is widely recognized that the quantification of the chemical forms of metals in soils is essential for the environmental evaluations of soil pollution (Gupta et al. 1996; Abollino et al. 2002). On the other hand, the use of sequential extraction provides information about the mode of occurrence of trace metals (Tessier et al. 1979); the proportion of bioavailable or mobile metals (exchangeable fraction, adsorbed to carbonate or specifically adsorbed) as well as the proportion of metals strongly held within the soil is normally unavailable to plants (residual fraction) (Li et al. 1995). The different fractions also reflect some of the important soil properties, such as soil pH (soil acidity), clay content, organic matter (OM), and the amount of sulfides and oxides.
Tessier´s sequential extraction scheme (Tessier et al. 1979; Li et al. 1995) was used to analyze the chemical behavior and partitioning of some trace elements in mine tailing samples, before and after biofilm colonization, to elucidate the possible effects of biofilm development on the speciation of trace elements. Extraction solutions and leachates were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS). The consequences of biofilms on metals speciation is discussed in detail.
Sampling site description
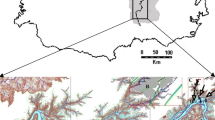
The tailing piles from the Valenciana Mine are 4 km northwest of the city of Guanajuato, Mexico (21˚01´–21˚14’ N and 101˚15’–101˚26’ W), at the lower elevation of the Guanajuato Mountain range (Fig. 1). The average elevation of the study site is 2,120 m above sea level in a semidry climate, with heavy rain during the summer months. There are three tailing piles in the area, two are continuous terraces and one is in different steps (different heights). These piles are composed from sand-like material derived from the exploitation of the gold and silver-rich epithermal veins. This material and the ore material itself do not generate acid mine drainage due to their high carbonate/sulfide ratio (12:1) characteristic of the quartz–calcite ore deposits (Carrillo-Chávez et al. 2003). The combined volume of the tailing piles is of 20 millions tones of material, covering an area of approximately 0.5 km2, and with a thickness of 10–15 m (Carrillo-Chávez et al. 2003). The most recent pile (abandoned in 1998) was chosen for the present work.
The major constituents of the mine tailings are silicates (mostly quartz and feldspars, 50–65%), carbonates (calcite, 12%), and gypsum (<10%); the minor compounds include clay minerals (6%) and Fe oxy-hydroxides (3%). Chemical analyses show minor amounts of heavy metals (Cr, Ni, Cu, Zn, As, and Pb) not detected in XRD mineralogy. The tailings have very low OM content (<1%), <1% sulfide, a relatively low cation exchange capacity (CEC), and low vertical permeability due to its texture type (sand-lime) and porosity (0.51) (García-Meza et al. 2004).
Ten samples of tailing material were collected from the youngest Valenciana tailing pile in November 1999. The samples were collected from the central part of the pile. A circle of 15 m in diameter was marked in the field, and the samples were taken from the surface material (less than 5 cm depth). All the samples from the same site were mixed thoroughly to obtain a single composite and homogeneous sample. The samples were set in double sealed plastic bags.
Methods
Sample analysis before the bioassay
The samples were analyzed for OM content by ignition at 550°C and by titration (Walkley 1947), CEC according to Bower (1952) and pH. The measurements were done in triplicate. Metal extraction was carried out by the five-step sequential extraction procedure (Tessier et al. 1979; Li et al. 1995). Each of the chemical fractions is defined as:
-
Exchangeable—tailings sample extracted with 8 ml of 0.5 M MgCl2 (pH 7) for 20 min, with continuous agitation at room temperature;
-
Bound to carbonates or specifically adsorbed—the residue from (i) was extracted with 8 ml of 1 M NaOAc (pH 5) for 5 h, with continuous agitation at room temperature;
-
Bound to Fe–Mn oxides—the residue from (ii) was extracted with 20 ml of 0.04 M NH2OH/HCl in 25% HOAc for 6 h, with occasional agitation at 96°C; after the extraction, the extract solution was diluted to 20 ml with deionized water (DIW) and subjected to continuous agitation for 10 min;
-
Bound to OM and sulfide—the residue from (iii) was extracted with 3 ml of 0.02 M HNO3 and 5 ml of 30% H2O2 (pH 2); the sample was heated progressively to 85°C and maintained at this temperature for 2 h with occasional agitation; then, 3 ml of 30% H2O2 was added, and mixture was heated to 85°C for 3 h with intermittent agitation; after cooling, 5 ml of 3.2 M CH3COONH4 were added, by diluting to a final volume of 20 ml with DIW, with the samples continuously agitated for 30 min;
-
Residual (metals present as scatters within the crystal lattice of the rocks and minerals)—the residue from (iv) was digested with 10 ml of HNO3 in a microwave oven according to the method EPA 3051.
Further information describing the sequential extraction procedure is available in Li et al. (1995).
A PQ3 (VG-Elemental) ICP-MS was used for trace elements analyses throughout this study. Ion lens setting and the X–Y–Z position of the torch were manually optimized for maximum sensitivity (5×105–1×106 counts/s) for isotope 115 of In and the minimum percentage for BaO/Ba2+ (below 3%) using a solution containing 10 μg/l of Be, Co, In, Tb, Bi, and Ba. Under routine conditions one element menu is used for the characterization containing the metals and metalloid to analyze. The measurements were done in duplicate at the Laboratory of Geochemistry of the Institute of Geophysics–UNAM and the Laboratory of Geochemistry of the University of Wyoming.
Bioassay setup
The mine tailing samples were distributed in six acrylic boxes or cells (24 cm height, 20.5 cm width and 40 cm length). The cells were filled with the material until 5 cm deep, for a final volume of 4,100 cm3. Four cells were inoculated with algae biofilms (cyanobacteria and green algae or chlorophytes) for the bioassay (BA). A control (no inoculums addition) was also carried out in duplicate. All the cells were humidified daily with 100 ml of sterile tap water (distilled water) and the leachates were collected using plastic flasks. The cells and flasks were previously acid cleaned. Each cell was illuminated with artificial sunlight. The light intensity was maintained constant at 48 μmol m–2 s–1 (LICOR LI−185B) following a 14–10 light–dark cycle. The room temperature was set at 25+1°C. The BA was performed per 21 weeks.
During 16 weeks, cyanobacteria, algae, and fungi determination was done by direct microscopic observations (1,000×) of the biofilms sampled placed in a sterile Petri dish with 1–2 ml of sterile water to assist biofilm segregation. Morphological and morphometric characteristics were used to identify the genera or species. Bacteria determination was done with biochemical tests sensu McFaddin (1990) and using API biochemical identification system (BioMeriux® biochemical tests, API-STAPH, API-20E, RAPID-ID324), after the isolation of the strains in anoxic cultures.
Analysis after the BA
The mine tailing samples of each cell were collected separately in black plastic bags. Samples and the leachates were previously acidified and stored at –4°C until their analysis. The treated tailing samples were also analyzed for OM, CEC, pH, and multi-elemental determination by a five-step sequential extraction procedure, as previously described (before the BA). The leachates were acidified with ultra-pure HNO3 (J.B. ULTREX II) for multi-elemental determination using ICP-MS. All the glasswares and plastic wares were cleaned with 10% HNO3 and rinsed with DIW.
Statistical analysis
Significant differences between treatments were tested with nonparametric test Kruskal and Wallis at a significance level of P<0.05, for n>2. For paired treatments, one-tailed student’s t tests (Sokal and Rohlf 1995) were used. Pearson correlation index was obtained, between fractions. The statistical tests were performed with SPSS 10.0.5 for Windows (Microsoft 1999).
Results
Trace element partitioning before the BA
Table 1 and Fig. 2 summarize the results of the sequential extracted tailings sample after the BA. The results indicated that most of the sequential extracted elements are present in the third fraction (bound to the Fe–Mn oxides), though any of these elements are also strongly bound to the tailing matrix (residual fraction), with the exception of Ti, the only inert element (87.7% of total Ti is in the residual fraction).
The exchangeable fraction represents the largest fraction of Zn (34.8%), As (27.0%), Se (15.8%), and Pb (11.9%). High proportions of Mo, Cd, Sb, and Tl are also exchangeable (57.7, 16.8, 18.7, and 30.1%, respectively).
The second fraction (metals bound to carbonates and specifically adsorbed phases) represents the second largest phase of Cd (31.5%), Mn (18.9%), and Ni (8.2%). While the fourth fraction (metals bound to OM and sulfides) represents the second most important phase of Cu (39.1%), Co (28.3%), total Cr (16.4 %), Ni (16.2%), and Be (14.2%). The Se is the only element adsorbed mostly within the fourth fraction (65.0%).
Trace element partitioning after the BA
In general, the results of the treated tailings samples (after the BA), indicate that the fractions remain relatively invariable (Table 2; Fig. 3).
Only the decrease of Co exchangeable, the increase of Cr total, Ni, and Cu in the OM-sulfide fraction, and the increase of Ni, Cu, As, and Pb on carbonates/specifically adsorbed are significantly different after the BA (P<0.05) than before the BA.
The content of metals in the leachates of the control sample is relatively low and does not show significant differences before and after the BA (P<0.05). The exceptions are Zn and Pb, the concentration of both in trial samples (after the BA), is significantly lower than the control leachates (P<0.05, Table 3).
The initial and final OM contents and CEC values of the tailing samples are shown in Fig. 4. The results indicate a significant increase (P<0.05) of the OM, [from 0.8+0.4% (before the BA) to 3.5+0.4% (after the BA)]. Furthermore, the OM represents a chemical surface with a higher activity, which may support the CEC increase after the BA (2.7+0.6–3.8+0.5 meq 100 g–1). On the other hand, there are no significant changes in pHs from the original and treated tailings samples (7.8+0.3).
Biofilm colonization
After 4 weeks, the development of biofilms becomes evident in the inoculated. Twenty weeks later, the biofilms covered up to 70% of the tailings surface. The microorganisms colonized the mine tailings samples during the BA as autotrophic biofilms, composed mainly by two unicellular green algae (Chlorococcals) and three species of filamentous cyanobacteria (Table 4). The colonies of green algae were well embedded into the cyanobacteria filaments network, which maintained the biofilm structure (microscopic observations). Heterotrophic aerobic bacteria (eight species), anaerobic bacteria (two species), and some fungi (three species) were also observed (Table 4). These groups of microorganisms were closely linked with cyanobacteria and algae, since they were directly isolated from the autotrophic biofilm.
Discussion
Microorganisms colonization during the BA
The presence of viable algae indicated a continuous performance of the photosynthesis, with the parallel processes of oxygen and organic carbon release. While the occurrence of nitrogen-fixing cyanobacteria (Anabaena sp) in the autotrophic biofilms could be linked with low sulfide concentration and/or oxygen-rich conditions; it may also be related with saturated conditions (Sabater et al. 2000).
The main bacteria genera present in the developed biofilms were Bacillus, Pseudomonas, and Corynebacterium (Table 4). Chappell and Craw (2002) also found the aerobic bacteria—Bacillus subtillis, Pseudomonas aeuroginosa, and Corynebacterium spp.—in an old Au-mine tailings of Otago, New Zealand. These authors suggest that the organic C (1%) and carbonates are present in high enough concentration to act as organic electron donors for growth of these heterotrophic bacteria. The observation in the present work suggests that the autotrophic biofilms are the primary C supply for the heterotrophic bacteria and fungi, since the cyanobacteria and algae were the initial colonizers.
The reason why autotrophic biofilms can grow on mine tailings and are metal tolerant is not well understood. An earlier study on metal exposed cyanobacteria and algae has shown that a thick packing of cells embedded in a dense mucus matrix increased overall tolerance. The biofilm matrix is mostly composed of extracellular polymeric substances (EPS), which provide a suitable microenvironment for microbial development and interactions. EPS can also act as a detoxification agent against metals, since they contain large amounts of negatively charged functional groups like carboxyl, phosphate and sulfate groups, and acting as metal binding sites (Wingender et al. 1999).
Mine tailings characteristics before the BA
The results of the sequential extracted mine tailings sample after the BA (Table 1) indicate that the trace elements from the Valenciana tailings are in a lower concentration than those obtained in soils affected by mine tailings of other countries (Brierley et al. 1989; He et al. 2002; Leonard 1995; Li and Thornton 2001; Simón et al. 2001). However, the total concentration of Zn and Se is above the acceptable limits for soils (Kabata-Pendias and Pendias 1992; Abollino et al. 2002), while Cr, Zn, Se, and Cd totals are above the environmental legislations of USA, and the European Community, and Cu and Pb mobilizables are over the Swiss Guide Values for pollutants in soils (Gupta et al. 1996).
Additionally, the sum of the first fraction (exchangeable) and second fraction (carbonate/specifically adsorbed) results in high proportion of some metals in these two nonresidual fractions (21.9% Cu, 47% Zn, 29.8% As, 21.9% Se, 47.2% Cd, and 22.2% Pb; Fig. 2), which could limit the plant development (Li et al. 1995; Ma and Rao 1997). The proportion of the exchangeable phase of Zn, As, Se, and Pb was up to 10%. According to Kabata-Pendias and Pendias (1992), metal availability above 10% indicates that metals have not been retained by soil compounds (oxy-hydroxides, clay, carbonates), so Zn, As, Se and Pb are potentially leachables. Finally, the residual fraction is the minor fraction of trace elements with the exception of Ti (Table 1 and Fig. 2). In soils, the nonresidual fractions are associated with exogenous material (Abollino et al. 2002); but the low proportion of residual fractions in Valenciana tailings could be a consequence of the degree of the material alteration during the mineral ores concentration.
On the other hand, the largest fraction of most of the elements is the third one (bound to Fe–Mn oxides) (Table 1 and Fig. 2). The Fe–Mn oxides are excellent binders of metals (Tessier et al. 1979) because the anionic character (Morgan and Stumm 1995) and the metals absorbed onto this fraction are normally unavailable to plants (Li et al. 1995). The amount of Fe-oxy hydroxides of the Valenciana mine tailing (3%) suggests that the Fe-oxy hydroxides may play a key role in the natural absorption processes of the elements. Results obtained by Carrillo-Chávez et al. (2003) indicated that Fe oxides are the most probable adsorbing surfaces in tailings of the Guanajuato mining area, controlling complexation reactions and adsorption processes onto the mineral surface.
Despite the high proportion of nonresidual fractions, the carbonate, oxides, and sulfide complexes ensure the metal immobilization due to the favorable (high) pH of Valenciana tailings, but especially if the pH remains unchangeable (Morgan and Stumm 1995; Stark et al. 1996).
Mine tailings characteristics after the BA
Results indicate that OM in the tailings material after the BA increased significantly. The pH remained slightly alkaline, mostly due to the high buffer capacity of the Valenciana tailings (high carbonate content, Oesler 2001). Additional OM came as a consequence of the biofilms colonization, and could be associated with the living and dying cells, as well as the EPS excreted by the microorganisms.
Both the OM increment and the pH constancy after 20 weeks of biofilms colonization (high buffer capacity) are promising facts for mine tailing remediation. The OM increment suggests a natural fertilization of the material, while the constant pH may ensure that trace elements are immobilized on metallic complexes.
Consequence of OM improvement in trace elements behavior
The organic surfaces contain functional groups (–OH, ≡ROH, R–COOH) that are able to act as coordinating sites forming stable complexes over wide ranges of pH and concentrations (Morgan and Stumm 1995). The presence of cellular envelopes and EPS also increases the metal ions sorption (Parmar et al. 2000) and hydrous oxides sorption (Phoenix et al. 2000) due to their polyanionic character (Wingender et al. 1999). Therefore, it is expected that under high OM amount, a higher level of some trace elements should be found in this fraction (Abollino et al. 2002).
After the BA, Cu showed a shift in the relative abundance in the fourth fraction (OM-sulfide), which becomes the first largest fraction of Cu (up to 48.4%), resulting in a significant increase of Cu (P<0.05) (Fig. 3). The results also indicate a strong correlation between Cu in the fourth fraction (OM; r=0.97) and the CEC (r=0.96). Balasoiu et al. (2001) also found more immovable Cu on the soil surface, according to the high OM amount quantified, while Adriano (1986) stated that the Cu behavior strongly correlated with the significant increase of the CEC, and, with pH. The Cu has more capability to interact chemically with the organic compound of the soils (Kabata-Pendias and Pendias 1992) due to the high formation constants of organic-Cu complexes (Morgan and Stumm 1995) or to be bond on sulfide minerals under unoxidized conditions (Carlsson et al. 2002; see below). Also, EPS of biofilms from the Valenciana mine tailings showed a strong affinity for Cu under oxidized conditions.
Consequence of biofilms colonization on trace elements precipitation
Additional to the OM build up, the biofilm may contribute to configure favorable conditions for metal precipitation. Particularly, the EPS of biofilms provide chemical gradients and physical conditions that are conducive to formation of insoluble metal precipitates or biomineralization (Podda et al. 2000). The biofilms, a tridimensional gel-like structure where the microorganisms are embedded (Wingender et al. 1999), have an internal pH higher than the pH of the surrounding environment (Liehr et al. 1995), due the CO2 consumption during the photosynthesis. An increase in pH enhances the precipitation of dissolved metals. Thus, the overall photosynthetic metabolism may promote metals precipitation as well as their sorption in the solid phase (Liehr et al. 1995; Rose et al. 1998; Parmar et al. 2000). In fact, the involvement of some cyanobacteria species in the formation of metallic carbonates is well documented (Podda et al. 2000).
The results suggest that the presence of biofilm might enhance the conformation of metal–carbonate complexes, as is indicated in the significant increase (P<0.05) after the BA of Ni, Cu, As, and Pb in carbonates/specifically adsorbed phase (Table 2).
In addition, under neutral pH and high alkalinity, metals may also precipitate as hydroxides. The metals bound to hydroxides have a strong capability of adsorbing other metals ions onto their surface that may result in more anionic hydroxi-metal complexes formation (Morgan and Stumm 1995; Peppas et al. 2000). These new surfaces may immobilize free ions through biomineralization processes. The study performed by Podda et al (2000) is a good example of the active role of the phototrophs in the biomineralization. In natural biofilms composed of the cyanobacteria Scytonema sp. (cyanobacteria) and the green algae Chlorella sp., the researchers reported the extracellular biomineralization of hydrozincite ([Zn5(CO3)2(OH)6]) on the envelopes of the (Scytonema sp.) as well as the alkalization of the microenvironment. They conclude that the microbial community is responsible for the natural polishing of metals (Ni, Cu, Cd, and Pb), by co-precipitation within biofilms. Furthermore, the mineralization is not detrimental to the photosynthetic microorganisms (Phoenix et al. 2000; Podda et al. 1999). The presence of metal precipitates coating the algal cells seems to reduce the metal toxicity (Admiraal et al. 1999). The former could also explain the biofilms occurrence on tailings surfaces during the BA.
Even when the photosynthesis involves the release of oxygen, the aerobic degradation of organic compounds implies oxygen consumption, with the organic material acting as an “oxygen trap,” thereby minimizing the oxidation of underlying tailings (Peppas et al. 2000). Actually, when the biofilms reach 10–25 μm thickness, the surface condition for the biofilms remains aerobic, while the deeper layer becomes anaerobic (Percival et al. 2000), which may mean that the biofilms generate a pH and gas gradient. The presence of anaerobic bacteria (Clostridium sp. and Peptococcus sp.) in our biofilms suggests that this effect may occur.
The biofilms act as a physical barrier against oxygen diffusion preventing formation of Fe–Mn oxides and sulfur oxidation (Peppas et al. 2000; Carlsson et al. 2002). This could explain why the third (Fe–Mn oxides) and fourth fraction (OM-sulfide) of most of the trace elements remains unchanged after the BA.
The sulfides minerals may also influence the trace elements immobilization, while the biofilm colonization seems to prevent sulfide oxidation, since Cr, Ni, and Cu in fourth fraction (OM-sulfide) increase after the BA, while Se remains mostly in the fourth fraction (Table 2, Fig. 3). In general, trace metals (as Ni, Cu, Zn, Cd) are frequently found associated with pyrite in organic-rich sediments (Wilkin and Ford 2002).
The Se adsorbed in the fourth fraction showed a strong correlation with its total concentration (r=0.99) and noncorrelation between Se and the OM amount. The Se leachates seem to be a consequence of its total concentration in mine tailings.
The biofilms colonization, the prevalence of oxidizing conditions, and the relative high pH in the surface might increase the As concentration within the second fraction because of the high solubility of As at alkaline pH and the formation of arsenic complexes with carbonate (Wilkin and Ford 2002). An important proportion of As may remain in the crystalline Fe–Mn oxides (25%), and the sulfide minerals (3.5%) under the anaerobic conditions in the lower layer. Arsenic is potentially associated with iron oxides or arsenic sulfides in reducing environments (Wilkin and Ford 2002). Additionally, the arsenate [As(V)] is continuously subject to microbial reduction to arsenite [As(III)] (more mobile and toxic) (Turpeinen et al. 2002). Arsenic(III) oxidation may also be catalyzed biologically as a microbial detoxification mechanism (Battaglia-Brunet et al. 2002).
Consequence of biofilms colonization on leaching and bioavailability of trace elements
The results indicate low metals leachates of both—the control and BA (Table 3) with the exception of Zn and Pb; the concentration of Zn and Pb in the BA leachates was significantly lower than the control leachates.
The results previously obtained by Carrillo-Chávez et al. (2003) with leachates from humidity cell tests using samples from Valenciana tailings also indicate a low concentration of trace elements in the leachates due to the neutral pH. The researchers suggested that the Zn in leachates may derive from Zn carbonate dissolution. If the carbonate dissolution is reduced because of the biofilms presence, it is expected to decrease trace elements within the leachates. Besides, the OM increment (or biofilm development) and the Zn in leachates are strongly correlated (r 2=0.97). Pb of the second fraction (carbonates/specifically adsorbed) and Pb in leachates show a strong and negative correlation (r=–0.90).
Even though Simms and collaborators worked in different tailing material, they also observed a decrease of metal releases and leaching in pre-oxidized mine tailings under water saturated (Simms et al. 2000). So, the biofilms could act as a physical barrier optimizing the water saturation. Biofilms, especially their EPS, contribute to maintain the surface moisture and to reducing water infiltration (Wingender et al. 1999, Peppas et al. 2000).
Although there is significant reduction of Zn in leachates in BA trials, its concentration still remains over the recommended limit for drinking water (Leonard 1995). While the Mn, As, Se, and Cd in leachates of both trial and control samples are over the limits of the European Community legislation for drinking water (Katsoyiannis et al. 2002).
Finally, the exchangeable fraction of the trace elements does not show significant differences after the BA; the exception is the Co exchangeable, significantly lower (r=0.01) after, than before the BA. Therefore, the availability of some trace elements remains over 10% (Zn 13.4%, As 32.7%, Se 10.8%, Mo 25%, Cd 17.4%, Sb 11.6%, and Tl 20.3%).
Conclusions
The low concentration of metal in natural leachates indicates that the surface complexation processes may play a key role in controlling metal mobility. The results of the BA suggest that the biofilms enhanced the trace element immobilization, either in their original phases, or promoting the decrease of some exchangeable forms (Co) and of some trace leachates (Zn and Pb), the increase of the proportion of carbonate/specifically adsorbed fraction (Ni, Cu, As, Pb), and the OM-sulfide fraction (Cr, Ni, Cu). Certainly, the carbonate/specifically adsorbed forms could shift to exchangeable forms if the environmental conditions change (i.e., pH, redox potential). However, the pH is expected to remain stable due to high buffer capacity of the tailings material.
As Bradshaw and Hüttl (2001) indicated, the ecosystem development involves more than just the establishment of a set of plant species; it concerns the development of complete mechanisms of nutrient accumulation, which ultimately implies that the health and activity of the microorganisms is important.
The persistence of the photosynthetically active forms during the BA resulted in a natural fertilization of the tailings samples building up an appropriate substrata for the further establishment of plants. Actually, the occurrence of growing photosynthetic cyanobacteria and algae, heterotrophic bacteria, nitrogen-fixing cyanobacteria, nitrogen-reducing bacteria as well as OM-decomposers fungi may optimize the metabolism and, thus, functional structure of biofilms. The interaction among microorganisms, within a suitable mucilaginous EPS matrix, allows the self-sustaining of the biofilm as a biological dynamic unit.
Due to the OM improvement, the biofilms could also be conceptualized as an organic cover, which (1) controls the acidity production, (2) sorbs metals in its matrix (EPS) or may trap metals as organic complexes, (3) optimizes the water saturation effects and limits the water and oxygen diffusion, (4) provides chemical gradients and physical conditions that are conducive for formation of insoluble metal precipitates (carbonates and oxides), and (5) enhances the metal biomineralization. Consequently, the biofilms, as dynamic biological units, represent an excellent opportunity for mine tailing remediation of the Valenciana mine tailings.
Nevertheless, the high Zn leachates, even under the biofilm presence conditions, and the nonsignificant reduction of other trace elements leaching after the BA (Se, As, and Cd) remain a potential environmental risk.
References
Abollino O, Aceto M, Malandrino M, Mentasti E, Sarzanini C, Petrella F (2002) Heavy metals in agricultural soil from Piedmont, Italy. Distribution, speciation and chemometric data treatment. Chemosphere 49:545-557
Admiraal W, Blanck H, Buckert-De-Jong M, Guasch H, Ivorra N, Lehmann V, Nyström BAH, Paulsson M, Sabater S (1999) Short-term toxicity of zinc to microbial algae and bacteria in metal polluted stream. Water Res 33:1989–1996
Adriano DC (1986) Trace elements in the terrestrial environment. Springer, Berlin Heidelberg NewYork
Allan RJ (1995) Impact of mining activities on the terrestrial and aquatic environment. In:Salmons W, Förstner U, Mader P, (eds) Heavy metals, problems and solutions. Springer, Berlin Heidelberg NewYork A, pp 120–140
Anagnostidis K, Roussomoustakaki M (1988) Cyanophytes from metal burdened substrates. Arch Hydrobiol Suppl 80:1–4
Balasoiu CF, Zagury GJ, Deschenes L (2001) Partitioning and speciation of chromium, copper, and arsenic in CCA-contaminated soils: influence of soil composition. Sci Total Environ 280:239–255
Battaglia-Brunet FF, Dictor MC, Garrido F, Crouzet C, Morin D, Dekeyser K, Clarens M, Baranger P (2002) An arsenic (III)-oxidizing bacteria population: selection, characterization, and performance in reactors. J Appl Microbiol 93:656–667
Bower CA (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73:252–261
Bradshaw AD, Hüttl RF (2001) Future mine site restoration involves a broader approach. Ecol Eng 17:87–90
Brierley CE, Brierley JA, Davidson M (1989) Applied microbial processes for metal recovery and removal from wastewater. In: Beveridge TJ, Doyle RJ, (eds) Metal, ions and bacteria. Wiley, New York, pp 359–382
Carlsson E, Thunberg J, Öhlander B, Holmström H (2002) Sequential extraction of sulfide-rich tailings remediated by the application of till cover, Kristineberg mine, northern Sweden. Sci Tot Environ 2002; 299:207–226
Carrillo-Chávez A, Morton-Bermea O, González-Partida E, Rivas-Solórzano H, Oesler G, García-Meza JV, Hernández E, Morales P, Cienfuegos E (2003) Environmental geochemistry of the Guanajuato mining district, Mexico. Ore Geol Rev 23:277–297
Chappell DA, Craw D (2002) Geochemical analogue for circumneutral pH mine tailings: implication for long-term storage, Macraes Mine, Otago, New Zealand. Appl Geochem 17:1105–1114
Ernst WHO (1988) Response of plants and vegetation to mine tailings and dredged materials. In: Salmons W, Förstner U, (eds) Chemistry and biology of solid wastes dredged material and mine tailings. Springer Berlin Heidelberg New York, pp 54–69
García-Meza JV (1999) Algas de jales mineros de Guanajuato (Algae from mine tailings of Guanajuato) National and Autonomous University of Mexico (UNAM), pp 183. Master thesis. In Spanish
García-Meza JV, Ramos E, Carrillo-Chávez A, Durán C (2004) Mineralogical and chemical composition of historical mine tailings from the Valenciana mine, Guanajuato, Mexico: environmental implications. Bull Environ Contam Toxicol 72:170–177
Gupta SK, Vollmer MK, Krebs R (1996) The importance of mobile, mobilisable and pseudo total heavy metal fraction in soil for three level risk assessment and risk management. Sci Total Environ 178:11–20
He B, Liang L, Jiang G (2002) Distribution of As and Se in selected Chinese coal mines. Sci Tot Environ 296:19–26
Kabata-Pendias A, Pendias H (1992) Trace elements in soils and plants.2nd edn. CRC Press, Boca Raton, Fl. pp 365
Katsoyiannis I, Zouboulis A, Althoff H, Bartel J (2002) As (III) removal from groundwaters using fixed-bed upflow bioreactors. Chemosphere 47:325–332
Leonard A (1995) Arsenic. In: Merian E (eds) Metals and their compounds in the environment. VCH, Weinheim, pp 751–767
Li X, Coles BJ, Ramsey MH, Thornton I (1995) Sequential extraction of soils for multielement analysis by ICP-AES. Chem Geol 124:109–123
Li X, Thornton I (2001) Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl Geochem 16:1693–1706
Liehr SK, Chen H-J, Lin S-H (1995) Metal removal by algal biofilms. Water Sci Tech 30:59–68
Ma LQ, Rao GN (1997) Chemical fraction of cadmium, copper, Nickel, and Zinc in contaminated soils. J Environ Qual 26:259–264
McFaddin JF (1990) Pruebas bioquímicas para la identificación de bacterias. Panamericana editorial. México DF
Morgan JJ, Stumm W (1995) Chemical processes in the environment, relevance of chemical speciation. In: Merian E (eds) Metals and their compounds in the environment. VCH, Weinheim pp 67–103
Oesler GP (2001) The impact of abandoned mine tailings on surface water: Monte de San Nicolas, Guanajuato, Mexico, Department of Geology and Geophysics, University of Wyoming (71 pp), Master thesis
Parmar N, Warren LA, Roden EE, Ferris FG (2000) Solid phase capture of strontium by the iron reducing bacteria Shewanella alga strain BrY. Chem Geol 169:281–288
Peppas A, Komnitsas K, Halikia I (2000) Use of organic covers for acid mine drainage control. Miner Eng 13:563–574
Percival SL, Walker JT, Hunter PR (2000) Microbiological aspects of biofilms and drinking water. CRC Press, Boca Raton, Fl. pp 229
Phoenix VR, Adams DG, Konhauser KO (2000) Cyanobacterial viability during hydrothermal biomineralisation. Chem Geol 169:329–338
Podda F, Zuddas P, Minacci A, Pepi M, Baldi F (2000) Heavy metal coprecipitation with hidrozincita [Zn5(CO3)2(OH)6] from mine waters cause by photosynthetic microorganisms. Appl Environ Microbiol 66:5092–5098
Rose PD, Boshoff GA, van Hille RP, Wallace LCM, Dunn KM, Duncan JR (1998) An integrated algal sulfate reducing high rate ponding process for the treatment of acid mine drainage wastewaters. Biodegrad 9:247–257
Sabater S, Guasch H, Romaní A, Muñoz I (2000) Stromatolitic communities in Meditarranean streams: adaptation of a changing environment. Biodivers Conserv 9:379–392
Simms PH, Yanful EK, St-Arnaud L, Aubé B (2000) A laboratory evaluation of metal release and transport in flooded pre-oxidized mine tailings. Appl Geochem 15:1245–1263
Simón M, Martín F, Ortiz I, García I, Fernández J, Fernández E, Dorrosoro C, Aguilar J (2001) Soil pollution by oxidation of tailings from toxic spill of pyrite mine. Sci Total Environ 279:63–74
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biology research. Freeman and Co., New York
Stark LR, Williams FM, Wenerick WR, Wuest PJ, Urban C (1996) The effects of substrate type, surface water depth and flow rate on manganese retention in mesocosmos wetlands. J Environ Qual 25:97–106
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for speciation of particulate trace metals. Anal Chem 5:844–851
Turpeinen R, Pantsar-Kallio M, Häggblom M, Kairesalo T (2002) Role of the microbes in controlling the speciation of arsines and production of arsines in contaminated soils. Sci Tot Environ 285:133–145
Walkley A (1947) A critical examination on a rapid method for determining organic carbon in soil. Soil Sci 63:451–464
Wilkin RT, Ford RG (2002) Use of hydrochloric acid for determining solid-phase arsenic partitioning in sulfidic sediments. Env Sci Technol. 36: 4921–4927
Wingender J, Neu TR, Fleming HC (1999) What are bacterial EPS? In: Wingender J, Neu TR, Fleming HC (eds) Microbial extracelluar polymeric substances. Springer, Berlin Heidelberg New York pp 1–9
Ye ZE, Shu WS, Zhang ZQ, Lan CY, Wong MH (2002) Evaluation of major constraints to revegetation of lead/zinc mine tailings using bioassay techniques. Chemosphere 47:1103–1111
Acknowledgements
The authors wish to thank very much to Ing. Ciro Márquez, Faculty of Chemistry, UNAM, and to Dr. Eberto Novelo and Luciano Hernández-Gómez, Faculty of Science, UNAM for the microorganisms identification. This work was possible in part to Mexican Science Fundation (CONACYT) scholarship (117255), Project CONACyT-SEMARNAT 2002-C01-1420, and Project UNAM PAPIIT IN114102-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Meza, J., Carrillo-Chávez, A. & Morton-Bermea, O. Sequential extractions on mine tailings samples after and before bioassays: implications on the speciation of metals during microbial re-colonization. Environ Geol 49, 437–448 (2006). https://doi.org/10.1007/s00254-005-0101-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-005-0101-4