Abstract
Water and dissolved ion transport in mineral tailings was studied at laboratory scale with three different tailings samples. Percolation rate, determined in column experiments, depends mainly on the granulometric distribution of the solid and varies between 3.0×10−4 cm/s for a tailings sample with coarser particles (d90>700 μm) to 4.0×10−5 cm/s for the tailings sample with higher content of fine particles (d90<200 μm). In stationary conditions, the ascending rate of water by capillarity through the solid bed is controlled by the evaporation rate at the top surface of the tailings. Rates between 2.6×10−5 cm/s (2.2 mm/day) and 8.1×10−5 cm/s (7.0 mm/day) have been obtained under forced evaporation at 40 °C. Ion transport in the percolation process is mainly controlled by the hydraulic diffusion of the solution through pores in the solid bed. In the capillarity process, ion transport is controlled by chemical diffusion of the dissolved ion in the ascending solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waste disposal has been recognized as a major environmental problem in the mining industry. Flotation tailings represent one of the most important mining wastes associated with copper production. In Chile, for each ton of copper produced, about 80 t of tailings are released and must be disposed of in a safe way. Physical stability of the tailing ponds has been a major concern for many years due to some historical disasters that occurred in the 1960s (Ritcey 1989; Robertson 1997). However, less attention has been paid to the geochemical stability of the wastes. Mineralogical and geochemical transformations associated with the weathering of sulfide minerals can produce a progressive acidification of the water and the slow release of dissolved metals. The potential of acid water generation associated with geochemical transformations of the sulfides depends on the mineralogical composition of the waste. The rate of the transformation processes involved will depend on the characteristics of the wastes and the climatic conditions in the disposal area (Dold and Fontboté 2001). An important factor is the mobility of elements in the tailings (Dold and Fontboté 2002). The transport of dissolved products is associated with the water transport through the deposit. This transport of solution in porous media is related to two mechanisms: transport by percolation under saturated conditions and hydrostatic pressure and transport by capillarity under unsaturated conditions. The rate of transport of solution through abandoned tailings depends essentially on the granulometric characteristics of the tailings (Choo and Yanful 2000) and on the hydrologic balance in the area of the deposit. In case of persistent and significant precipitation, vertical solution transport will proceed downward by percolation. Different types of low permeability cover have been proposed to limit the infiltration of water into the tailings (Woyshner and Yanful 1995; Yanful and others 1999). In the case of dry conditions with significant surface evaporation, the solution retained in the tailings will move up by capillarity.
In the present work, an experimental system has been designed to study both mechanisms and to determine the rate of solution and solute transport under different climatic simulated conditions.
Materials and methods
tailings samples
Experimental study was carried out using three samples of copper ore tailings ponds.
-
Sample A, with the coarser particles and a wider granulometric distribution, comes from an operating tailing pond facility in central Chile (Ovejería, Division Andina, CODELCO Chile).
-
Sample B, of medium granulometric distribution comes from an abandoned tailing pond in central Chile.
-
Sample C, which presents fine particles and the narrowest granulometric distribution, comes from an operating tailing pond facility in north Chile (Cie. Minera Candelaria, Copiapó).
Figure 1 shows the granulometric distribution of the three selected samples.
Table 1 shows some of the physical characteristics of the tailings samples. Mz is the medium size, defined as the sum of the weighted sizes, and d10 and d90 are the passing diameter of 10% weight and 90% weight of the sample, respectively.
Experimental system
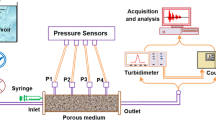
Experiments were performed in acrylic columns. Percolation rate was determined using 10-cm-diameter and 50-cm-height columns (Fig. 2a). Hydraulic pressure can be applied by controlling the level of water above the tailings sample. Capillarity columns (6 cm diameter and 100 cm height) were connected on their lower end to a reservoir of which the vertical position can be controlled to simulate different hydraulic pressures (Fig. 2b).
Percolation and capillarity tests
The percolation rate of pure distilled water through tailings samples was determined at steady state under different hydraulic charges. In each experiment, a 3 kg of dry tailings sample was placed in the acrylic column.
In smaller columns (20 to 30 cm height) submitted to continuous percolation of solution fed at the exact percolation rate to maintain a steady state flow, aluminum sulfate was added to the supplied solution and the rate of transport was determined by monitoring the solution conductivity in the output flow.
The capillary ascending rate was determined using dry tailings samples and by measuring the vertical rate of ascending pure water by visual observation of the wet interface. The mean saturation grade of the mineral bed was calculated by measuring the volume of consumed water. Local saturation grade was also determined further at different heights by wet and dry weight determinations on humid samples. Once the liquid front has reached the top of the column, the wet tailings were submitted to a forced evaporation process produced by an infrared lamp operated by an automatic temperature control system. Evaporation and solution transport rate were determined by water balance.
Aluminum sulfate was then added to the water tank. After a few days of operation, the columns were dismounted and tailings samples from different heights in the column were washed and the solution was analyzed for its aluminum content by conductivity determination.
Results and discussion
Rate of percolation
Figure 3 shows the results of percolation experiments performed with the three tailings samples under different water overloading (expressed as height of water on the top of the column). The rate of solution increases almost lineally with the water overloading. The permeability, which corresponds to the rate of solution transport through the porous solid without overloading, was 4.0×10−5 cm/s for sample C, with the finest particles, 7.0×10−5 cm/s for sample B, the intermediate tailings sample and 3.0×10−4 cm/s for sample A with the coarsest particles.
The true medium rate of solution through the bed can be calculated by dividing the permeability by the porosity, assuming that the ratio between effective area for solution transport to total transversal surface is the same as the ratio between void volume and total volume of the bed. Then, the inter-pore liquid rates for the different samples are: 7.3×10−4 cm/s for sample A, 1.4×10−4 cm/s for sample B and 7.0×10−5 cm/s for sample C.
Rate of capillary transport
The vertical rate of the solution ascending through the bed by capillarity was calculated from the observed position of the liquid front line and is presented in Fig. 4. In the lower part of the column, the ascending rate of the solution decreases almost logarithmically with the elevation of the liquid in the column and then tends to stabilize at an almost constant value. The volume of ascending water and the corresponding grade of saturation of the porous bed decreases with height for samples A and B with values ranging from 40 to 15% of saturation but is almost constant for sample C and corresponds to about 43% of porosity. The finest material, which also presents the higher porosity, is then more susceptible to capillarity.
In a further experiment, when evaporation of the solution at the top of the column was stimulated by infrared radiation at constant superficial temperature, the rate of water evaporation was determined at different surface temperatures and compared with the evaporation under the same condition of a free water surface. At 40 °C, when total evaporation on a free water surface was 12 mm/day (1.4×10−5 cm/s), the evaporation under the same conditions at the C sample surface was only about 7.5 mm/day, showing that the solid retains liquid by capillarity. For samples A and B, the evaporation rate was about 8.5 mm/day, slightly higher than for sample A. Even at this very high evaporation rate, the solution rise at a higher rate and the liquid front is maintained very near the surface. In a stationary condition, the rate of solution transport is lower than the values reported above because the system is controlled by evaporation of the solution from the unsaturated surface of the tailings sample. Rates of about 2.6×10−5 cm/s (2.2 mm/day) to 8.1×10−5 cm/s (7.0 mm/day) have been obtained under these conditions, depending on the sample. The last value (7.0 mm/day) corresponds to a very high evaporation rate only observed in very dry area. These results show that, even under very dry conditions, vertical water transport is controlled by the evaporation and not by capillarity.
Rate of dissolved ion transport
Figure 5 shows the input and output conductivity of the solution with a pulse of dissolved aluminum flowing through a 200-mm-height column containing tailings sample A. The solution was added at the top of the column and percolated at a flow of 1.4 ml/min (3.0×10−4 cm/s). The system behaves as a combination of a series of plug flow and well mixed reactors. From the delay between the input and output signals, the equivalent volume of the plug flow reactor can be estimated to 280 ml, which corresponds to about 44% of the total volume of solution in the column. The behavior of the system corresponds to the hydraulic diffusion of the solution through the porous solid sample. The concentration of the dissolved ion in the output solution is determined by the mixing of the solution coming out through channels of different lengths.
When the solution percolates through the porous media, the rate of dissolved ion transport is determined by the distribution of the solution between the different channels. For sample C, which presents a higher porosity (57%), the hydraulic diffusion is higher and the volume of the plug flow reactor is proportionally lower (34% of the total liquid volume). Due to the lower rate of percolation, the delay between the pulse and the output concentration change is much lower but the signal is also more attenuated.
Similar experiments were performed in capillarity columns. Figure 6 shows the conductivity of solution samples at different height of the capillarity column of sample B after 17 days of forced evaporation. The total amount of water evaporated during this time was 107.3 cm3, which corresponds to a medium evaporation rate of 2.2 mm/day (2.6×10−6 cm/s). However, this rate was calculated considering the whole surface of the column. As in the case of percolation, an inter-pore rate can be calculated dividing the total evaporation flow by the pore surface. The estimated inter-pore rate is then 4.6 mm/day (5.3×10−6 cm/s). On the other hand, this can be seen in the figure; the rate of dissolved ion was much higher and its presence was detected at about 15 cm from the bottom, which corresponds to an ascending rate of 8.8 mm/day (1.0×10−5 cm/s). In this case, it can be assumed that this higher rate of transport of dissolved ion is mainly due to chemical diffusion in the solution.
Conclusions
The rate of solution and dissolved ions transport in a tailings pond will depend on the granulometric distribution of the material in the waste deposit and on the climatic conditions on the site. When the tailings are affected by rain or snow precipitation, the solution percolates at a rate that depends on the granulometric distribution and on the hydraulic height of the water over the tailings surface. Percolation rates vary between 3.0×10−4 cm/s for a tailings sample with coarser particles to 4.0×10−5 cm/s for the tailings sample with a higher content of fine particles.
In the absence of rain or snow precipitation, under dry conditions, water will evaporate at the tailings surface and move upward by capillarity. The ascending rate of water through the solid bed is controlled by the evaporation rate at the top surface of the tailings. Rates between 2.6×10−5 cm/s (2.2 mm/day) and 8.1×10−5 cm/s (7.0 mm/day) have been obtained under forced evaporation at 40 °C.
Ion transport in the percolation process is mainly controlled by the hydraulic diffusion of the solution through pores in the solid bed. However, in the capillarity process, ion transport is mainly controlled by chemical diffusion of the dissolved ion in the ascending solution.
References
Choo LP, Yanful EK (2000) Water flow through cover soils using modeling and experimental methods. ASCE J Geotech Geoenviron Eng 126:324–334
Dold B, Fontboté L (2001) Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J Geochem Explor 74:3–55
Dold B, Fontboté L (2002) A mineralogical and geochemical study of element mobility in sulfide tailings of Fe oxide Cu-Au deposits from the Punta del Cobre belt, northern Chile. Chem Geol 189:135–163
Ritcey GM (1989) Tailing management: problems and solutions in the mining industry. Elsevier, Amsterdam
Robertson A (1997) The influence of depositional methods on the engineering properties of tailings deposits. Steffen Robertson & Kirsten, Vancouver, Canada
Woyshner MR, Yanful EK (1995) Modelling and field measurements of water percolation through an experimental soil cover on mine tailings. Can Geotech J 32:601–609
Yanful EK, Simms PH, Payant SC (1999) Soil cover for controlling acid generation in mine tailings: a laboratory evaluation of the physics and geochemistry. Water Air Soil Pollut 114:347–375
Acknowledgments
The present work was supported by CONICYT through the Fondecyt Research Project 1010428.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiertz, J.V., Marinkovic, F.A. Dissolved pollutant transport in tailings ponds. Env Geol 47, 237–240 (2005). https://doi.org/10.1007/s00254-004-1148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-004-1148-3