Abstract
An investigation was conducted at Astrolabe Park landfill, a decommissioned municipal landfill in Sydney, Australia, to assess the physical and chemical processes affecting the distribution of inorganic constituents in the leachate plume. The plume is migrating from the landfill towards a groundwater-fed pond into which leachate-impacted groundwater discharges. Borehole geophysical logging and depth-discrete groundwater sampling were used to delineate the distribution of the leachate plume along two groundwater flow paths between the landfill and the shore of the pond. Borehole geophysical logs indicate a strong correlation between bulk and fluid electrical conductivity (EC) values, and help to identify small-scale heterogeneities that comprise a major constraint on contaminant transport within the aquifer. Variations in the distribution of several indicator parameters (EC, HCO3 −, pH, Eh, NH4 +/NO3 −, S2−/SO4 2−) are used to assess the dominant processes affecting contaminant distribution along the flow path, including mixing of fresh and contaminated groundwater, oxidation/reduction reactions and ion exchange.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disposal of waste in landfills is an integral part of waste management strategies around the world. The production of leachate as a by-product of organic and inorganic decomposition in landfills poses a serious threat if released to the environment (Kimmel and Braids 1974; Baedecker and Back 1979; Arneth and others 1989; Kjeldsen 1993; Jankowski 1997). Andreottola and Cannas (1992) describe leachate pollution as the result of a mass transfer process between the waste and leaching water that has infiltrated into the waste layers. Physical, chemical and microbial processes transfer pollutants from the waste material to the infiltrating water, resulting in a contaminated liquid containing high concentrations of organic and inorganic contaminants. If the leachate is released into the underlying aquifer, it forms a complex contaminant plume that fundamentally alters the chemical properties of the aquifer (Baedecker and Back 1979; Nicholson and others 1983; Lyngkilde and Christensen 1992; Bjerg and others 1995; Jankowski 1997; Jankowski and Acworth 1997; Ludvigsen and others 1998; Cozzarelli and others 1999; Christensen and others 2001). For this study, groundwater samples were collected from four multi-level piezometers that comprise the endpoints of two parallel groundwater flow paths between the Astrolabe Park landfill and a groundwater-fed pond located hydraulically downgradient from the landfill (Lachlan Pond No.5). Several groundwater quality parameters and inorganic elements (EC, HCO3 −, pH, Eh, NH4 +/NO3 −, S2−/SO4 2−) are examined to assess the variations in contaminant concentrations and spatial distribution between the landfill and the pond.
Environmental setting
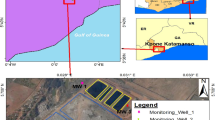
The Astrolabe Park landfill is approximately 6 km south of the Sydney central business district (CBD) in the suburb of Daceyville (Fig. 1). The site is within the northern portion of the Botany Basin, an erosional depression in the Triassic Hawkesbury Sandstone bedrock (Albani 1981; Griffin 1963; Rickwood 1998). The Botany Basin consists of several deep, steep-sided paleochannels incised into the Triassic Hawkesbury Sandstone bedrock that were filled with a sequence of Quaternary sedimentary deposits that comprise the Botany Sands aquifer (Fig. 1).
The sedimentary sequence observed in the Botany Sands aquifer represents depositional environments ranging from marine to terrestrial. From most recent to oldest, this sedimentary sequence has been divided into four units (Albani 1981): recent marine deposits (Unit 1); aeolian deposits (Unit 2); fresh to brackish lagoon deposits (Unit 3); and marine/estuarine deposits (Unit 4). In the vicinity of the Astrolabe Park landfill, the aeolian deposits of Unit 2 comprise the most significant water bearing formation.
Site description
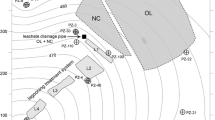
The study site is comprised of the Astrolabe Park landfill (which was completed as a sports field upon decommissioning), a portion of the adjacent Eastlakes Golf Course, and a portion of the eastern shore of Lachlan Pond No. 5 (Fig. 2). The site is underlain by an approximately 30-m-thick deposit of predominantly aeolian quartz sand comprising the upper sequence of the Quaternary Botany Sands aquifer (Unit 2, discussed in the previous section). The upper portion of the aquifer in this area is unconfined, while the occurrence of silty, clayey and peaty lenses creates semi-confined conditions in the lower portion of the aquifer. On a regional scale these lenses are discontinuous, but they are locally extensive in the vicinity of Astrolabe Park and comprise a significant constraint on groundwater flow and solute transport in the shallow aquifer. The landfill is located up hydraulic gradient from a series of groundwater-fed ponds known as the Lachlan Ponds.
Mining of heavy mineral sands occurred at this site using a floating suction dredge between 1964 and 1973; this is believed to have extended to a maximum depth of 17 m below the original ground surface (Hitchcock unpublished data 1991). Following the conclusion of the mining activities, the water-filled sand pits were backfilled with solid wastes of mostly domestic origin. The total volume of waste deposited was approximately 2.04×105 m3 (Hitchcock unpublished data 1991). The lateral extent of the landfill was estimated to be 4.7 ha, based upon examination of a series of aerial photographs, resulting in a calculated average waste thickness of approximately 4.35 m.
The regional groundwater flow direction within the Botany Sands aquifer is generally towards the south-southwest. However, in the vicinity of Astrolabe Park the shallow, unconfined groundwater flow is controlled by local topography and flows approximately in a west-southwest direction, as determined in this and previous investigations (Jankowski and Knight unpublished data 1991; Hitchcock unpublished data 1991).
Landfill processes and leachate production: a brief overview
Degradation and stabilization of waste in landfills has been observed to occur according to an ordered succession of physical, chemical and biological processes over time, as described in detail by Ehrig (1983), Andreottola and Cannas (1992) and Qasim and Chiang (1994). These include an initial, short-lived aerobic degradation phase followed by three phases of anaerobic degradation: (I) acid-fermentation, (II) intermediate anaerobiosis, and (III) methanogenesis. Each stage represents a progression in the process and rate of decomposition of organic matter present in the wastes, and the resultant leachate produced can be identified by characteristics indicative of each stage.
In general, the organic waste material is ultimately reduced to CO2(aq), CH4, and H2O (Baedecker and Apgar 1984; Christensen 1992; Ehrig 1983). In the typical pH range of 6.0–7.0 for methanogenic phase leachate the CO2(aq) is converted to HCO3 − (Baedecker and Back 1979; Ehrig 1983; Nicholson and others 1983). The amount of HCO3 − introduced to the groundwater system through the degradation of organic matter can be very large; for leachate plumes in low salinity aquifers containing a low percentage of carbonate material, HCO3 − is an excellent indicator of the plume location and relative concentration.
The various populations of microbes responsible for the degradation of organic matter in landfills utilize oxygen, or other oxygen-bearing species in the absence of free oxygen, to convert organic compounds to the simple end-members CO2(aq), CH4 and H2O (Baedecker and Apgar 1984; Andreottola and Cannas 1992; Ehrig 1983). Anaerobic microbial oxidation of organic matter results in the depletion of solid and dissolved oxidants in the aquifer system and accumulation of soluble reduced redox species in groundwater, along with a resultant decrease in the redox potential (measured in the field as Eh) of the groundwater. A comparison of the distribution of oxidised and reduced redox species provides insight into the specific redox processes occurring within the landfill and leachate plume.
It should be noted that while field measurement of Eh values may provide a relative indication of changing redox conditions within a groundwater system, they are not suitable for the determination of the specific redox processes occurring within the system (Baedecker and Cozzarelli 1992; Lindberg and Runnells 1984).
Materials and methods
Borehole geophysical logging
Borehole geophysical investigation techniques were used to provide an additional degree of confidence to the interpretation of groundwater chemistry and site-scale stratigraphy. The geophysical program consisted of borehole bulk electrical conductivity and gamma emission logging. The bulk conductivity logs are used to provide a detailed assessment of the leachate plume location and the gamma logs are intended to aid in the delineation of the subsurface stratigraphy.
The field equipment consists of a Geonics EM39 geophysical logging system, which includes an electrically powered variable speed winch with 100 m of cable. The cable passes over a tripod-mounted pulley system that comes equipped with a shaft encoder to measure the logging speed. The individual sondes used for performing the borehole measurements are attached to the end of the cable and lowered down the borehole at a predetermined speed. The signal from the sonde is recorded using a DOS program on a portable computer, which allows for a real time plot of the data to be generated (McNeill 1990).
The EM-39 sonde used for borehole measurement of bulk electrical conductivity consists of a three-coil induction system. A transmitter coil in the sonde induces eddy currents in the formation surrounding the bore, which in turn generate a magnetic field that is measured by a coaxial receiver coil in the sonde. The intensity of the generated magnetic field is directly proportional to the electrical conductivity of the formation. A more detailed discussion is provided by McNeill (1986).
The gamma logs were generated with the same equipment as the bulk conductivity logs, only using a gamma sonde. The gamma sonde detects and quantifies gamma emissions from the degradation of naturally occurring radioactive isotopes in the aquifer, especially Potassium-40. Potassium is an abundant component of feldspars, which weather to produce clays. Thus, gamma anomalies are often interpreted as increases in clay concentration in the subsurface.
The borehole geophysical logging occurred in April 2001, and fluid EC values measured concurrent to the logging program are provided for comparison to the bulk EC logs. The diameter of the central casing for piezometer 30 is too small to accommodate the geophysical logging equipment, and the geophysical logs for piezometer 10 were measured in 1995. Thus, the logs for piezometers 3 and 4A are provided as the most relevant to this study.
Groundwater sampling and analysis
The data for this investigation were collected from a network of multi-level piezometers: four of these were installed in two parallel flow path transects (30→4A and 10→3) between the edge of the landfill and the shore of Lachlan Pond No. 5, approximately 60m downgradient from the landfill (Fig. 2). Sample points on the multi-level piezometers were positioned at 0.5-m intervals across the vertical section that defines the maximum vertical extent of the leachate plume, and then at 1.0 m intervals for an additional 5–10 m below the plume in the uncontaminated regional groundwater. Piezometers 30 and 4A were sampled in February 2002, following the installation of piezometer 30 in December 2001. The results are compared to the most recent sample data from piezometers 10 and 3, collected in July 2001; it should be noted that variations in plume geometry and contaminant concentrations between the two transects are partially a result of the discordant timing of the sampling events.
The sample points were purged using a Geopump 2 peristaltic pump, and groundwater samples were collected once the general parameters (pH, Eh, dissolved oxygen [DO], electrical conductivity [EC] and temperature) had stabilized. The unstable chemical constituents were analysed in the field immediately upon sample collection to minimize the analyte alteration. An alkalinity titration method was used for the field determination of HCO3 − using 0.01 M HCl as the titrant and a bromocresol green indicator (APHA 1998). Analyte fixation and spectrophotometric analysis methods were used for the field determination of Fe2+, S2−, NO3 −, NH4 + and PO4 3+ concentrations using a HACH DR/2010 spectrophotometer (HACH 2000). One 60-mL sample was filtered using a 0.45-μm Millipore cellulose acetate membrane filter and preserved in the field by acidification using 0.4 mL of concentrated, analytical grade nitric acid for analysis of major and minor elements by inductively coupled plasma atomic emission spectrometry (ICP-AES), and one 120-mL sample was filtered and used for Cl− determination in the laboratory using an argentometirc titration method (APHA 1998).
It should be noted that in environments with elevated concentrations of dissolved organic material, such as landfill leachate, ionically charged organic acids can contribute significantly to the total alkalinity (Kehew and Passero 1990; Baedecker and Cozzarelli 1992). However, the presence of organic acids in significant concentrations in leachate is primarily associated with the early acetogenic phase of waste decomposition, whereas leachate from mature landfills in the methanogenic phase is dominated by refractory, non-polar organic compounds (Chian and DeWalle 1977; Ehrig 1983; Andreottola and Cannas 1992). Taking into consideration the relative age and maturity of Astrolabe Park Landfill, total alkalinity has been calculated as HCO3 − with the recognition that an unidentified minor percentage may be contributed by organic acid anions.
The accuracy and precision of the ICP analyses were assessed by analysis of spiked samples, deionized water blanks, and duplicate sample analyses as per standard laboratory protocols. All spiked samples, blanks and duplicates were within ±10% relative percent difference (RPD), calculated for sample spike recoveries duplicate analyses. Also, the charge balance error for each sample was calculated as the percent difference in cation and anion concentrations (as meq/L); all samples were within the control range of ± 5%.
Duplicate spectrophotometer analyses were performed in the field following suspect results; the instability of the analytes following sample collection, as well as budget constraints, precluded a direct, relevant comparison of the spectrophotometer results with alternative laboratory analysis techniques. However, the strong quality control results, coupled with the correlation in overall data trends observed across the three different analytical methods between six sampling events, suggest that these analytical methods accurately identified spatial and temporal trends in chemical concentrations as they occurred within the leachate plume.
Results and discussion
Borehole geophysical logs
The results of the borehole geophysical logging program for piezometers 3 and 4A are presented in Fig. 3. The plots include the bulk electrical conductivity logs, the gamma logs, and fluid EC values measured concurrent to the geophysical logging for comparison to the bulk EC logs. A clear electrical anomaly is observed in the upper portion of the bulk and fluid EC logs for both piezometers. There is a strong correlation between the bulk and fluid EC profiles at each piezometer, indicating that the variations in bulk EC are primarily caused by presence of the leachate plume in groundwater as opposed to changes in the electrical properties of the aquifer sediments. The difference in the bulk and fluid EC values is explained by the additional electrical resistance of the aquifer sediments that is included in the bulk EC measurements. The electrical anomaly created by the presence of a leachate plume with an elevated solute load within an aquifer otherwise characterised by low conductivity groundwater underscores the value of bulk EC logging for delineation of the plume.
Small peaks in the gamma logs indicate the presence of thin clayey horizons within the sandy aquifer. The peak at approximately 10 m below ground surface (bgs) at piezometer 4A exhibits a strong correlation with the lower boundary of the leachate plume. This provides a strong piece of evidence to suggest that solute transport in the shallow aquifer is constrained by the presence of thin layers of low permeability material present in the sandy aquifer at this location. Several small peaks in the gamma log are also observed at piezometer 3, although there is no clear correlation with the changes in the bulk and fluid EC. However, a clayey horizon was detected at 8 m bgs at this location during a previous site investigation by cone penetrometer testing that correlates well with the lower margin of the leachate plume as defined by the bulk and fluid EC (Jankowski and Knight unpublished data 1991). The gamma logging equipment is most useful for detecting clayey layers that are greater than 1 m thick, and tend to lose sensitivity for thinner layers.
Groundwater chemistry
A statistical summary of the results of groundwater chemical analyses for selected indicator parameters is presented in Table 1. Several indicator parameters have been used to assess the spatial distribution and relative attenuation of inorganic contaminants in groundwater between the Astrolabe Park landfill and Lachlan Pond No. 5, including: EC, HCO3 −, pH, Eh, NH4 +/NO3 −, and S2−/SO4 2−. Vertical profiles of the concentrations of these indicator parameters are plotted graphically along groundwater flow paths according to sample point elevations between piezometers 30 and 4A and piezometers 10 and 3 (Figs. 4 and 5, respectively). These figures will be used in the following discussion to make direct comparisons of the relative concentrations and spatial distribution of the indicator parameters between the landfill and the pond.
EC and HCO3 −
The relationship between EC and HCO3 − for all samples associated with this research project (n=498) is plotted in Fig. 6. This plot indicates that there is an excellent correlation between these two values (r2=0.9645), and that both can be used to characterise the relative degree of leachate impact to regional groundwater.
EC and HCO3 − show significant variations in maximum concentration and spatial distribution between piezometer 30 and 4A (Fig. 4), 50 m downgradient. Maximum EC values decrease from 1,429 to 1,060 μS/cm, a 26% decrease, and the elevation of the peak value dropped by approximately 4.4 m between the piezometers. Likewise, the maximum HCO3 − concentration decreased from 847 to 622 mg/L, a 27% decrease, with a vertical displacement nearly identical to that of the EC.
The decrease in EC and HCO3 − concentrations between these piezometers is most likely due to dilution of the leachate plume from longitudinal and lateral dispersion. The decrease in elevation of the centre of mass may be due to a number of factors, including density differences between the fresh and contaminated water, the presence of preferential flow paths in the subsurface, or depression of the plume from infiltrating precipitation and lawn watering on the golf course between the landfill and the pond.
The observed variation between piezometers 10 and 3 is slightly different. The maximum EC value decreased from 1,650 to 1,381 μS/cm, a decrease of 16%, while the maximum concentration of HCO3 − decreased from 1,008 to 816 mg/L, a decrease of 19%. The elevation of the maximum concentration for both parameters only dropped by approximately 1 m. Assuming that the effects of dispersion and lawn watering infiltration on the leachate plume are similar in the two closely spaced transects, the most likely explanation for the discrepancy in variation between the 30 to 4A and 10 to 3 transects is the timing of the sampling events (February 2002 and July 2001, respectively) relative to rainfall events. Also, a slight increase in the fraction of fine sediment present between piezometers 30 and 4A as compared to 10 and 3 may be contributing to the increased attenuation of the inorganic contaminants.
pH and Eh
Both transects display a similar pattern of increased pH values and decreased Eh values with respect to regional groundwater within the leachate plume, but the individual profiles vary significantly.
The pH of the contaminated groundwater at piezometer 30 (Fig. 4) is tightly constrained between values of 6.4 to 6.7 for approximately 6 m, below which a sharp decrease is observed between an elevation of 8.5 to 7.5 m above sea level (a.s.l.). A similar pattern is observed with Eh values, which rise dramatically within the same interval. The pH values at piezometer 4A (Fig. 4) are also constrained between 6.4 to 6.7 in the contaminated zone, and then gradually returns to background pH values of 5.0–5.5, which are characteristic for the uncontaminated part of the aquifer (Jankowski and Beck 2000), within the 6-m vertical section between 10 to 4 m a.s.l.
Likewise, the Eh profile at piezometer 4A (Fig. 4) displays a much narrower vertical section of highly reducing water with a more gradual transition to fresh water. The variation in the transitional zones may be due to vertical mixing between the two piezometers, although the severity of the transitional zone at piezometer 30 may also indicate the presence of a laterally discontinuous confining layer at an elevation of approximately 8 m a.s.l. The maximum Eh value at piezometer 4A is lower than that of piezometer 30, this discrepancy is probably due to localized variations in the sedimentary composition of the aquifer, resulting in variable rates of microbiological activity.
The pH profiles at piezometers 10 and 3 (Fig. 5) also exhibit pH values constrained between values of 6.0 to 6.5 within the leachate plume, although this zone appears to be approximately 1.5–2 m thicker at piezometer 3, most likely as a function of vertical dispersion of the plume between the two piezometers. The Eh profiles between the two piezometers are slightly erratic, although both display a decrease in Eh values that corresponds to the zone of increased pH values. The variation in the shapes of the profiles is likely attributable to the presence of locally discontinuous peat lenses containing iron sulfides, which act as substrates for SO4 2−-reducing bacteria. This may be responsible for the sharp drops in Eh observed at elevations of 6 and 2 m a.s.l. at piezometer 10 (Jankowski and Acworth 1997). Comparison of the shape of the Eh profiles to the S2−/SO4 2− profiles appears to support this assertion.
NH4 + and NO3 −
The profiles of N-species at all four piezometers clearly display the expected relationship between the redox couples within and below the leachate plume.
Piezometer 30 (Fig. 4) displays a near absence of NO3 − within the leachate plume, but then gradually increases with depth below 9 m a.s.l. NH4 + concentrations exhibit the opposite trend, with the highest concentrations observed within the plume, decreasing along a steep vertical gradient between the elevations of 10 to 8 m a.s.l. A similar relationship is observed for piezometer 4A, although the maximum concentration of NH4 + decreases from 35 to 24 mg/L, a decrease of 30%. Likewise, the zone of maximum NH4 + concentration drops from 12–11 to 10–9 m a.s.l., and appears to narrow vertically by approximately 2 m. Also, a spike of high NO3 − concentration is observed at piezometer 4A at an elevation of 12 m a.s.l. This is likely due to ammonium oxidation along the edge of the plume as it reacts with oxygen-enriched recharge water, and perhaps by the application of nitrogen-based fertilizers on the golf course.
A much narrower vertical section of elevated NH4 + concentrations is observed in piezometers 10 and 3 (Fig. 5), although the maximum concentration values are nearly the same as that of piezometer 30. Surprisingly, the maximum concentration of NH4 + actually increases between piezometers 10 and 3, from 40 to 43 mg/L, an increase of 8%. There was no observed change in the elevation range of the maximum NH4 + concentration. Also, a spike of NO3 − is observed at the top of the profile at piezometer 3; the most likely source of this additional nitrogen is from fertilizers used by the golf course. The high NO3 − concentrations at the water table at piezometer 3 may be contributing to the increased NH4 + concentration within the leachate plume.
It seems likely that any potential contribution of nitrogen to groundwater from nitrogen-based fertilizers used by the golf course would be similar for the two transects as they are relatively close to each other, similar in length and cross the same portion of the golf course. By this assumption, the discrepancy between the observed NH4 + attenuation, or lack thereof, for the two transects may be due to a significantly increased percentage of fine-grained silt, clay and peat material present in the upper portion of the aquifer between piezometers 30 and 4A that is not present between piezometers 10 and 3. This may cause some attenuation of the NH4 + in groundwater due to ion exchange on the clay surfaces.
S2− and SO4 2−
The SO4 2− concentrations in all profiles display similar patterns of decreased concentrations within the leachate plume and elevated concentrations above and below the leachate plume. The increase in SO4 2− concentrations below the plume is relatively sharp (10–30 mg/L increase in the first 2 m below the plume) for all piezometers except for piezometer 3. The Eh values and the NO3 − and SO4 2− concentrations all recover much more slowly with depth beneath the plume at piezometer 3 than is observed in the other piezometers. It is possible that the presence of lenses of organic material provide an additional substrate for microbial activity, which preserves the reducing environment to a greater depth than is observed in the other piezometers.
It is interesting to note the elevated SO4 2− concentration immediately above the plume at piezometer 3. Research by Ulrich and others (2003) at Norman Landfill in Oklahoma also observed this phenomenon, and suggested that it was due to microbial oxidation of iron sulfides in response to fluctuations in the water table. The water table at piezometer 3 is strongly controlled by the water level of Lachlan Pond No.5, which is subject to rapid fluctuations in response to storm events as it receives a significant volume of stormwater discharge from local drainage networks. During storm events, the groundwater flow direction has been observed to temporarily reverse due to elevated water levels in the pond, causing pond water to infiltrate into the aquifer (Acworth personal communication 2003). The infiltration of oxygen-enriched pond water into the normally reducing zone of the leachate plume at piezometer 3 may instigate a brief period of aerobic oxidation of iron sulfides, thus generating excessive SO4 2− concentrations. The groundwater sampling at piezometer 3 occurred immediately after 10 days of excessive rainfall.
Sulfide is absent within the zone of greatest leachate contamination as defined by the EC, HCO3 − and NH4 + profiles. As SO4 2− concentrations appear to be reduced within the plume, the expected result would be a concomitant increase in S2−. The most plausible explanation for the absence of S2− in the plume is that it is rapidly precipitating as FeS in the presence of elevated concentrations of Fe2+ in the leachate plume (Postma and Jakobsen 1996).
The highest S2− concentrations in all profiles occur immediately below the leachate plume (Jankowski and Acworth 1997). This zone also happens to correspond to a relatively continuous layer of pyrite-bearing peat material present in the investigation area identified in previous research. In contrast to the EC, HCO3 − and NH4 + concentrations for piezometers 30 and 4A (Fig. 4), which appear to decrease in concentration and elevation as they are transported downgradient from the landfill, the S2− concentration increases downgradient from the landfill and does not display any vertical displacement. A similar trend is also present between piezometers 10 and 3 (Fig. 5). Further, the peaks in S2− concentration occur at nearly the same elevation in all of the piezometers. This combined evidence suggests that the pyrite-bearing peat layer is the most likely source of the increased S2− concentrations observed in the profiles (Jankowski and Beck 2000). The excess acidity generated during the oxidation of these pyritic aquifer sediments is sufficient to overcome the pH buffering capacity of the leachate plume, and is likely responsible for the abrupt decrease in pH values observed at the same depth interval.
Conclusions
In general, a decrease in the maximum value/concentration of EC and HCO3 − was observed between the Astrolabe Park landfill and Lachlan Pond No. 5, as well as a decrease in the elevation at which the maxima were detected, although this was most pronounced in the piezometer 30 to 4A transect. As the groundwater flows away from the landfill, and thus away from the source of the HCO3 − production, it is assumed that EC values and HCO3 − concentrations decrease as a result of dispersion along the flow path and mixing with fresh regional groundwater. The relative differences in the concentration decreases is likely due to differences in precipitation at the time of the two sampling events.
The pH profiles for both transects display well buffered values within the leachate plume (Kehew and Passero 1990; Jankowski and Acworth 1997), which recover to background values below the plume. Once again, there is a greater observable change in the thickness of the buffered zone along the 30 to 4A transect than along the 10 to 3 transect, most likely attributable to different weather patterns due to the discordant timing of the sampling events. While generally displaying depressed values within the leachate plume, the Eh profiles varied significantly between each piezometer. Local variations in the sedimentary composition of the aquifer appear to have a significant impact on the variability of redox potentials with depth at each piezometer, which makes Eh values a difficult parameter from which to draw any significant conclusions regarding the specific effects of transport on leachate contaminants.
The profile for each piezometer displayed the expected relationship between NH4 + and NO3 − within and below the leachate plume. The 30 to 4A transect showed a significant decrease in the maximum concentration of NH4 + along the flow path, most likely due to a combination of dispersion/dilution and attenuation of NH4 + on exchange site surfaces within the geological material of the aquifer. Unexpectedly, the maximum NH4 + concentration was increased between piezometers 10 and 3. The only possible cause for this occurrence is an additional influx of nitrogen along the flow path. The extremely high NO3 − concentration immediately above the plume in piezometer 3 suggests that nitrogen may be flushed into the groundwater system from nitrogen-based fertilizers used on the golf course; and nitrate-reducing bacteria may have reduced a portion of the NO3 − to NH4 +, accounting for the observed increase in concentration along the flow path. Once again, the difference in the timing of the sampling events may explain why this phenomena was not observed along the 30 to 4A transect.
The SO4 2− concentrations followed a similar pattern to NO3 −: decreased concentrations within the leachate plume with an observed recovery below the plume. The expected concomitant increase in S2− concentrations within the plume was not observed; any S2− produced within the plume as a result of SO4 2− reduction is most likely precipitated as FeS in the Fe2+-enriched plume (Postma and Jakobsen 1996). Elevated SO4 2− concentrations at piezometer 3 are likely due to aerobic oxidation of iron sulfides in response to water table fluctuations following a significant storm event (Ulrich and others 2003).
In contrast, the highest concentrations of S2− were observed immediately below the plume, where previous research has identified a locally extensive, pyrite-bearing peat layer (Jankowski and Acworth 1997). The fact that the elevated S2− concentrations occurred at approximately the same elevation in each profile, and the maximum concentrations increased along the flow path, suggests that the S2− is produced naturally within the aquifer as a by-product of microbial degradation occurring within the peaty horizon where oxidation of FeS2 followed by subsequent reduction of SO4 2− is occurring (Jankowski and Beck 2000). The excess acidity generated during the oxidation of the pyritic aquifer sediments may be responsible for the abrupt decrease in pH values observed at the same depth intervals.
While dispersion, dilution and sorption processes along groundwater flow paths appear to be responsible for the decrease in concentrations of some components of the leachate plume, the influence of precipitation needs further investigation to determine whether the relative variations observed between the transects are indeed due to weather variations as a result of the discordant timing of the sampling events. Also, the relative nitrogen contribution from fertilizer along the flow path use needs to be investigated to explain the unexpected increase in NH4 + concentration between piezometers 10 and 3.
References
Albani AD (1981) Sedimentary environments and Pleistocene chronology of the Botany Basin, NSW. Geo-Mar Lett 1:163–167
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA-AWWA-WET Washington, DC
Andreottola G, Cannas P (1992) Chemical and biological characteristics of landfill leachate. In: Christensen TH, Cossu R, Stegmann R (eds) Landfilling of waste: leachate. E & FN Spon, London, pp 65–88
Arneth J-D, Milde G, Kerndorff H, Schleyer R (1989) Waste deposit influences on groundwater quality as a tool for waste type and site selection for final storage quality. In: Baccini P (ed) The landfill, vol 20, (Lecture Notes in Earth Sciences). Springer, Berlin Heidelberg New York, pp 399–424
Baedecker MJ, Apgar MA (1984) Hydrogeochemical studies at a landfill in Delaware. In: Groundwater contamination, studies in geophysics. National Academy Press, Washington, DC, pp 127–138
Baedecker MJ, Back W (1979) Hydrogeological processes and chemical reactions at a landfill. Ground Water 17(5):429–437
Baedecker MJ, Cozzarelli IM (1992) The determination and fate of unstable constituents of contaminated groundwater. In: Lesage S, Jackson RE (eds) Groundwater contamination and analysis at hazardous waste sites. Marcel Dekker, New York, pp 425–461
Bjerg PL, Rugge K, Pederson JK, Christensen TH (1995) Distribution of redox-sensitive groundwater quality parameters downgradient of a landfill (Grinsted, Denmark). Environ Sci Technol 29:1387–1394
Chian ESK, DeWalle FB (1977) Characterization of soluble organic matter in leachate. Environ Sci Technol 11(2):158–163
Christensen TH (1992) Attenuation of leachate pollutants in groundwater. In: Christensen TH, Cossu R, Stegmann R (eds) Landfilling of waste: leachate, E & FN Spon, London, pp 441–483
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen H-J, Heron G (2001) Biogeochemistry of landfill leachate plumes. Appl Geochem 16:659–718
Cozzarelli IM, Suflita JM, Ulrich GA, Harris SH, Scholl MA, Schlottmann JL, Jaeschke JB (1999) Biogeochemical processes in a contaminant plume downgradient from a landfill, Norman, Oklahoma. U.S. Geological Survey Water Resources Investigations Report # 99–4018C, Reston, VA
Ehrig H-J (1983) Quality and quantity of sanitary landfill leachate. Waste Manage Res 1:53–68
Griffin RJ (1963) The Botany Basin. Geol Surv New South Wales Bull 18, 110 pp
HACH (2000) DR/2010 Spectrophotometer handbook – Procedures manual. Hach Company, Loveland, Colorado, 872 pp
Jankowski J (1997) Generation and migration of chemical constituents in a leachate plume. In: McNally GH (ed) Collected case studies in engineering geology, hydrogeology, and environmental geology, 3rd series, Geol Soc Aust pp 181–205
Jankowski J, Acworth RI (1997) Development of a contaminant plume from a municipal landfill: Redox reactions and plume variability. In: Chilton J, Hiscock K, Morris B, Nash H, Tellam J, Hennings S (eds) Proceedings of the XXVII Congress of the International Association of Hydrogeologists: Groundwater in the Urban Environment, Nottingham, UK, 21–27 September 1997. Balkema, Rotterdam, pp 439–444
Jankowski J, Beck P (2000) Aquifer heterogeneity: hydrogeological and hydrochemical properties of the Botany Sands aquifer and their impact on contaminant transport. Aust J Earth Sci 47:45–64
Kehew AE, Passero RN (1990) pH and redox buffering mechanisms in a glacial drift aquifer contaminated by landfill leachate. Ground Water 28(5):728–737
Kimmel GE, Braids OC (1974) Leachate plumes in a highly permeable aquifer. Ground Water 12(6):388–392
Kjeldsen P (1993) Groundwater pollution source characterisation of an old landfill. J Hydrol 142:349–371
Lindberg RD, Runnells DD (1984) Ground water redox reactions: an analysis of equilibrium state applied to Eh measurements and geochemical modeling. Science 225:925–927
Ludvigsen L, Albrechtsen H-J, Heron G, Bjerg PL, Christensen TH (1998) Anaerobic microbial redox processes in a landfill leachate contaminated aquifer (Grinsted, Denmark). J Contam Hydrol 33:273–291
Lyngkilde J, Christensen TH (1992) Redox zones of a landfill leachate pollution plume (Vejen, Denmark). J Contam Hydrol 10:273–289
McNeill JD (1986) Geonics EM39 borehole conductivity meter—theory of operation. Geonics Limited, Technical note TN-20
McNeill JD (1990) Use of electromagnetic methods for groundwater studies. In: Ward SH (ed) Geotechnical and environmental geophysics, vol 1: review and tutorial. Soc Explor Geophys Tulsa, Oklahoma, pp 191–218
Nicholson RV, Cherry JA, Reardon EJ (1983) Migration of contaminants in groundwater at a landfill: a case study. 6. Hydrogeochemistry. J Hydrol 63:131–176
Postma D, Jakobsen R (1996) Redox zonation: equilibrium constraints on the Fe(III)/SO4-reduction interface. Geochim Cosmochim Acta 60(17):3169–3175
Qasim SR, Chiang W (1994) Sanitary landfill leachate: generation, control and treatment. Technomic Publishing Company, Lancaster, Pennsylvania 323 pp
Rickwood PC (1998) The Botany Basin: a convenient definition. In: McNally GH, Jankowski J (eds) Environmental geology of the Botany Basin, collected case studies in engineering geology, hydrogeology and environmental geology, 4th series. Geol Soc Aust pp 173–178
Ulrich GA, Breit GN, Cozzarelli IM, Suflita JM (2003) Sources of sulfate supporting anaerobic metabolism in a contaminated aquifer. Environ Sci Technol 37:1093–1099
Acknowledgements
This research was made possible through the financial assistance of Sydney Water. The authors thank Karina Morgan, Bethany O’Shea, Sarah Groves and Wendy McLean for their assistance during sample collection and Dorothy Yu for her help with chemical analysis in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jorstad, L.B., Jankowski, J. & Acworth, R.I. Analysis of the distribution of inorganic constituents in a landfill leachate-contaminated aquifer: Astrolabe Park, Sydney, Australia. Env Geol 46, 263–272 (2004). https://doi.org/10.1007/s00254-004-0978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-004-0978-3