Abstract
Panvel Basin of Raigarh district, Maharashtra, India is the study area for groundwater quality mapping using the Geographic Information System (GIS). The study area is typically covered by Deccan basaltic rock types of Cretaceous to Eocene age. Though the basin receives heavy rainfall, it frequently faces water scarcity problems as well as water quality problems in some specific areas. Hence, a GIS based groundwater quality mapping has been carried out in the region with the help of data generated from chemical analysis of water samples collected from the basin. Groundwater samples show quality exceedence in terms of chloride, hardness, TDS and salinity. These parameters indicate the level of quality of groundwater for drinking and irrigation purposes. Idrisi 32 GIS software was used for generation of various thematic maps and for spatial analysis and integration to produce the final groundwater quality map. The groundwater quality map shows fragments pictorially representing groundwater zones that are desirable and undesirable for drinking and irrigation purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
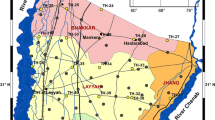
Water quality analysis is one of the more important issues in groundwater studies. The hydrogeochemical study reveals the zones and quality of water that are suitable for drinking, agricultural and industrial purposes. Further, it is possible to understand the change in quality due to rock water interaction or any type of anthropogenic influence. Groundwater often consists of seven major chemical elements- Ca+2, Mg+2, Na+1, K+1, Cl−1, HCO3 −1 and SO4 −2. Hence, hydrogeochemical studies can be conducted by analyzing water samples based on these components. The study area for the present work is the Panvel Basin due north of the Raigarh district of Maharashtra (Fig. 1). In the present study, post- monsoon water samples were collected (December 2001 and January 2002) from 19 locations. Pre-monsoon (May 2002) water samples were collected from 25 locations. The water quality of dug well and bore well samples for both seasons were compared to determine any changes in the concentration of various constituents due to evaporation and precipitation. The physical parameters taken into consideration are color, odor, turbidity and temperature. The chemical parameters taken into consideration are hydrogen ion concentration, specific conductance, Total Dissolved Solids (TDS), total hardness and all major cations and anions. The purpose of the study is to understand the groundwater quality in the basin and pictorially represent it using the Geographic Information System (GIS). GIS is an effective tool for storing large volumes of data that can be correlated spatially and retrieved for the spatial analysis and integration to produce the desirable output. GIS has been used by scientists of various disciplines for spatial queries, analysis and integration for the last three decades (Burrough and McDonnell 1998). A number of studies were conducted to determine potential sites for groundwater exploration in diverse geological set ups using remote sensing and GIS techniques (Krishnamurthy and Srinivas 1995; Kamaraju 1997; Srivastava and others 1997; Srivastava and Battacharya 2000). Most of the groundwater studies based on GIS are concentrated on the preparation of hydrogeomorphological maps, interpretation of lineaments and integrated terrain analysis. Several hydrogeochemical studies have been conducted in the surrounding areas. Lekha Siraz (1996, unpublished PhD thesis) has demarcated fresh groundwater potential zones and salt water intrusion along Vasai-Palghar coastal tracts of Western Deccan Volcanic province, India. She found that the majority of the coastal groundwater samples are of Ca2+ - Mg2+ - Cl− - SO4 2− type. The Groundwater Survey and Development Agency (GSDA 1991 unpublished report) has carried out chemical analysis of groundwater samples in the Panvel Basin both from bore wells and dug wells for three seasons: October 1990, February 1991 and May 1991. Lunkard and Raymahashay (1986) studied the water chemistry of the basaltic aquifers in India. They concluded that groundwater from basaltic aquifers are essentially of Mg2+- HCO3 type. Locsey and Cox (2003) arrived at the same conclusion that groundwater in the basaltic region is mostly of Mg-Ca-Na- HCO3 type.
Panvel Basin is occupied by the Deccan basaltic rocks of Cretaceous to Eocene age. The area falls within the Bushe Formation of Lonavala Subgroup (Subbarao 1988). The geology map available with the GSDA report is used as the basis for this study. The map was updated and new lithological contacts were interpreted through digitally processed IRS IC LISS III satellite data. The various lithological boundaries were interpreted from False Color Composite (FCC) and Principle Component Analysis (PCA) of satellite data. Das and Mukherjee (2002) have used standard FCC bands of 2, 3 and 4 from IRS 1B LISS II data to delineate various basaltic units for the Konkan coastal tract. The same procedure was adopted for the present study with the help of Principal Component Analysis. The geological succession of the area given in the GSDA report (GSDA 1991 unpublished report) has been used as a reference. There are two units identified within the Deccan traps. The upper unit is of amygdaloidal and vesicular type while the lower unit is massive trap rock. In the upper unit vesicles are spherical in shape and are not interconnected. The lower unit is feebly jointed and fractured and moderately weathered. There is no marker separating these two units. Amygdaloidal basalt, massive basalt and weathered basalt were delineated using Principal Component 1 (Fig. 2). Field data has been used for cross checking the geology map generated with the help of digitally processed output.
Massive basalts occur as pockets in the plains and sometimes grade to amygdaloidal basalt type. Most of the hills and ridges are of the massive basalt type. Massive basalt is exposed in very few areas such as road cuttings, along riverbeds and well sections and is very fine grained, compact, dark gray in color with negligible amounts of amygdales. Spheroidal weathering is commonly observed in this type of basalt. Massive basalts are resistant to weathering; hence, it remains as such while its surroundings are reduced to plains. This results in the formation of residual hills and ridges. Massive basalt gives less reflectance than the amygdaloidal type and appears dark in the PCA image. Massive basalts are mostly exposed in the eastern part of the basin.
In the study area, hilltops in the eastern region are covered with thick vegetation. This is due to thick soil cover formed by in-situ weathering of basalts. These areas are seen as dark red patches in the FCC. These areas can be differentiated in the PCA image due to the change in reflectance pattern. The amygdaloidal basalt is light to dark brown in color and fine-grained in the hand specimen. The size of the amygdales varies and they are filled with secondary minerals like zeolite, calcite and cryptocrystalline silica. This is the major rock type found in the study area and is exposed in many wells. Zeolites are seen in macroscopic as well as in microscopic study. Some open vesicles were also found, which are spherical, elliptical, pipe or irregular in shape. In many places the rock was highly weathered to the extent that the grains have become loose. The cavities filled with secondary minerals like zeolite give amygdaloidal basalt a spotted appearance in the FCC and a brighter reflectance in the PCA image. Amygdaloidal basalts are exposed in many locations either in a fresh or weathered state. It has also been exposed towards the western central part of the basin. This is in agreement with the lithology delineated from digitally processed satellite data.
Weathered basalts are either massive or amygdaloidal and have undergone weathering by different physical and chemical agents. These will have a yellowish white appearance in the FCC and can be easily distinguished in the PCA image where it shows a yellowish color. A majority of the plains in the Panvel Basin are comprised of weathered basalts of amygdaloidal or massive type.
In the study area, there are several dykes of basaltic nature with trends mostly in a N-S direction. Along Bombay-Pune National Highway, a basaltic dyke has been encountered in a road cutting. This dyke has been determined from the satellite image as a lineament extending several kilometers. The basaltic dyke can be distinguished from the country rock due to the presence of biscuit-type joints that are a conspicuous feature. Recent sediments of lacustrine-type mud deposits ranging in thickness from 5 to 10 m are deposited along the Panvel creek and in between the inter-stream areas. A fresh water alluvial deposit also occurs along the Panvel creek.
Methodology
The study is carried out with the help of four major components: input from remote sensing data, topographic sheets, data available from GSDA and data collected during field visits (Fig. 3). The IRS IC satellite data of 1996 acquired from the National Remote Sensing Agency for the Panvel Basin was used for the generation of various digitally processed outputs like FCC and PCA. From these outputs the original geology map was updated. Field visits were carried out in the months of December, 2001 to January, 2002 for the post-monsoon period and during May, 2002 for the pre-monsoon period. The fieldwork included water level measurements, well inventory and collection of water samples from dug wells and bore wells and the study of geological and geomorphological features. Observation well data, rainfall data and litholog data for bore holes as well as geological and hydrogeological data were collected from GSDA, Belapur. Laboratory work included petrographic studies of a few samples collected during field visits and chemical analysis for major element constituents in groundwater samples. Followed by water quality analysis, thematic maps were generated and digitized using Idrisi 32 GIS software developed by Clark Labs (Idrisi 32 Release2 2001). Using Idrisi GIS software, spatial analysis and integration were carried out for drinking and irrigation water quality mapping in the basin.
Chemical analysis
In the study area, the monsoon continues for quiet a long duration. It starts in June and continues up to the month of October. Post-monsoon water samples were collected from 19 locations during the period of December 2001 and January 2002. Pre-monsoon water samples were collected at 25 locations during the month of May 2002 (Fig. 4). Chemical analyses were carried out for the major ion concentrations of the water samples collected from different locations in the basin. The analytical data can be used for the classification of water for utilitarian purposes, solving problems of saline water intrusion, or ascertaining various factors on which the chemical characteristics of waters depend. For greater interpretability, the analytical data are represented in Piper–Trilinear plots. Maximum and minimum concentrations of major ions for groundwater samples collected in both seasons are given in Table 1. The analytical data were used for the spatial analysis and integration for groundwater quality mapping.
The groundwater samples collected from the Panvel Basin are colorless, odorless and free from turbidity. Measurement of temperature and pH was done during the field visits. The temperature measured for groundwater samples is found to be constant at 27 °C during post-monsoon field visits (December 2001 and January 2002) and 30 °C during pre-monsoon field visits (May 2002). Water to be used for drinking purposes should be free from color, turbidity, odor and microorganisms. However, these do not fall in the realm of chemical quality. Chemically, the water should be soft, low in dissolved solids and free from poisonous constituents.
The Piper diagram is extensively used to understand problems concerning the geochemical evolution of groundwater. The diagram consists of three distinct fields – two triangular fields and a diamond shaped field. The percentage equivalents per mole (epm) values are used for the plot (Todd 1980). The overall characteristic of the water is represented in the diamond- shaped field by projecting the position of the plots in the triangular fields. Different types of groundwater can be distinguished by their plotting position, occupying certain sub-areas of the diamond-shaped field. Piper–Trilinear plots were made for the samples collected during pre-monsoon and post-monsoon field visits. Aquachem software is used for the plotting of piper-trilinear diagrams (Scientific Software Group, Utah 1998). From the plots, it can be found that calcium and magnesium ions are the dominant cations. Most of the points fall in the field in which strong acids exceed weak acids. Compared to the post-monsoon water sample plots, pre-monsoon plots show higher concentrations of chloride and sulfate ions along with calcium and magnesium ions (Fig. 5a, b). This can be explained in terms of the concentration process, which is dominant during the pre-monsoon period when evaporation exceeds precipitation.
The GSDA, in Alibagh has carried out chemical analyses of groundwater samples in the Panvel Basin from bore wells and dug wells for three seasons: October 1990, February 1991 and May 1991. These chemical data have been used to plot Piper-Trilinear diagrams. For all three seasons, the dug well samples when compared with bore well samples have lower chloride concentration. Dug well samples show a calcium and magnesium ions dominancy and weak acid exceeds strong acid. The dominancy of calcium and magnesium ions is attributed to the basaltic lithology of the basin.
Spatial analysis for groundwater quality
Groundwater quality gives a clear picture about the usability of the water for different purposes. There are specific standards for quality of water for different purposes. Drinking water should satisfy many quality criteria, as it is the most sensitive among various uses. The standard quality for drinking water has been specified by the World Health Organization (WHO 1971). It has given the permissible and desirable limits for the presence of various elements in groundwater (Table 2). Among various elements, from the analysis carried out in Panvel Basin, it has been revealed that the chloride concentration, TDS and hardness exceed the desirable limit at many locations. Pre-monsoon groundwater samples show maximum deviation, as this is the time when precipitation is less so that the concentration of ions will increase considerably. Electrical conductivity, which is a measure of salinity also shows variation and falls in three different classes—low, medium and high salinity hazard classes. Overlay analysis was performed for generating a quality map where the various quality maps were overlaid one above the other (Eastman 1995). It is based on Boolean logic where only two values are assigned, i.e. ‘1 ‘and ‘0’ (Burrough and McDonnell 1998). In this context ‘1’ stands for desirable and ‘0’ for undesirable water quality.
GIS and groundwater quality mapping for drinking water
The quality of the groundwater samples has been analyzed separately for drinking and irrigation purposes. As there is no major industry in the study area, quality analysis for industry is not significant. Groundwater quality for drinking water purposes was analyzed by considering the WHO (1971) standards. It has been found that some samples show chloride, hardness and TDS values above desirable limits. The values were plotted in the respective sample locations and contours were generated using the simple method of triangulation and interpolation techniques. Water quality maps were generated for chloride, TDS and hardness in the study area showing areas falling under desirable limits and areas falling under undesirable limits. A salinity hazard map was also prepared after generating contours. The salinity hazard map shows regions with low, medium and high salinity hazards. Though groundwater samples were not taken from mud flat areas, the area was classified as a region of undesirable quality since from knowledge it is known that this area falls within the creek region and has a higher concentration of chloride, hardness and TDS (Reddy 2002, unpublished M.Tech Thesis).
Chloride concentration
The chloride ion is the most predominant natural form of the element chlorine and is extremely stable in water. The range of chloride is found to vary between 32 to 227 mg/l for post-monsoon water samples and 37 to 390 mg/l for pre-monsoon samples. As per WHO (1971) and Indian standards (ISI 1983) the desirable limit for chloride is 250 mg/l. For the study area it has been found that in certain locations the chloride concentration exceeds this limit for pre-monsoon samples. Chloride concentration at different locations was plotted and using the triangulation method and values were interpolated to generate contours. The contour map was digitized and imported into the GIS environment as a parameter for quality analysis. Areas with chloride concentrations above the desirable limit were delineated and differentiated from areas having values below the desirable limit (Fig. 6). Ranking was assigned for these classes. A rank of ‘10’ was assigned for areas having chloride values within the desirable limit and a rank ‘1’ for areas having chloride concentration above the desirable limit.
Total dissolved solids
Total dissolved solids (TDS) denote the various types of minerals present in water in the dissolved form. In natural waters, dissolved solids are composed of mainly carbonates, bicarbonates, chlorides, sulfate, phosphate, silica, calcium, magnesium, sodium and potassium. Concentrations of TDS are an important parameter in drinking water and other water quality standards. Caroll (1962) has proposed four classes of water based on TDS values (Table 3). In a majority of the water samples TDS in the study area falls within the fresh water class of Caroll’s classification. However, in some locations the pre-monsoon values are above the desirable limit of WHO (1971) standards. Hence, the study area was delineated into two classes: desirable and undesirable. A rank of ‘10’ was assigned for the desirable class and a rank of ‘1’for the undesirable class (Fig. 7).
Hardness
Water hardness is caused primarily by the presence of cations such as calcium and magnesium and anions such as carbonate, bicarbonate, chloride and sulfate in water. Water hardness has no known adverse effects; however, some evidence indicates its role in heart disease (Schroeder 1960). Hard water is unsuitable for domestic use. In the Panvel Basin, the total hardness varies between 100 to 440 ppm for the post-monsoon period. For the pre-monsoon period, the value varies from 155 to 835 ppm. According to Sawyer and McCartly’s (1967) classification for hardness, 3 samples fall under the moderately hard class and 16 samples fall under the hard class for post-monsoon water samples. The hardness classification is given in Table 4.
The hardness values for the study area are found to be high for almost all locations for pre-monsoon and post-monsoon water samples and determined to fall above the desirable limit of WHO’s specification (WHO 1971). Hence, the Indian standard given by Indian Standard Institution (ISI 1983) is taken into consideration for this parameter where the desirable limit is higher. The desirable limit for WHO is 100 mg/l (WHO 1971) while the Indian standard is 300 mg/l (ISI 1983). Contours were generated using the same procedure as for chloride to delineate areas of desirable hardness value from areas with the undesirable hardness value (Fig. 8). A rank of ‘10’ is assigned for areas with desirable hardness and ‘1’ for areas with undesirable hardness values.
Spatial integration and quality mapping
The three thematic maps with parameters such as chloride concentration, hardness and TDS having desirable and undesirable classes were integrated using the Boolean logic of the overlay module available in Idrisi32 GIS software. The function performed is ‘addition’ (Eastman 1995). The final output is a pictorial representation of groundwater quality for drinking purposes in the Panvel Basin (Fig. 9). Nearly two thirds of the area is covered by groundwater with a decent quality. About one third of the basin falls in the undesirable category. The zones of the undesirable category are located towards the northern and southern parts of the basin.
Groundwater quality analysis for irrigation
Water quality, soil types and cropping practices play an important role in irrigation. Agriculture is found to be one of the major land-use practices in the study area. Most of the cultivated lands in the Panvel Basin are irrigated by groundwater either from dug wells or bore wells. Therefore, it is necessary to perform the analysis of chemical quality of groundwater for irrigation purposes. The important chemical constituents that affect the suitability of water for irrigation are the total concentration of soluble salts, relative proportion of bicarbonate to calcium and magnesium and relative proportion of sodium to calcium. Water quality problems in irrigation include salinity and alkalinity. Excessive sodium content in water makes it unsuitable for irrigation purposes. In addition to this, the quantity of bicarbonate and carbonate in excess of alkaline earth elements also influences the suitability of water for irrigation purposes (Table 5). This excess is denoted by Residual Sodium Carbonate (RSC) and is determined by the formula given below (Karanth 1987).
The sodium hazard in irrigation water is expressed by the Sodium Absorption Ratio (SAR) which is given by the relation (Karanth 1987)
in which concentrations are expressed in equivalent per mole values. Low sodium water (S1) can be used for irrigation on almost all soils. As both pre-monsoon and post-monsoon groundwater samples fall in the low sodium (S1) category, there is no hazard of alkalinity (Richards 1954, Table 6).
Salinity hazard
Most of the groundwater samples fall in the medium salinity hazard (C2) as per the salinity hazard classification in the basin. One sample falls in the low salinity hazard category (C1) while a few of the samples fall in the high salinity hazard category (C3). Groundwater samples that fall in the low salinity hazard class (C1) can be used for irrigation of most crops and the majority of soils. However, some leaching is required, but this occurs under normal irrigation practices except in soils of extremely low permeability. Groundwater that falls in the medium salinity hazard class (C2) can be used if a moderate amount of leaching occurs. Plants with moderate salt tolerance can be grown in most cases without any special practices for salinity control. Hence, no special measures are required in the study area for salinity hazards. However, a few samples fall in the high salinity hazard class (C3) (Table 7). High salinity water (C3) cannot be used on soils with restricted drainage. Even with adequate drainage, special management for salinity control may be required and plants with good salt tolerance should be selected. Such areas need special attention as far as irrigation is concerned.
The quality of water for irrigation purposes depends on the salinity and alkalinity. The EC value for the samples will give the salinity value for the area. The pre-monsoon water samples from the study area fall in three classes of salinity hazards: excellent, good and doubtful. Contours were constructed and the entire basin was divided into these three classes. Thus, three classes of polygons were digitized. The salinity hazard map shows that the majority of the basin area falls in ‘good’ category (Fig. 10). Similar to the drinking water quality map, the doubtful category falls towards the northern and southern parts of the basin.
Integrated quality mapping
The spatial integration for quality mapping was carried out using overlay analysis in Idrisi32 GIS software. Groundwater quality for irrigation can be known from the salinity map. The groundwater quality map for drinking purposes was integrated with the groundwater quality map for irrigation. Integrating groundwater quality for drinking purposes and the salinity map can pictorially represent groundwater zones favorable for drinking purposes, irrigation purposes, zones for both drinking and irrigation purposes and zones not favorable for either drinking or irrigation purposes (Fig. 11). Prioritization of zones on the basis of quality for drinking and irrigation can be used for the planning and preservation of groundwater resources.
Conclusions
In the present study, the GIS technique has successfully demonstrated its capability in groundwater quality mapping of the Panvel Basin. The final output has given the pictorial representation of groundwater quality suitable or unsuitable for drinking and irrigation purposes in the basin. From the hydrogeochemical analysis, it is inferred that the excess concentration of chloride, TDS and hardness at some locations has determined an undesirable quality for drinking purposes. Similarly, considerable areas in the basin are having high salinity hazards. Such zones require special care and an alternative salt tolerance-cropping pattern. The reasons for excess concentration of various elements and salinity levels require further detailed investigation.
References
Burrough PA, McDonnell RA (1998) Principles of Geographical Information Systems. Oxford University Press, Oxford, 333 pp
Caroll D (1962) Rain water as a chemical agent of geological processes- a review. USGS Water Supply 1533:18–20
Das AK, Mukherjee S (2002) Study of weathering and erosional status of basaltic rocks using remote sensing data. J Indian Soc Remote Sens 30(3):149–156
Eastman JR (1995) Idrisi for Windows- A users guide. Version1.0, Clark Labs, Worcester, MA, 120 pp
Indian Standards Institution (1983) Indian Standard Specification for drinking water, IS 10500
Kamaraju MVV (1997) Ground-water potential evaluation of West Godavari district, Andhra Pradesh State, India- A GIS approach. Ground Water 34(2):318–334
Karanth KR (1987) Groundwater assessment, development and management. Tate McGraw Hill, New Delhi, 720 pp
Krishnamurthy J, Srinivas G (1995) Role of geological and geomorphological factors in ground water exploration: a study using IRS LISS data. Int J Remote Sens 16(4):2595–2618
Locsey KL, Cox EM (2003) Statistical and hydrochemical methods to compare basalt and basement rock hosted groundwaters: Atherton Tablelands, northeastern Australia. Environ Geol 43:698–713
Lunkard SK, Raymahashay BC (1986) Groundwater quality in weathered Deccan basalt of Malwa Plateau, India, Q J Engg Geol 19:389–398
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agric Handbook 60, US Dept Agric, Washington, DC, 160 pp
Sawyer GN, McCartly DL (1967) Chemistry of sanitary Engineers, 2nd Ed. McGraw Hill, New York, 518 pp
Schroeder HA (1960) Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. J Chron Disease 12:586–591
Srivastava PK, Bhattacharya AK (2000) Delineation of groundwater potential zones in a hard rock terrain of Bargarh district, Orissa using IRS Data. Photonirvachak- J Indian Soc Remote Sens 28(2):129–140
Srivastava A, Tripathi NK, Gokhale GK (1997) Basement topography and aquifer geometry around Ken Garden, India. Int J Remote Sens 20(11):2295–2305
Subbarao KV (1988) Deccan flood basalts. Memoir 10, Geol Soc India, Bangalore, 393 pp
Todd DK (1980) Groundwater hydrology. Wiley, New York, 535 pp
World Health Organization (1971) International Standards for Drinking Water, Geneva
Acknowledgements
The authors would like to acknowledge the Groundwater Survey and Development agency (GSDA), in Belapur, in general and Dr. P. Aurangbadker, Deputy Director, GSDA, Belapur in particular for providing the necessary data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anbazhagan, S., Nair, A.M. Geographic Information System and groundwater quality mapping in Panvel Basin, Maharashtra, India. Env Geol 45, 753–761 (2004). https://doi.org/10.1007/s00254-003-0932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0932-9