Abstract
Increased offshore development in the Alaskan Arctic has stimulated interest in assessing potential impacts to the environment before the onset of any adverse effects. Concentrations of trace metals in sediments are used in this paper to provide one sensitive indicator of anthropogenic inputs from offshore activity over the past several decades. Sediments in coastal waters of the western Beaufort Sea are patchy with respect to sediment granulometry, organic carbon content, and concentrations of trace metals. However, results for surface sediments and age-dated cores show that nearly all samples contain natural concentrations of Ag, Ba, Be, Co, Cr, Cu, Hg, Ni, Pb, Sb, Tl, V and Zn, with metal/Al ratios that have been constant for many decades. Metal concentrations for incoming river-suspended matter compare well with sediment metal values and, along with vertical distributions in sediments, show no discernible diagenetic impacts that distort the sedimentary record for metals, except for Mn, As and possibly Cd. Slightly elevated concentrations of Ba, Hg, Ag, Sb and Zn were observed in a total of eight instances or in only 0.7% of the 1,222 data points for metals in surface sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As oil development in the Alaskan Arctic continues to move offshore into the coastal Beaufort Sea, a variety of studies are being carried out to identify, perhaps even predict, where subtle perturbations in the natural system may occur before the onset of deleterious environmental effects. Such assessments are best formulated from studies of spatial and temporal trends for selected indicators. Concentrations of trace metals in surface sediments and in age-dated cores, along with sediment granulometry and total organic carbon, are used in this paper to help evaluate the cumulative impacts of industrial activity in the coastal Beaufort Sea.
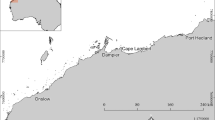
The first major discovery of oil near Prudhoe Bay, Alaska, (Fig. 1) in 1968 followed more than 20 years of exploration (Strohmeyer 1997). To date, more than 5,000 wells have been drilled on the Northslope of Alaska and more than 13 billion barrels of oil have been carried along the ~1,300-km route of the Trans-Alaskan Pipeline System to Valdez since 1977 (Alaska Department of Natural Resources 2002). Most (>90%) of the oil recovered to date from the Alaskan Arctic has come from onshore sites (Alaska Department of Natural Resources 2002).
Map showing study area along the western portion of the coastal Beaufort Sea with inset maps of Alaska, the oil production site at Northstar Island and the proposed future drilling site at Liberty Prospect. Diversity in schemes for station identification results from reusing the numbering system from the 1989 Beaufort Sea Monitoring Program for some stations (Boehm and others 1990; number-letter and number-number) and introducing a new station identification system for study of impacts at Northstar Island (N) and Liberty Prospect (L)
Offshore activities during the 1970s led to the discovery of the Endicott, Point McIntyre and other reservoirs. Production from the Endicott Field began in 1987, following construction of two gravel islands and an 8-km-long causeway to the mainland. To date, about 30 offshore exploratory wells have been drilled in the Beaufort Sea. During 2000, a gravel island was constructed ~10 km offshore with a subseafloor pipeline to bring the Northstar Prospect into production during 2002. The coastal Beaufort Sea continues to be a dynamic area for oil development, seasonal barge and supply boat traffic, as well as the completion of thousands of kilometers of seismic lines.
Trace metals can be useful indicators of impacts from industrial activity because they are commonly enriched in raw and finished materials used by modern industry. For example, barite (BaSO4) is a major component of fluids used during petroleum drilling operations, and concentrations of Ba in these fluids are often at levels of >100,000 µg/g of solids (Trefry and others 1985) relative to typical Ba levels of 200 to 700 µg/g in Beaufort Sea sediments (Crecelius and others 1991). Other metals from industrial activities also can be concentrated in bottom sediments where they are often sensitive indicators of cumulative inputs from a variety of anthropogenic sources.
Previous studies of trace metals in sediments from the coastal Beaufort Sea have shown that metal concentrations are highly variable, but generally at natural levels with minimal localized inputs from development (Sweeney and Naidu 1989; Snyder-Conn and others 1990; Crecelius and others 1991; Naidu and others 1997, 2001; Valette-Silver and others 1999). Snyder-Conn and others (1990) identified elevated levels of Ba, Cr, Pb and Zn in areas adjacent to one or more disposal sites for drilling effluent. Crecelius and others (1991) found elevated levels of Ba at a few sites in western Harrison Bay and Cr near the mouth of the Canning River, with no other indications of metal contamination.
A spatial patchwork of metal concentrations, such as observed in sediments from the coastal Beaufort Sea (Crecelius and others 1991; Macdonald and Thomas 1991), can result from natural variations, anthropogenic inputs or diagenetic impacts. Identifying differences in metal concentrations that result from natural variations in grain size and mineralogy often can be carried out by normalizing (ratioing) concentrations of metals to Al or Fe (Moore 1963; Bruland and others 1974). Normalization to Al and Fe also can be used to help identify instances of metal contamination in sediments (Trefry and Presley 1976; Schropp and others 1990). Finally, the impacts of chemical diagenesis on concentrations of metals in sediments often can be identified by examining vertical profiles for metals in sediments (Ridgway and Price 1987; Shaw and others 1990; Gobeil and others 1997) or by comparing concentrations of metals in sediments with source-suspended material from rivers (Trefry and Presley 1982). All the considerations mentioned above are used in this manuscript to help resolve the complex trends observed for metals in sediments from the study area.
Sediment samples for this study were collected during 1999 (n=44 surface samples), 2000 (n=44 surface samples) and 2001 (n=104 samples from six cores) at 51 different sites that extended from west of Prudhoe Bay (~150°W) to Pole Island (~146°W) and included intensive sampling from areas near the Northstar Island before and during construction of this offshore island for oil development (Fig. 1). Intensive sampling was also carried out in an area identified as Liberty Prospect (Fig. 1) where development may occur in the future. Concentrations of Ag, Al, As, Ba, Be, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, Tl, V and Zn were determined for surface sediment (0–2 cm) and sediments from cores that were age-dated using vertical profiles for 137Cs and excess 210Pb.
Study area
The geology of the region has been summarized by Payne and others (1951) and Mull and Adams (1989). In the Brooks Range (100 to 150 km south of the Beaufort Sea), shales from Triassic to Pennsylvanian age are widespread as are the Lisburne limestone and dolomite group from the Pennsylvanian and Mississippian ages. The Gubik formation (Quaternary riverine and marine sediments) underlies the coastal plain. In addition to Quaternary sediments, older Cretaceous and Tertiary sandstones, conglomerates, and siltstones are exposed in the foothills province. Sediments from these formations are carried seaward from the Brooks Range and Northslope by several rivers including the Colville River, the largest in northern Alaska with a drainage basin of ~50,000 km2 and an annual sediment load of 5 to 10 million metric tons (Arnborg and others 1967; Naidu and Mowatt 1974).
The depositional environment of the inner shelf of the Beaufort Sea grades gently from the shoreline to a water depth of 30 m and is interrupted by low deltaic mudflats, sandbars, and narrow gravel and sand barrier islands. Clay-size (<2 µm) sediments make up an average of 13±9% of the sediments on the inner shelf (Crecelius and others 1991). The sand- and silt-rich sediment on the shelf is generally <5 m thick (Reimnitz and Barnes 1974). Sediment deposition is patchy (Weiss and Naidu 1986; Naidu and others 2001) and Reimnitz and Wolf (1998) suggest that the entire area is a net erosional environment during the Holocene. Coastal waters are characterized by nine to 11 months of nearly complete sea ice cover. Sea ice generally begins to form during late September to October and break-up is usually complete by the beginning of August. The presence and movement of ice along the shelf can greatly influence sediment transport, deposition, and reworking.
Methods
Surface sediments (0–2 cm) were collected using a modified-ponar grab sampler. Sediment cores were collected using a 10-cm-diameter, 1-m-long, gravity corer. Sediments were removed from the sampler or cores using Teflon spatulas, then placed into 75-ml plastic vials and kept cold until they were returned to the laboratory, where they were stored frozen. Sediment cores were subsectioned in 0.5-cm intervals from 0 to 5 cm and in 1.0-cm intervals at depths of >5 cm. Samples for grain size analysis were wrapped in plastic bags and stored at 4 °C. River water samples were collected directly in acid-washed, low-density polyethylene bottles and filtered through acid-washed, 0.4-µm polycarbonate membrane filters to obtain suspended sediments (Rember and Trefry 2003).
Prior to analysis for trace metals, 0.4 g of freeze-dried, homogenized sediment and a certified reference material (CRM) were totally digested in Teflon beakers using concentrated, high-purity HF-HNO3-HClO4 as described in Trefry and Metz (1984). Sediment samples to be analyzed for Hg were digested separately by heating 2 to 4 g of wet sediment in acid-washed, sealed, polypropylene centrifuge tubes with 4 ml HNO3 and 2 ml H2SO4 following the method of Adeloju and others (1994). Filters containing 1 to 15 mg of suspended sediment were digested using the method described by Trefry and Trocine (1991). Briefly, the filters bearing suspended sediments were placed in stoppered, 15-ml Teflon test tubes and the sediments were completely decomposed and dissolved using Ultrex II HNO3, HF and HCl.
Labware used for sample preparation was acid washed with hot, 8 N HNO3 and rinsed three times with distilled, deionized water (DDW). Two procedural blanks, two duplicate samples and two CRMs were prepared with each set of 40 samples. The CRM used was the marine sediment MESS-2 issued by the National Research Council of Canada (NRC).
Bottom sediment samples, CRMs, and procedural and reagent blanks were analyzed as follows: (1) Al, Cr, Cu, Fe, Mn, Ni, V and Zn by flame atomic absorption spectrometry (FAAS) using a Perkin-Elmer (PE) Model 4000 instrument, (2) Ag and As by Zeeman graphite furnace atomic absorption spectrometry (GFAAS) using a PE Model 5100 instrument, (3) Ba, Be, Cd, Co, Pb, Sb, and Tl by inductively coupled plasma-mass spectrometry (ICP-MS) using a PE Model ELAN 5000 instrument, and (4) total Hg by cold vapor atomic absorption spectrometry (CVAAS) using a Laboratory Data Control Model 1235 Mercury Monitor. The above methods are similar to methods described by the United States Environmental Protection Agency (EPA) for Series 7000 FAAS and GFAAS, Series 7470 CVAAS and Series 6010A ICP-MS (United States Environmental Protection Agency 1991). Analytical precision (coefficient of variation, CV), based on replicate analysis of 15 sediment samples, averaged ≤2% for Al, Ba, Co, Cu, Mn, Ni and Zn; ≤3% for Cd, Cr, Fe, Pb, Sb, Tl, and V; ≤5% for As, Be and Hg and 6% for Ag. Concentrations of Al, As, Ba, Cr, Cu, Fe, Pb and Zn in suspended sediments from rivers were determined by similar techniques, as described in Rember and Trefry (2003).
The total organic carbon (TOC) content of the sediments was determined following treatment with H3PO4 to remove inorganic carbon. Then, 200 to 400 mg of carbonate-free sediment were combusted, after the addition of powdered vanadium pentoxide, at 900 °C in a Shimadzu TOC-5050A instrument linked to a solid sampling module (SSM-5000A). A calibration curve was constructed with pure sucrose and checked at every 10 samples by analyzing the CRM MESS-2. Final concentrations of TOC were corrected to account for the increase in sediment mass following the H3PO4 treatment; precision averaged 2.4%. Grain size was determined by the wet sieving and pipette methods of Folk (1974).
Sediment geochronology was determined using excess 210Pb and 137Cs following methods described by Kang and others (2000). Vials containing about 10 g of freeze-dried sediments were counted for 2 to 3 days until peak areas were sufficient to provide <10% counting error for total 210Pb. The activities of 210Pb, 214Pb, 214Bi and 137Cs were determined using a well-type, intrinsic germanium detector (WiGe, Princeton Gamma Tech). Detector efficiency was determined using the following: NBS 4350B, river sediment and NBS 4354, freshwater lake sediment from National Institute of Standards and Technology (NIST) and RGU-1 and RGTh-1 from the International Atomic Energy Agency. The specific activity (dpm/g) of each sediment sample was calculated from the detector efficiency, gamma intensity, geometry factor, and sample weight (Kang and others 2000). All values were decay-corrected to the date of coring. Errors are shown on the basis of 1-σ counting statistics.
Quality assurance and quality control during this work included analysis of procedural blanks, spiked and replicate sample, as well as CRMs. Results for concentrations of metals and TOC in the CRMs agreed within the 95% confidence interval of certified concentrations.
Results and discussion
Normalizing sediment metal concentrations
Considerable variability was found for concentrations of all 18 metals and TOC in surface and subsurface sediments from the study area as suggested by the ranges of values in Table 1. Crecelius and others (1991) showed that sediment grain size was a primary variable controlling concentrations of metals in surface sediments from the study area. Levels of silt plus clay from the present study ranged from 1 to 98.8% (Table 1). Granulometry data from the present study show no simple distribution patterns for sand, silt and clay throughout the study area. Furthermore, the fractional amounts of sand, silt and clay found at some nearshore sites vary from year to year. Samples from the sediment cores contained a greater fraction of silt + clay (Table 1) because a concerted effort was made to sample finer-grained sediments during the coring effort.
To help resolve observed variability, sediment metal values from this study were initially normalized to concentrations of Al. Natural levels of Al and many trace metals vary collectively as a function of sediment grain size, organic carbon content and mineralogy, with higher metal levels in fine-grained aluminosilicates (clays) and lower metal levels in coarse-grained quartz sand and carbonate shell fragments. Normalization is a useful precursor to more detailed evaluation of spatial and historical trends as well as possible diagenetic effects on metal concentrations, as discussed below.
In sediments from this study, positive linear relationships are observed for Al versus percent silt+clay (r=0.89; Fig. 2a), percent clay (r=0.75) and TOC (r=0.74). Aluminum concentrations also correlate well with levels of Fe (r=0.94; Fig. 2b) throughout the study area. Aluminum and Fe are present at percent levels in the sediment, relative to concentrations in parts per million (µg/g) for trace metals; and, Al and Fe are not commonly introduced to marine sediments in sizeable amounts by anthropogenic processes. Therefore, any fractional changes in concentrations of Al and Fe are expected to be small relative to possible shifts in concentrations of trace metals due to anthropogenic or diagenetic influences. Concentrations of Fe can be altered during early chemical diagenesis; however, the net effect on solid-phase concentrations of Fe is generally small (e.g., <10% change in Fe/Al ratio; Trefry and Presley 1982).
Concentrations in sediment for Al versus a silt+clay, b Fe, and c V. Equations are from linear regression calculations, r is the correlation coefficient and n is the number of data points. Dashed lines show 99% prediction interval. Points marked with large letters on the Fe graph are for suspended sediment from the Sagavanirktok (S), Kuparuk (K) and Colville (C) rivers
Mean concentrations of Al and Fe in suspended sediments collected during 2000 and 2001 from the Sagavanirktok and Colville Rivers that supply sediments to the study area plot within the 99% prediction interval developed for bottom sediments (Fig. 2b). Furthermore, concentrations of Al and Fe in the river-suspended sediments plot at the higher end of the continuum in Fig. 2b due to a greater fraction of clay-rich particles suspended in the rivers. Suspended sediments from the Kuparuk River had higher levels of Fe during part of the summer when concentrations of suspended solids were low (~4 mg/l; Rember and Trefry 2003), thereby shifting the average value in Fig. 2b above the upper prediction interval.
Under natural conditions, concentrations of selected trace metals in sediments will commonly follow a strong linear trend versus Al and/or Fe in a given depositional environment. For example, concentrations of V correlate well with Al (r=0.97, Fig. 2c) and Fe (r=0.96) in all surface and subsurface sediment samples collected. The broad range in V concentrations, yet good linear fit for Al (and Fe) versus V, is consistent with mixing of relatively uniform composition, metal-rich aluminosilicate phases with metal-poor quartz sand and carbonates. Vanadium levels in natural sediments from the Beaufort Sea are predicted to follow the trend presented in Fig. 2c. Thus, the strong relationship for Al versus V in Fig. 2c also supports a lack of anthropogenic inputs of V and no impact on V levels due to sediment diagenesis. The three points that plot slightly above the upper prediction interval in Fig. 2c exceed that limit by <10% and are consistent with the statistical boundaries of a 99% prediction interval. Crecelius and others (1991) used V, in the absence of data for Al and Fe, to normalize concentrations of other trace metals in sediments from the coastal Beaufort Sea. Aluminum was chosen for normalization in the present study because it is least affected by chemical weathering and diagenesis.
Graphs for Al versus Pb, Cu, Cr and Ni (Fig. 3) also show strong (r>0.87) linear relationships with no points that plot at more than 10% above the upper prediction interval. Correlation coefficients for Al versus Co (0.85), Sb (0.84) and Tl (0.86) also are strong with no data points that plot at >10% above the upper prediction interval. Collectively, the results support the conclusion that no discernible anthropogenic inputs of these seven metals can be identified. Available metal data for suspended sediments from source rivers (Pb, Cu, Cr, Zn; Fig. 3) show that the metal/Al ratios for river particles fit within, or very close to, the prediction intervals found for bottom sediments in the coastal Beaufort Sea. These similarities in metal/Al ratios for river source material and bottom sediment, when linked to data for sediment cores discussed below, are used to evaluate whether diagenetic impacts distort the historical record for these metals in area sediments. Concentrations of metals in the river-suspended matter plotted at the higher end of the metal/Al continuum (Fig. 3) as previously described for Al and Fe.
Concentrations in sediment for Al versus Pb (a), Cu (b), Cr (c) , Ni (d), Zn (e), Hg (f), Ba (g) and As (h). Equations are from linear regression calculations, r is the correlation coefficient and n is the total number of data points. Dashed lines above and below the regression line show the 99% prediction interval. Points marked with large letters on selected graphs are for suspended sediment from the Sagavanirktok (S), Kuparuk (K) and Colville (C) rivers. Data for sites identified on the graph were not included in the regression calculations
In contrast with the metals discussed above, concentrations at one or more locations were >10% above the upper prediction interval on the metal versus Al plots for Zn, Hg and Ba (Fig. 3). An anomalous Zn value was observed for site 5H (near Endicott Island), and anomalous values for Hg and Ba were found for sediments collected near Northstar Island (Fig. 3). Considerable industrial activity is common to both areas; however, the degree of metal enrichment averaged <25% more than the value at the upper prediction limit for a given concentration of Al.
In addition to the anomalies from the 1999 to 2001 data for Ba described above, concentrations of Ba in samples collected during 1989 (Boehm and others 1990) from sites 7A and 7G in western Harrison Bay also plot above the upper limit of the 99% prediction interval (Fig. 3). Elevated Ba levels at sites 7A and 7G in Harrison Bay during 1989 are consistent with exploratory drilling and drilling residues in the area (Snyder-Conn and others 1990; Crecelius and others 1991). The sensitivity of normalizing to Al is demonstrated by calculating the excess Ba concentration as total Ba minus natural Ba, where the natural Ba level determined from Fig. 3g is the value for Ba at the upper prediction interval for a given Al concentration. The most anomalous sample point in Fig. 3g from station N22 has an excess Ba level of 509 μg/g (859–350 μg/g) that can be explained by the presence of barite at only 0.09% of the total sediment mass, where pure barite contains Ba at 588,000 μg/g. Subtle enhancement of Ba concentrations at site L8 (1999) may be a remnant of exploratory drilling in the area in 1982 and 1997 (URS 2001). Although these various anomalies are minor, they do support the sensitivity of Al versus metal graphs and can help focus future field investigations.
Concentrations of Ag and Be were low and somewhat more variable (Table 1); therefore the correlations versus Al were weaker (Ag, r=0.57; Be, r=0.69). Background levels of As in the study area were high relative to average marine sediment. This point was previously noted throughout the Beaufort Sea by Valette-Silver and others (1999). In the present study, the authors found As levels in suspended sediments from local rivers to average 15±5 µg/g (n=17). Data shown on Fig. 3h for As are more scattered with a lower r value of 0.53 and several points above the upper prediction interval, (Fig. 3h). These trends for As also were observed for Mn and Cd (Table 2) and are related to diagenetic impacts as discussed below.
Temporal distribution of sediment metal concentrations
The historical record of metal levels in sediments from the coastal Beaufort Sea is developed here from age-dated cores. Metal data from sediment cores, coupled with results for river-suspended particles, also are used to help identify possible diagenetic effects. Collecting sediment cores suitable for age-dating in the study area is complicated by bottom-fast ice, ice gouging, low net sediment accumulation rates, low activities of excess 210Pb and 137Cs, and storm-induced resuspension and transport of sediments offshore into deeper water. Even when coring sites were chosen based on bathymetry (i.e., semi-restricted basins) or surface sediment composition (i.e., >90% silt plus clay), only one in four cores was viable for establishing a geochronology over the past 50 to 100 years. In many instances, extremely low levels of excess 210Pb (<0.2 dpm/g) or 137Cs (<0.02 dpm/g) were found, even in the top 0.5 cm of sediment. Such observations are consistent with previous reports that characterize this coastal area as a net erosional environment (Reimnitz and Wolf 1998).
Past efforts to reconstruct recent geochronology for coastal sediments from this nearshore area of the Beaufort Sea (Weiss and Naidu 1986; Naidu and others 2001) have encountered many of the same difficulties reported here. Weiss and Naidu (1986) used vertical profiles for the activity of total 210Pb to calculate sedimentation rates of 0.6 to 1 cm/year at sites in Simpson Lagoon, near stations 6A and 6G (Fig. 1); however, the activities for total 210Pb averaged <2 dpm/g with variable texture in each core. In recent work, Naidu and others (2001) reported no excess 210Pb and no detectable 137Cs in a core from Simpson Lagoon whereas they found levels of excess 210Pb levels at 0.9 to 1.2 dpm/g and 137Cs activities of 0.2 dpm/g at a site near our station 3B (Fig. 1). Based on inherent difficulties with area sediments, a primary goal of the geochronology effort for the present study was to collect some representative sediment that was deposited prior to the onset of development during the late 1960s and early 1970s and some sediments that was deposited post-development.
Detailed results for geochronology were obtained for three sites: (1) station P1 in Prudhoe Bay, (2) station E1, just east of Endicott Island near the mouth of the Sagavanirktok River, and (3) station 6G in the eastern section of the Colville River delta (Fig. 1). At stations L2, 3A, N2 and 6A, either no detectable excess 210Pb and 137Cs were found or very low levels were found only in the top 0.5 cm. The locations of these sites with little or no detectable recent sediments extend across the study area and support the contention that deposition of present-day sediment is patchy and thin.
In Prudhoe Bay (station P1), the maximum activity of excess 210Pb in the surface layer of sediment was 0.84 dpm/g with detectable decay to a depth of ~5 cm and a calculated sedimentation rate of 0.11±0.02 cm/year (Fig. 4a). The vertical profile for 137Cs supports the results from excess 210Pb with a sedimentation rate of 0.10 cm/year based on the 1950 appearance of 137Cs at ~5 cm and the observed 1963 peak at ~3.75 cm (Fig. 4b). Samples from depths >4 cm were most likely deposited before development began during the 1960s in the area of Prudhoe Bay. Preservation of such detail in the geochronological record over such a short depth interval for this site is surprising; however, boat traffic in the inner portion of the Prudhoe Bay is rare and water depths in the deepest portion of the secluded bay (~3 m) are sufficient to minimize the effects of bottom-fast ice and ice gouging. Even if a combination of sediment deposition and winnowing at station P1 created an apparent sedimentation rate, it seems reasonable to suggest that the top 1 to 2 cm contain post-development sediments and that sediments found deeper than 4 to 5 cm were deposited prior to development.
At station E1, the activity of excess 210Pb was 1.1 dpm/g at 0 to 0.5 cm and 1.5 dpm/g at 0.5 to 1.0 cm (Fig. 4c). The calculated sedimentation rate based on excess 210Pb is about 0.04±0.02 cm/year. Activities of 137Cs were detectable to a depth of 3.25 cm, yielding a sedimentation rate of ~0.06 cm/year (Fig. 4d), a value that is reasonably consistent with that obtained from the profile for excess 210Pb considering the uncertainty in the data. These data for station E1 support the likelihood that sediments at depths >4 cm pre-date development.
Additional support for low sedimentation rates at stations P1 and E1 can be developed from data for river inputs of sediment. The Sagavanirktok River, the major river carrying sediments into this area, is estimated to have an annual sediment load of about 0.3×106 mt (Rember and Trefry 2003). The depositional area for these sediments in the coastal Beaufort Sea is at least 1,000 km2 to yield an estimated deposition rate of ~0.02 cm/year based on a sediment bulk density of 1.6 g/cm3 ([0.3×1012 g dry sediment/1,000×1010 cm2]×[(1.6 g wet sediment/cm3)/(2.6 g dry sediment/cm3)]). As previously noted, the study area may be net erosional at this time (Reimnitz and Wolf 1998).
In the Colville River delta at station 6G, the maximum activity of excess 210Pb was 0.76 dpm/g and the calculated sedimentation rate was 0.04±0.02 cm/year (Fig. 4e). The 137Cs profile supports a sediment accumulation rate of ~0.06 cm/year (Fig. 4f). Once again, the record of sediment input since the 1950s is sequestered in the top 4 to 5 cm of sediment. At nearby station 6A, detectable levels of excess 210Pb at 0.27 dpm/g were observed only in the top 0.5 cm of the sediment column. This latter result is consistent with that of Naidu and others (2001) for the same area.
Concentrations of trace metals were determined for 104 samples from six cores (P1, E1, 3A, 6A, 6G and N2). Some variability in concentrations of metals was observed in each core (Table 1 and Figs. 5, 6), mainly due to variations in amounts of fine-grained sediment. However, the CV for metal/Al ratios averaged 10% in each of the six cores for Ni, V, Zn, Fe, Cr, Ba, Co, Tl, Be, Pb, Sb and Cu (Table 2). Such uniform metal/Al ratios support long-term deposition of sediments with uniform composition and no identifiable impact from diagenesis for these metals. These conclusions are further supported below through detailed evaluation of cores from stations P1 and 6G and from data for river-suspended sediments.
Vertical profiles for concentrations and ratios to Al in sediment core from Prudhoe Bay (station P1) for Fe and Al, Ba, Pb, Cr, V, Zn, As, total organic carbon (TOC), and Mn. Triangles plotted at 0 cm show concentrations and metal/Al ratios for suspended sediment from the Sagavanirktok River. Numbers in parentheses show coefficient of variation for metal/Al ratio. Graphs with no line identifying a sediment age of 1950 (As, TOC and Mn) lack geochronological significance due to post-depositional diagenesis/diffusion
Vertical profiles for concentrations and ratios to Al in sediment core from Colville River delta (station 6G) for Fe and Al, Ba, Pb, Cr, V, Co, Mi, Mn and total organic carbon (TOC). Triangles plotted at 0 cm show concentrations and metal/Al ratios for suspended sediment from the Colville River. Numbers in parentheses show coefficient of variation for metal/Al ratio. Graphs with no line identifying a sediment age of 1950 (Mn and TOC) lack geochronological significance due to post-depositional diagenesis/diffusion
In Prudhoe Bay (station P1), concentrations of Al and Fe follow parallel trends down core (Fig. 5). Variations in concentrations of Al and Fe in the core result from shifts in the fraction of sand, silt and clay deposited during a given time period. Vertical distributions for Ba, Pb, Cr, V and Zn (Fig. 5), as well as Be, Cu, Ni, Sb and Tl, follow trends similar to those observed for Al and Fe with the CVs for the metal/Al ratios all <8%. These vertical profiles support long-term deposition of sediments with no discernible shifts in metal/Al ratios or anthropogenic inputs. Metal concentrations and the metal/Al ratios for Fe, Al, Pb, Cr, Zn in suspended sediments from the Sagavanirktok River are plotted at the top of each vertical profile in Fig. 5 and are coincident with values found in the surficial layers of the core. This continuity, in conjunction with the vertical profiles, supports no discernible diagenetic impacts in the vertical distributions for Ag, Ba, Be, Co, Cr, Cu, Hg, Ni, Pb, Sb, Tl, V and Zn.
Concentrations of TOC (and the TOC/Al ratio) are elevated by about 30% in the top 0.5 cm and by a factor of ~2 at about 20 cm relative to other sections in the core (Fig. 5). Coincident with elevated levels of TOC in the surface layer of sediment are higher values of As/Al and slightly lower levels of Mn/Al (Fig. 5). Furthermore, the Mn/Al ratios are enriched in the layers at ~20 cm where concentrations of TOC are high. Diagenetic impacts on Mn in sediments are well studied and can lead to a variety of perturbations in concentrations of Mn (Trefry and Presley 1982; Gobeil and others 1997). In the top 0.5 cm of the core from Prudhoe Bay, concentrations of Mn are about double levels found in subsequent layers to a depth of 15 cm, yet the Mn and Mn/Al levels in the top 0.5 cm of sediment are about 25% lower than in river-suspended sediment. One possible explanation for this observation is that particles deposited in the sediment lose Mn via reductive dissolution and diffusion of dissolved Mn2+ from the sediments to the overlying water column (e.g., Gobeil and others 1997). The onset of this process in Prudhoe Bay occurs in the top layer of sediment and reaches completion at depths >1 cm. Such behavior (reducing conditions in the top 1 cm) seems inconsistent with a sedimentation rate of 0.1 cm/year and may reflect processes that occur in a stagnant, thin (<1-m-thick) layer of water trapped under 2 m of ice during 8 months of the year. A similar impact on As levels is observed in this core. The loss of As from the sediments is related to release of As from sediments to the overlying water during diagenetic remobilization under reducing conditions (Farmer and Lovell 1986). Overall, diagenetic effects alter the vertical distributions of Mn, As and, to some lesser degree, Cd, but none of the other metals studied are impacted.
At station 6G, on the Colville River delta, post-development sediments appear to be restricted to the top 3 cm of the sediment column. No discernible differences in metal/Al ratios are observed for all metals except Mn (Fig. 6). Available data for suspended sediments from the Colville River show that concentrations of Fe, Al, Pb and Cr are higher than observed for sediments at station 6G; however, the metal/Al ratios are similar (Fig. 6). No indications of anthropogenic inputs of metals are found in the core from station 6G and only concentrations of Mn are impacted by diagenesis.
Metal data from other cores in the area of Pole Island (station 3A) to Northstar Island, including stations 3A, L2, E1 and N2, show similar trends with uniform metal/Al ratios throughout the cores (Table 2). In some cases, the surficial layer of sediment could be quite old as demonstrated by undetectable levels of 137Cs and excess 210Pb. Concentrations of Mn, As and Cd show varying amounts of distortion due to diagenetic effects. Overall, concentrations of Ag, Ba, Be, Co, Cr, Cu, Hg, Ni, Pb, Sb, Tl, V and Zn in cores from these five sites are unimpacted by anthropogenic inputs or diagenesis.
Conclusions
Region wide, nearly all sediments collected contained natural levels of Ag, Ba, Be, Co, Cr, Cu, Hg, Ni, Pb, Sb, Tl, V and Zn. About eight exceptions (or 0.7% of the 1,222 data points from 94 surface samples) were identified by clear, positive anomalies (>10% above upper prediction interval) on the metal/Al plots. These exceptions include the following: Ag (N14 in 1999), Ba (N14 in 1999; L08 in 1999 and N22 in 2000), Hg (N10 and N17 in 2000), Sb (5(10) in 1999) and Zn (5H in 1999). Most of these minor deviations were found in the most active areas between West Dock (near station 5D) and Northstar Island; however, the instances and magnitude of the deviations show that, with respect to trace metals, the sediments are essentially pristine. In a 1989 study that incorporated a larger geographical area (Boehm and others 1990), concentrations of Ba and/or Cr were elevated at locations in Harrison Bay (7A, 7B and 7G) and near the mouth of the Canning River (2E). Thus, in addition to the patchy distribution of sediment types in the study area, a few instances of minor contamination have been observed.
Early detection of potential environmental problems near industrial sites is the goal at many locations around the Earth, including the coastal waters of the western Beaufort Sea. Because many trace metals are a ubiquitous part of modern industry, metals in sediments can often help identify subtle increases in the accumulation of potential pollutants before they lead to an adverse environmental consequence. For example, in sediments from the coastal Beaufort Sea with an Al concentration of 6.0%, natural Pb levels are predicted to be 15± 5µg/g with 99% confidence (Fig. 3a). Data for two sediment samples represented on Fig. 3a with slightly >6% Al show that Pb levels of 20.3 and 21.5 µg/g plot slightly above the upper prediction limit. These samples are from stations N23 and 5(10), respectively. The slight degree of Pb enrichment and the locations of these two sites near Northstar Island help focus future efforts in this area.
Overall, the constancy of metal/Al ratios in (1) river-suspended sediment, (2) the surface layers of bottom sediments and (3) deeper, older layers in bottom sediments show that anthropogenic inputs of trace metals have not significantly elevated metals levels in sediments in areas of offshore oil exploration and production in the coastal Beaufort Sea. The results presented in this study provide a framework for continued efforts to assess any cumulative impacts of offshore development in the coastal Beaufort Sea. However, the sediments in this region are dynamic and care must be taken to assure that investigators know whether sediment samples that are collected are representative of the most recent inputs.
References
Adeloju SB, Dhindsa HS, Tandon RK (1994) Evaluation of some wet decomposition methods for mercury determination in biological and environmental materials by cold vapour atomic absorption spectroscopy. Anal Chim Acta 285:359–364
Alaska Department of Natural Resources (2002) Division of Oil and Gas 2002 Report. Anchorage, Alaska
Arnborg L, Walker HJ, Peippo J (1967) Suspended load in the Colville River, Alaska, 1962. Geogr Ann 49A: 131–144
Boehm P, LeBlanc L, Trefry J, Marajh-Whittemore P, Brown J, Schutzberg A, Kick A (1990) Monitoring hydrocarbons and trace metals in Beaufort Sea sediments and organisms. Final Rep to US Dept Int, MMS, Anchorage, Alaska
Bruland KW, Bertine K, Koide M, Goldberg ED (1974) History of metal pollution in southern California coastal zone. Environ Sci Technol 8:425–432
Crecelius EA, Trefry JH, Steinhauer MS, Boehm PD (1991) Trace metals in sediments from the inner continental shelf of the western Beaufort Sea. Environ Geol Water Sci 18:71–79
Farmer JG, Lovell MA (1986) Natural enrichment of arsenic in Loch Lomond sediment. Geochim Cosmochim Acta 50:2059–2067
Folk RL (ed) (1974) Petrology of sedimentary rocks. Hemphill, Austin
Gobeil C, Macdonald RW, Sundby B (1997) Diagenetic separation of cadmium and manganese in suboxic continental margin sediments. Geochim Cosmochim Acta 61:4647–4654
Kang W-J, Trefry JH, Nelsen TA, Wanless HR (2000) Direct atmospheric inputs versus runoff fluxes of mercury to the lower Everglades and Florida Bay. Environ Sci Technol 34:4058–4063
Macdonald RW, Thomas DJ (1991) Chemical interactions and sediments of the western Canadian Arctic shelf. Cont Shelf Res 11:843–863
Moore JR (1963) Bottom sediment studies, Buzzard Bay, Massachusetts. J Sediment Petrol 33:511–558
Mull CG, Adams KE (1989) Dalton Highway, Yukon River to Prudhoe Bay, Alaska. Alaska Div of Geol Geophys Surv, Guidebook 7, vols 1 and 2
Naidu AS, Blanchard A, Kelley JJ, Goering, JJ, Hameed MJ, Baskaran M (1997) Heavy metals in Chukchi Sea sediments as compared to selected circum-arctic shelves. Mar Pollut Bull 35:260–269
Naidu AS, Goering JJ, Kelley, JJ, Venkatesan MI (2001) Historical changes in trace metals and hydrocarbons in the inner shelf sediments, Beaufort Sea: prior and subsequent to petroleum-related industrial developments. Final Rep US Dept Int, MMS2001–061
Naidu AS, Mowatt TC (1974) Depositional environments and sediment characteristics of the Colville and adjacent deltas, north Arctic Alaska. In: Broussard MLS (ed) Deltas for subsurface exploration. Houston Geol Soc, pp 283–309
Payne TG, Dana SW, Fischer WA, Yuster ST, Krynine PD, Morris RH, Lathram E, Tappan H (1951) Geology of the arctic slope of Alaska. US Geol Surv Oil Gas Invest Map OM-126
Reimnitz E, Barnes PW (1974) Sea ice as a geologic agent on the Beaufort Sea shelf of Alaska. In: Reed JC, Sater JE (eds) The coast and shelf of the Beaufort Sea. Arctic Inst No Am, Arlington, pp 301–353
Reimnitz E, Wolf SC (1998) Are north slope surface alluvial fans pre-Holocene relicts? USGS Prof Paper 1605
Rember RD, Trefry JH (2003) Increased concentrations of dissolved trace metals and organic carbon during snowmelt in rivers of the Alaskan Arctic. Geochim Cosmochim Acta (in press)
Ridgway IM, Price NB (1987) Geochemical associations and post-depositional mobility of heavy metals in coastal sediments: Loch Etive, Scotland. Mar Chem 21:229–248
Schropp SJ, Lewis FG, Windom HL (1990) Interpretation of metal concentrations in estuarine sediments of Florida using aluminum as a reference element. Estuaries 13:227–235
Shaw TJ, Gieskes JM, Jahnke RA (1990) Early diagenesis in differing depositional environments: the response of transition metals in pore water. Geochim Cosmochim Acta 54:1233–1246
Snyder-Conn E, Densmore D, Moitoret C, Stroebele J (1990) Persistence of trace metals in shallow arctic marine sediments contaminated by drilling effluents. Oil Chem Pollut 7:225–247
Strohmeyer J (1997) Extreme conditions: big oil and the transformation of Alaska. Cascade Press, Anchorage
Sweeney MD, Naidu AS (1989) Heavy metals in sediments of the inner shelf of the Beaufort Sea, Northern Arctic Alaska. Mar Pollut Bull 20:140–143
Trefry JH, Metz S (1984) Selective leaching of trace metals from sediments as a function of pH. Anal Chem 56:745–749
Trefry, JH, Presley BJ (1976) Heavy metals in sediment from San Antonio Bay and the northwest Gulf of Mexico. Environ Geol 1:283–294
Trefry JH, Presley BJ (1982) Manganese fluxes from Mississippi Delta sediments. Geochim Cosmochim Acta 46:1715–1726
Trefry JH, Trocine RP (1991) Collection and analysis of marine particles for trace elements. In: Hurd DC, Spencer DW (eds) Marine particles: analysis and characterization. Am Geophys Union Monogr 63, pp 311–315
Trefry JH, Trocine RP, Proni JR (1985) Drilling-fluid discharges into the northwestern Gulf of Mexico. In: Duedall IW, Kester DR, Park PK, Ketchum BH (eds) Wastes in the ocean, vol. 4. Wiley, New York, pp 195–222
United States Environmental Protection Agency (1991) Methods for the determination of metals in environmental samples. EPA/600/4-91/010. USEPA, Cincinnati
URS (2001) Liberty development 2001 sediment quality study. Final Rep to B.P. Exploration (Alaska), Inc
Valette-Silver N, Hameed MJ, Efurd DW, Robertson A (1999) Status of the contamination in sediments and biota from the western Beaufort Sea (Alaska). Mar Pollut Bull 38:702–722
Weiss HV, Naidu AS (1986) 210Pb flux in an arctic coastal region. Arctic 39:59–64
Acknowledgments
The authors thank Captain Mark Mertz for his efforts in sample collection and Simone Metz for her assistance with some of the metal analyses and in subsequent discussions. Paul Boehm's continued efforts and inputs to the program are greatly appreciated. Special thanks to Dick Prentki and Cleve Cowles of MMS, Anchorage, for continued discussions and support, as well as assistance in the field. The study was supported by Minerals Management Service (Contract No. 143501-99-CT-30998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trefry, J.H., Rember, R.D., Trocine, R.P. et al. Trace metals in sediments near offshore oil exploration and production sites in the Alaskan Arctic. Env Geol 45, 149–160 (2003). https://doi.org/10.1007/s00254-003-0882-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0882-2