Abstract
A method of combining hydrochemical data logging and in situ titrating with measurement of stable carbon and oxygen isotopes was used to reveal the hydrochemical and isotopic characteristics in the Baishuitai travertine scenic area of SW China. It was found that the travertine-forming springs have a very high concentration of calcium and bicarbonate, and accordingly very high CO2 partial pressures, which are not likely to be produced by biological activity in soil alone. Further analysis of the stable carbon isotopes of the springs shows that the high pressure of CO2 is mainly related to an endogenic CO2 source. That means the Baishuitai travertine is endogenic in origin. This is contrast to the commonly accepted saying that the travertine deposition in this study simply is a product of warm and humid conditions in a karst ecological environment. Rapid CO2 degassing from the water is triggered by the much higher partial pressures in water than that of the surrounding air. Consequently, as the waters flow downstream of the spring the pH increases, the waters become supersaturated with respect to calcite, and travertine is deposited. The preferential release of 12CO2 to the atmosphere results in a progressive increase of travertine δ13C downstream. This is concluded with a preliminary discussion of variation in travertine-forming water temperatures, according to differences in stable oxygen isotopic compositions of the travertine formed in different epochs at Baishuitai. It was found that the change in water temperature is as high as 13 °C, i.e., from 23 °C at about 2500 years b.p., to 10 °C at present. This may mainly reflect that the effect of geothermal source on water temperature is decreasing. The problems involved in paleoenvironmental reconstruction with endogene travertine are also discussed. They are the impacts of "dead carbon" in radiocarbon dating and the enrichment in 13C of travertine by endogenic CO2 and degassing of CO2 from water, which has to be considered in paleovegetation reconstruction when using δ13C data of the endogene carbonate deposits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
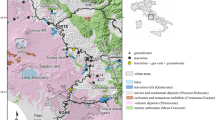
Baishuitai is in southeastern Zhongdian County, Yunnan Province, SW China, about 100 km from the county town. The altitude ranges from 2,380 m to 2,600 m a.s.l. Baishuitai Springs, which are abundant in calcium, flow out from the mountainside of Mahuishan in a forested area, which is near Gudu Village (Fig. 1). About 100 m from the springs, a travertine platform with layered CaCO3 has formed. It is a typical karst landscape, which has been formed by calcite-supersaturated aqueous solutions, and covers about 3 km2 (Zhao and others 1998). It is believed to be one of the largest travertine deposits in China, and becomes an important natural tourism resource due to its beauty and as the cradle of Donba Culture of the Naxi Minority (Fig. 1).
Since the Ming Dynasty (about 600 b.p.), Baishuitai has become a famous tourist and religious attraction in Yunnan Province. However, the origin of the Baishuitai travertine has not been well understood. It has been considered to be a product of a special karst ecological environment (Zhu 1992), associated with a warm and humid climate (Zhao and others 1998). There has also been speculation that the travertine may be endogenic, or related to an endogenic CO2 source (Pentecost and Zhang 2001). These inconsistent interpretations suggest that further research is necessary to clarify the origin and forming-processes of the travertine at Baishuitai. Moreover, there is a possibility of paleoenvironmental reconstruction within this kind of travertine deposit.
Climatic, botanical and geological background at Baishuitai
Though Baishuitai lies in the subtropics, the altitudes are highly variable. There are thus four climatic zones present simultaneously, depending on the elevations on the mountain slope: a warm-temperate zone (altitude 2,200–2,500 m), a temperate zone (altitude 2,500–3,000 m), a cold-temperate zone (altitude 3,000–3,500 m) and a frigid zone (altitudes above 3,500 m). Because of these climatic variations, plants near Baishuitai also have distinct vertical zonation. The native plants in the upper course of the Baishuitai River are protected quite well, therefore the forest cover can be up to 80% or more (Zhao and others 1998). However, native plants were seriously destroyed in the lower reaches of the river, therefore the major component is the secondary shrub below an altitude of 2,600 m a.s.l.
Baishuitai is on Shisanjiao Mountain with limestone of the Triassic Beiya Formation. Due to intensive neotectonic movement (Zhao and others 1998) and rock weathering, the rock is highly fractured, and faults and fissures are well developed. This provides good conditions for rainfall infiltration and groundwater flow. However, due to the cut-off by a major northwest oriented fault, groundwater resurges at an altitude of about 2,600 m a.s.l, and forms the Baishuitai Spring. There are three main springs: spring No.1-1 to No.1-3 (Fig. 1). During the contact of spring water with the atmosphere, a large amount of CO2 is released and calcium carbonate has been deposited, and through time this natural wonder of colored travertine scenery has formed (Figs. 1, 2).
Cross section showing the geological conditions for the origin and formation of the Baishuitai travertine (modified after Zhao and others 1998). Monitoring site: 1 Baishuitai spring; 2 flowing water 100 m from the spring; 3–8 travertine pool Nos. 5–10 respectively; 9-site for hydrochemical data logging, about 500 m downstream from the spring
The temperature (about 11 °C) of the springs is approximately 3° higher than the annual mean air temperature (8 °C) at the altitude where the springs flow out (Song and others 1994). This suggests that the spring water may be heated by a deep-seated geothermal source, especially considering that the high altitude recharge waters (ca. 4,000 m) are significantly colder. Further support of this is shown by the following analysis of hydrochemistry and stable carbon isotopes.
Methods
The methods include hydrochemical data logging, in situ titrating and sample analysis in the laboratory.
The MultiLine P3 multi-parameter instrument (made by WTW-Wissenschaftlich-Technische-Werkstätten in Germany) automatically records temperature, pH and electrical conductivity of water in situ, with resolutions of 0.1 °C, 0.01 pH and 1 μs/cm, respectively. The time interval of data acquisition ranged from 15 to 60 min. By automatic recording, values of the water's physicochemical characteristics and their diurnal changes were obtained. In situ titrating was used to measure the HCO3 − and Ca2+ of water with the Aquamerck alkalinity test and hardness test. The resolutions are 6 mmol/l and 1 mg/l, respectively.
Samples of water and travertine were collected for analysis of major hydrochemical components and the compositions of stable carbon and oxygen isotopes (δ13C,δ18O, ‰, PDB) according to standard procedures in laboratory. The water samples for δ13C of dissolved inorganic carbon were treated in situ with NaOH (2 N) to increase pH to above 12.00 (special attention was paid to avoiding absorption of CO2 by the alkali from atmosphere), then with a saturated BaCl2 solution to precipitate BaCO3. They were then filtered and the BaCO3 analyzed in the laboratory using a VG903 isotope mass spectrometer.
Results
Table 1 shows the hydrochemical and stable carbon isotopic compositions of the springs, and the stable carbon and oxygen isotopic compositions of travertines can be seen in Table 2.
The downstream hydrochemical evolution at Baishuitai is shown in Fig. 3, and Fig. 4 shows the diurnal change in temperature, pH-value, and electrical conductivity of the Baishuitai downstream water at site No.9 in Fig. 2. The details of the results are explained in the following section.
Downstream hydrochemical evolution at Baishuitai. Note that [HCO3 −], [Ca2+], pH and conductivity are those measured in situ, and SI and Pco2 are obtained by running the SOLMINEQ88 software. Monitoring sites of 1–8 are indicated in Fig. 2
Diurnal Change in temperature, pH and electrical conductivity of downstream flowing water at the monitoring site No.9 in Fig. 2
Discussion and conclusions
Origin of the Baishuitai travertine
If the CO2 partial pressure of spring water is higher than that of the surrounding atmosphere, CO2 will outgas from the water, which can lead to calcite supersaturation and deposition (Herman and Lorah 1987; Dreybrodt and others 1992; Liu and others 1995, 1997; Liu and Yuan 2000; Pentecost and Zhang 2001; Song and others 1994; Zhao and others 1998). That is, research on travertine origin must deal with the analysis of the source of carbon, water and calcium of the three components. Considering the background of the climate, botany and geology at Baishuitai, it is inferred that the Baishuitai spring water originates from the infiltration of rainfall, the calcium in the water is related to dissolution of Triassic limestone, and CO2 (which dissolves limestone) comes from either the biological CO2 in soil or the endogenic CO2, or a mixture of both. In the following, the source of the CO2 will be clarified further through analysis of hydrochemical and stable carbon isotopic data.
The spring water has very high concentration of calcium and bicarbonate (Table 1). They are 200 and 700 mg/l, or 5 and 11.5 mmol/l, respectively, and the corresponding CO2 partial pressure is more than 14,400 Pa. (Note: The water of spring No.1-3 was diluted by snow-melting water nearby, and so not considered here). According to observations in China (Liu and Yuan 2000), even in the warm and humid tropical and sub-tropical zones, the partial pressure of biological CO2 in soil is no more than 10,000 Pa, and the concentration of calcium and bicarbonate are typically less than 150 and 450 mg/l, respectively. Pentecost and Zhang (2001) also found that concentrations of dissolved inorganic carbon that comes from limestone dissolution caused by biological CO2 rarely exceeds 10 mmol/l (usually 2–5 mmol/l). Therefore, the hydrochemical features of the Baishuitai springs cannot be explained by biological activity in soil alone (Zhao and others 1998), and also, 8 °C is too cold for significant biogenic CO2 production. A more important role by endogenic CO2 is likely.
According to Deines and others (1974), if an isotopic exchange equilibrium condition has been met, one has a relationship as follows:
where T is absolute temperature in K.
At Baishuitai, from the data by this study the temperature of spring water is 10.8 °C (or absolute temperature T=283.95 K) and the composition of the stable carbon isotopes of bicarbonate ion (δ13CHCO3) is −1.23‰ (the average value in Table 1). Therefore, with the relationship above, the composition of stable carbon isotope of CO2 in spring water can be obtained. The value is δ13CCO2=−10.32‰. This is the stable carbon isotopic composition of the CO2 for the origin of the Baishuitai travertine. It is obvious that the value is different from that of pure biological origin CO2 in soil (δ13Csoil CO2 ≈ −25‰; Galimov 1966), but much closer to that of endogenic CO2 gas on Yunnan-Sichuan-Tibet plateau (Liu and others 1993, 1995, 1997, 2000). More recently, the δ13C value for the endogenic CO2 gas from a hot spring (31.4 °C) near Baishuitai (about 4 km away) has been obtained. It is –7.87‰. From this it can be determined that the proportions come from the endogenic CO2 and biological CO2 sources, respectively at Baishuitai.
If the CO2 from the endogenic CO2 source is considered as x percent of the total CO2 content in Baishuitai springs, then the biological CO2 contributes (100-x) percent. Therefore, according to mass conservation of isotope, one has:
.
Therefore, x≈14, i.e., 14% of the total CO2 content in Baishuitai springs comes from soil CO2, and the other (86%) is endogenic, possibly related to a mixture of metamorphic and mantle CO2 sources (Liu and others 1993, 1995, 1997, 2000). The existence of the endogenic CO2 source explains why the Baishuitai spring water has such high concentrations of CO2, [HCO3 −] and [Ca2+]; and, the latter provides abundant material source for travertine deposition.
Formation process of the Baishuitai travertine
The spring water at Baishuitai is near equilibrium with respect to calcite, and slightly undersaturated with respect to calcite (saturation index <0, Table 1). This would seem to be unfavorable to travertine formation. However, as the spring water emerges, a large amount of CO2 is released from the water to the atmosphere due to the much higher CO2 partial pressure of water than that of atmosphere (less than 40 Pa). After about 500 m downstream, the CO2 partial pressure of water decreased from 14,400 Pa to 300 Pa, pH increased from 6.61 to 8.10, and the calcite saturation index increased from near zero in the spring to more than 1.0. Correspondingly, calcium carbonate, the travertine's major component, is deposited. With the deposition of travertine along the stream bed, the concentration of Ca2+ and HCO3 − of water decreases from 200 and 700 mg/l to 110 and 480 mg/l, respectively (Fig. 3).
The changes above are also reflected in the stable carbon isotopic composition of travertine. As shown in Table 2, from active travertine pool No.5 to No.10 (with the height difference of about 8 m and horizontal distance about 6 m), the δ13C increases from 3.62 to 4.98‰. The increase of δ13C of travertine is due to CO2 degassing, which preferentially releases 12CO2, with the resultant travertine becoming enriched as one moves downstream. This phenomenon was also found in active travertine on tree branches in a canal constructed in November 1999. As seen in Table 2, from the active travertine on tree branch No.B-1 to No. B-6 (separated by about 2 km, Fig. 1), δ13C increased from 3.71 to 6.04‰.
Moreover, after groundwater emerged, the water changed from a closed system to open to the atmosphere. Thus, its hydrochemistry and isotope characteristics experience not only spatial change, but also remarkable temporal change. Figure 4 shows the diurnal variations in pH, conductivity and temperature recorded at site No.9, about 500 m downstream of the springs (Fig. 2). All parameters show distinct diurnal changes. The decrease in temperature at night is obvious and expected. However, this is likely to affect the degassing rate of CO2, which should be lower at night because its solubility increases. Consequently, the pH drops, which theoretically should decrease calcite supersaturation levels and therefore calcite precipitation. This, in turn, should lead to an increase in the concentrations of calcium and bicarbonate ions in solution, and thus the conductivity of the water. At daytime, the contrary situation occurs, i.e. temperature and pH increase, and conductivity drops. It can be inferred from this finding that travertine deposition takes place mainly at daytime, particularly under sunny conditions.
Variations in water temperature recorded by Baishuitai travertines
According to ages (Table 2), the sampled Baishuitai travertines can be divided into three categories as follows:
-
1.
the old travertine, including 41 samples with ages of 2,530–2,570 years:
-
2.
the older travertine, including also 41 samples with ages of 1,970–2,010 years:
-
3.
active travertine, i.e., the others except categories (1) and (2) in Table 2.
It can be seen from Table 2 that there is remarkable difference in stable oxygen isotopic composition among the three categories of travertines: the old travertine has the lowest δ18O (mean, –15.32‰), the active travertine has the highest δ18O (mean, −12.39‰), and the older travertine is in the middle (mean, –14.48‰). This indicates that the travertine-forming water temperature decreased with time. From the difference in stable isotopic composition of the travertines, variations in water temperature can be estimated.
O'Neil and others (1969) found that if there is an oxygen isotopic exchange equilibrium between calcite precipitation and water, the following relationship prevails:
According to Song and others (1994), δ18Owater is –44.15‰(PDB). Suppose this value does not change during the process of travertine deposition, the travertine-forming water temperatures can be calculated by using the formula above. They are 23, 19 and 10 °C for the old travertine, the older travertine and the active travertine, respectively. (The consistency between the calculated and actual present temperature proves that the oxygen isotopic exchange equilibrium assumption for the calculation is reasonable.) This may reflect, on one hand, the effect of strong neotectonic uplift of the area (up to 900 m in 200 ka; Zhao and others 1998), and on the other hand the decreasing effect of geothermal source on water temperature. However, the influence of the uplift during recent 2,500 years should be very small due to the short time compared to 200 ka. Therefore, the major decrease in water temperature was attributed to less intensive geothermal activity in the area during the last 2,500 years.
The problems in paleoenvironmental reconstruction with endogene travertine
Problems in 14C dating of endogene travertine
The principle of 14C dating of travertine is as follows:
Before the deposition of travertine, 14C in water is active in the natural exchange cycle, i.e., the content of 14C in water is the same as that in exchangeable carbon reservoir. With the beginning of deposition, travertine no longer has exchange with this reservoir (e.g. the atmosphere and water). Because there is no replenishment of 14C, the original radio 14C decreases by a decay law, and timing begins. The formula for dating is:
where AON and ASN are specific radioactivities of 14C in the exchangeable reservoir and sample t years after the end of exchange, respectively (Stuiver and Polach 1977).
Therefore, a prerequisite for 14C dating is that there is radio 14C in the measured sample.
However, for endogene travertine, such as the travertine at Baishuitai, there is almost no 14C, because the dissolution of old carbonate rock (with no 14C in it) was mainly driven by endogenic source CO2, which also has no 14C. This is considered to be "dead carbon" (Chen and others 1988). Thus, it is impossible to determine the real age of such travertine with the 14C dating method. If there is 14C in the travertine, the 14C is from the exchange with atmosphere or /and biosphere after deposition. Thus, the age of travertine determined by this kind of 14C is questionable (Chen and others 1988). For example, for the old travertine mentioned above, the 14C age of 18,000 to 18,800 years was determined, which is much higher than those (2,530~2,570 years) by TIMS dating method (Table 2). Valero-Garces and others (1999) also noted that the dilution effect by large quantities of 14C-free CO2 hinders accurate 14C chronology of the lake records based on lacustrine organic matter and aquatic plants. This situation shows the necessity and importance of clarifying whether the travertine is endogenic or meteogenic (associated with atmospheric and soil CO2; Pentecost 1995) before 14C dating. For endogene travertine, TIMS-Uranium dating may be a better choice than radiocarbon dating (Yong and Benson 1988; Hall and Henderson 2001).
Problems in paleo-vegetation reconstruction with stable carbon isotopes of endogene travertine
Biological effects, variations in carbon source inputs, and variations in the exchange rate with atmospheric CO2 are commonly considered to be the main controls on the carbon isotope values of travertine. However, the geochemical evidence presented above favors physical process CO2-degassing as an important control on 13C enrichment. This finding, coupled with the discussion on the origin of the Baishuitai travertine, indicates that the endogenic CO2 source at Baishuitai, and the physical fractionation may have a greater significance than commonly accepted to explain travertine carbon isotope records. Similar results were also obtained by Melezhik and Fallick (2001). This situation shows that one has to be careful in paleo-vegetation reconstruction when using δ13C data of the endogene carbonate deposits.
References
Chen X, Zhu X, Zhou X (1988) A study on isotopes of karst water and travertine deposits at the Huanglong Scenic Spot. Carsol Sin 7(3):209–212
Deines P, Langmuir D, Harmon RS (1974) Stable carbon isotope ratios and the existence of a gas phase in the evolution of carbonate waters. Geochim Cosmochim Acta 38:1147–1164
Dreybrodt W, Buhmann D, Michaelis J, Usdoswski E (1992) Geochemically controlled calcite precipitation by CO2 outgassing: Field measurements of precipitation rates to theoretical predictions. Chem Geol 97:287–296
Galimov EM (1966) Carbon isotopes of soil CO2. Geochem Int 3:889–897
Hall B L, Henderson G M (2001) Use of uranium-thorium dating to determine past 14C reservoir effects in lakes: examples from Antarctica. Earth Planet Sci Lett 193:565–577
Herman JS, Lorah MM (1987) CO2 outgassing and calcite precipitation in Falling Spring Creek, USA. Chem Geol 62:251–262
Kharaka Y K, Gunter W D, Affarwall P K, Perkins E H, De Braal J D (1988) Solmineq88: a computer program code for geochemical modelling of water–rock interactions. USGeological Survey Water Investigations Report 88-05
Liu Z, Yuan D, Dreybrodt W, Svensson U (1993) The formation of tufa in Huanglong, Sichuan (in Chinese with English abstract). Carsol Sin 12:185–191
Liu Z, Svensson U, Dreybrodt W, Yuan D, Buhmann D (1995) Hydrodynamic control of inorganic calcite precipitation in Huanglong Ravine, China: Field measurements and theoretical prediction of deposition rates. Geochim Cosmochim Acta 59:3087–3097
Liu Z, Yuan D, He S (1997) Stable carbon isotope geochemical and hydrochemical features in the system of carbonate-H2O-CO2 and their implications—evidence from several typical karst areas of China. Acta Geol Sin 71:446–454
Liu Z, Yuan D (2000) Features of geochemical variations in typical epikarst systems of China and their environmental significances (in Chinese with English abstract). Geol Rev 46:324–327
Liu Z, Yuan D, He S, Zhang M, Zhang J (2000) Geochemical features of the geothermal CO2-water-carbonate rock system and analysis on its CO2 sources. Sci China (D) 43:569–576
Melezhik VA, Fallick AE (2001) Palaeoproterozoic travertines of volcanic affiliation from a 13C-rich rift lake environment. Chem Geol 173:293–312
O'Neil JR, Clayton RN, Mayeda TK (1969) Oxygen isotope fractionation in divalent metal carbonates. J Chem Phys 51:5547–5558
Pentecost A (1995) The Quaternary travertine deposits of Europe and Asia Minor. Quat Sci Rev 14:1005–1028
Pentecost A, Zhang Z (2001) A review of Chinese travertines. Cave Karst Sci 28:15–28
Song L, Zhao W, Shen R (1994) The forming mechanism of tufa landscape at Baishuitai, Yunnan, and its improvement and protection. In: Song L (ed) Karst landscape and cave tourism. China Environmental Science Press, pp 59–65
Stuiver M, Polach H (1977) Discussion: reporting of 14C data. Radiocarbon 19(3):355–363
Valero-Garces BL, Delgado-Huertas A, Ratto N, Navas A (1999) Large 13C enrichment in primary carbonates from Andean Altiplano lakes, northwest Argentina. Earth Planet Sci Lett 171:253–266
Yong L, Benson L (1988) Uranium-series age estimates and paleoclimatic significance of Pleistocene tufas from the Lahontan basin. Quat Res 30:165–176
Zhao X, Li T, He S (1998) The Baishuitai of China (in Chinese). Tourism Press of China, Beijing, p 68
Zhu D (1992) China karst tourism resources and their position in the world (in Chinese with English abstract). Carsol Sin 11:332–339
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 40073026), Ministry of Science and Technology of China (grant No. 2002CCA05200), Ministry of Land and Resources (grant No.9806), the Natural Science Foundation of Guangxi (grant No. 0144010). Special thanks are extended to Dr. Chris Groves at Western Kentucky University (USA), Dr. Russell Drysdale at the University of Newcastle, Australia, and an anonymous reviewer for their help in modifying the manuscript, and Dr. Cheng Hai at Minnesota University (USA) for the TIMS dating of the travertine samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, M., Li, Q. et al. Hydrochemical and isotope characteristics of spring water and travertine in the Baishuitai area (SW China) and their meaning for paleoenvironmental reconstruction. Env Geol 44, 698–704 (2003). https://doi.org/10.1007/s00254-003-0811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-003-0811-4