Abstract
Beta-glucosidase (Bgl) is an enzyme with considerable food, beverage, and biofuel processing potential. However, as many Bgls are inhibited by their reaction end product glucose, their industrial applications are greatly limited. In this study, a novel Bgl gene (Bgl1973) was cloned from Leifsonia sp. ZF2019 and heterologously expressed in E. coli. Sequence analysis and structure modeling revealed that Bgl1973 was 748 aa, giving it a molecular weight of 78 kDa, and it showed high similarity with the glycoside hydrolase 3 (GH3) family Bgls with which its active site residues were conserved. By using pNPGlc (p-nitrophenyl-β-D-glucopyranoside) as substrate, the optimum temperature and pH of Bgl1973 were shown to be 50 °C and 7.0, respectively. Bgl1973 was insensitive to most metal ions (12.5 mM), 1% urea, and even 0.1% Tween-80. This enzyme maintained 60% of its original activity in the presence of 20% NaCl, demonstrating its excellent salt tolerance. Furthermore, it still had 83% residual activity in 1 M of glucose, displaying its outstanding glucose tolerance. The Km, Vmax, and kcat of Bgl1973 were 0.22 mM, 44.44 μmol/min mg, and 57.78 s−1, respectively. Bgl1973 had a high specific activity for pNPGlc (19.10 ± 0.59 U/mg) and salicin (20.43 ± 0.92 U/mg). Furthermore, molecular docking indicated that the glucose binding location and the narrow and deep active channel geometry might contribute to the glucose tolerance of Bgl1973. Our results lay a foundation for the studying of this glucose-tolerant β-glucosidase and its applications in many industrial settings.
Key points
• A novel β-glucosidase from GH3 was obtained from Leifsonia sp. ZF2019.
• Bgl1973 demonstrated excellent glucose tolerance.
• The glucose tolerance of Bgl1973 was explained using molecular docking analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta-glucosidase (Bgl, EC 3.2.1.21, synonyms include gentiobiase, cellobiase, or β-D-glucosidase), an essential member of the glycosidase family, can hydrolyze β-glucosidic linkages present in either disaccharides, oligosaccharides, or so-called conjugated glucosides, from non-reducing termini and release β-D-glucose (Godse et al. 2021; Ketudat Cairns and Esen 2010). Bgl has been reported in a wide range of species, such as plants, animals, bacteria, and fungi (Singh et al. 2016), and even in many metagenomic samples (Su et al. 2021). This enzyme has many important applications in food and beverage production, biofuels, and medical fields (Bhatia et al. 2002; Chamoli et al. 2016; Godse et al. 2021; Singh et al. 2016; Zhang et al. 2021b). Bgls also play an important role in the winemaking process to promote the production of aromatic compounds in wine (Zhang et al. 2021b). Bhatia et al. argued that the supplementation of immobilized Bgl to the wine vinification process could significantly enhance the aroma of wine (Bhatia et al. 2002). In addition, Bgl has been applied in simultaneous saccharification and fermentation (Wu et al. 2018). Most phytochemicals in foodstuffs exist in a glycosylated form that cannot be easily absorbed in the intestine (Rossi et al. 2013). Bgls have been applied to release the aglycones of phytochemicals, including isoflavone (Kim et al. 2012), anthocyanidin (Monteiro et al. 2019), and polydatin (Zada et al. 2021), thereby increasing their bioavailability. However, one major drawback of Bgl in industrial applications is that their activities are vulnerable to inhibition by their final products, especially glucose (Salgado et al. 2018).
In the current CAZY database (http://www.cazy.org/Glycoside-Hydrolases.html), glycoside hydrolases (GHs) are divided into 173 families according to their amino sequence and structural similarities. Therein, Bgls are mainly distributed in the GH1, GH2, GH3, GH5, GH16, GH30, GH39, and GH116 families (Kaushal et al. 2021), and they are especially enriched in the GH1 and GH3 families (Sathe et al. 2017). Bgls belonging to the GH1 and GH3 families show distinct structures, explaining the diversity of their enzymatic properties (Tiwari et al. 2016; Zhang et al. 2018). Numerous studies have revealed that most GH1-family Bgls have higher glucose tolerance capabilities than GH3 family Bgls (Godse et al. 2021; Mariano et al. 2014; Salgado et al. 2018). GH1 family Bgls with the inhibition constant (Ki) for glucose higher than 1 M have been isolated from different microbes, such as BglNB11 from Saccharomonospora sp. NB11 (Ki more than 4 M), Bgl from Bacillus subtilis (Ki 1.9 M) (Chamoli et al. 2016), BGL-1 from Talaromyces amestolkiae (Ki 3.78 ± 0.13 M) (Méndez-Líter et al. 2020), Bgl1269 (Ki 4.28 M) (Li et al. 2012), and Bgl6 (Ki 3.5 M) (Cao et al. 2015) from microbial metagenome. On contrast, the enzyme activity of most Bgls from the GH3 family was shown to be inhibited at glucose concentrations less than 100 mM (Bohlin et al. 2013; Harnpicharnchai et al. 2009; Qu et al. 2020; Xia et al. 2016), with some exceptions, such as MpBgl3 from the fungus Malbranchea pulchella (tolerant to 1 M glucose) (Monteiro et al. 2020), Mg9373 from Scleractinian coral-associated bacteria metagenomics (retaining 51.9% activity at 833 mM glucose) (Su et al. 2021), and a GH3 Bgl from Mucor circinelloides (retaining 84% activity at 140 mM glucose) (Huang et al. 2014). Generally speaking, Bgl is the dominant enzyme in the GH3 family, and its catalytic efficiency in the GH3 family is significantly higher than that of the GH1 family (Teugjas and Väljamäe 2013). Therefore, based on the considerable amount of Bgls in the GH3 family, it is worth identifying glucose-tolerant GH3 family Bgls, due to their broad applications in many fields.
The genus Leifsonia, a member of the Microbacteriaceae family in the order Actinomycetales, is characterized as Gram-positive, obligate aerobic, non-motile, rod-shaped, or filamentous bacteria (Evtushenko et al. 2000). Leifsonia.sp.ZF2019, a mesophilic bacterium belong to genus Leifsonia, was isolated from the gut of Artipe eryx larvae, a Lepidoptera pests of Gardenia jasminoides, by our laboratory. In this study, a novel glucose-resistant GH3 Bgl, Bgl1973, was identified and cloned from Leifsonia sp. ZF2019. Bgl1973 was expressed in E. coli and the enzymatic properties were investigated in detail. In addition, the influence of metal ions, organic solvents, NaCl, and chemical reagents, as well as glucose, on Bgl1973 enzyme activity was analyzed. Moreover, the possible mechanism of its tolerance to glucose was studied using molecular docking simulation. Our research provides a new insight into the future application of Bgl in several industries.
Materials and methods
Chemical reagents
The 4-nitrophenyl-β-D-glucopyranoside (pNPGlc), 4-nitrophenyl-β-D-galactopyranoside (pNPGal), 4-nitrophenyl-β-D-xylopyranoside (pNPX), 4'-nitrophenyl-2-acetamido-2-deoxy-β-glucopyranoside(pNPNag), p-nitrophenyl-α-D-glucopyranoside (α-pNPG), isopropyl-β-D-thiogalactopyranoside (IPTG), and glucose oxidation–reduction (GOD-POD) kits were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). p-nitrophenol (pNP) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China). Kits for genomic DNA and plasmid DNA extraction were purchased from Tiangen Biotech Co., Ltd. (Beijing, China). DNA markers, protein makers, Taq DNA polymerase, restriction endonucleases, and T4 DNA ligase were purchased from Promega (Madison, WI, USA). Nucleic acid dyes were purchased from Thermo Fisher Scientific Co., Ltd. (Shanghai, China). All other reagents used were of analytical grade.
Gene sequence analysis and structure modeling
Leifsonia sp. ZF2019 was isolated from the larvae of a pest of Gardenia jasminoides Ellis fruits, and had been deposited in China Center for Industrial Culture Collection (CICC 25134). The whole genome was recently sequenced (GenBank CP065037 and CP065038). IT072_19730 (GenBank UAJ79388.1) was annotated as a glycosyl hydrolase and was designated as Bgl1973 here. Its signal peptide was analyzed previously (Nielsen et al. 2019) using the SignalP 5.0 sever (http://www.cbs.dtu.dk/services/SignalP/). The BLASTP algorithm in NCBI was employed to further compare the gene for this enzyme with the non-redundant (NR) NCBI protein database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). MEGA7 software was employed to construct a phylogenetic tree based on the Neighbor-Joining algorithm. The self-development test Bootstrap values repeatedly for 1000 times were marked on the branches to evaluate the confidence of this phylogenetic tree (Altschul et al. 2005; Kumar et al. 2016). Bgls from the GH3 family, whose enzyme activities and structures had been determined, were retrieved from the CAZy database (Lombard et al. 2014). CLUSTAL-Omega software was used for multiple sequences alignment (Sievers et al. 2011), and ESPrit3.0 online software (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi) was used for secondary structure prediction (Robert and Gouet 2014). ExPASy (https://web.expasy.org) online software was used to predict the isoelectric point and molecular mass of this protein.
Gene cloning and protein expression
The genomic DNA of Leifsonia sp. ZF2019 was obtained using a bacterial DNA extraction kit, according to the manufacturer’s instructions. The Bgl1973 gene was amplified by PCR using a forward primer (5′-TTT TTT CAT ATG GTC GAC CGT CTC GTC-3′, italics indicated the recognition sequence for NdeI) and reverse primer (5′-TT GAA TTC TAC CGT TCG CCG CGG CGA CCA-3′, italics indicated the recognition sequence for EcoRI). The PCR-amplified DNA fragment was then digested with restriction enzymes including EcoRI and NdeI, and this fragment was ligated to the pET-28-HMT expression vector, which contained an HMT tag (6 × His tag, MBP, and TEV protease cleavage site) at the N-terminus. The resultant ligation product was transformed into E. coli DH5α competent cells and screened on LB plates containing 40 μg/L kanamycin. After DNA sequencing by Qingke Biotechnology Co., Ltd (Hangzhou, China), the recombinant plasmid pET-28-HMT-Bgl1973 was transformed into E. coli BL21 (DE3) for protein expression.
This Bgl1973 expression strain was cultured in LB broth containing 40 μg/L kanamycin and 20 μg/L chloramphenicol at 37 °C with 200 rpm shaking, to an OD600 of 0.4–0.7. The protein expression of Bgl1973 was induced at 30 °C for 6 h by adding IPTG (final concentration 0.3 mM). After harvest, cells were resuspended in lysis buffer (20 mM Tris–HCl and 500 mM NaCl, pH 7.0) and disrupted by sonication (30 min with 14 s on and 20 s off). The crude lysate was then centrifuged at 4 °C with 10,000 rpm (10,380 × g) for 20 min, and 20 mL of the supernatant was collected and mixed with 1 mL of Ni–NTA resin that had been pre-equilibrated with lysis buffer (QIAGEN GmbH, Hilden, Germany). The resin was then washed with 20 mM imidazole, and the protein was finally eluted with 300 mM imidazole. To obtain tag-free Bgl1973, the protein was digested with TEV protease at 4 °C for 16 h to cleave the HMT tag and then dialyzed against lysis buffer for 24 h (Austin et al. 2009). Next, the protein was loaded onto Ni–NTA resin to absorb uncleaved HMT-Bgl1973, HMT tag, and TEV protease. Tag-free Bgl1973 in the flow-through fraction was collected (Wu et al. 2012). The purified tag-free Bgl1973 was dialyzed against PBS (10 mM, pH 7.4) for 48 h (changing the dialysate every 6 h) and stored at − 80 °C in PBS with 10% (w/v) glycerol. The relative molecular mass and purity of Bgl1973 protein was determined by SDS-PAGE.
Enzyme assays
The protein content was determined by the Bradford method using BSA as a standard (Bradford 1976). The enzyme activity of Bgl1973 was then determined using pNPGlc as a substrate as previously described (Kaushal et al. 2021). Briefly, the reaction mixture (200 μL) comprised of 2 mM pNPGlc, 130 μL phosphate-citrate buffer (pH 7.0), and 0.2 μg of enzyme was incubated at 50 °C for 20 min. The reaction was then stopped by adding 50 μL of 2 M Na2CO3; the absorption value at 405 nm was determined using a Multiskan Fc microplate reader (Thermo Fisher Scientific, MO, USA). Reaction mixture without enzyme was used as blank. The amount of enzyme needed to release 1 μmol pNP/min was referred to as one unit of enzyme activity.
Determination of optimum temperature and temperature stability of Bgl1973
The enzyme activity at different temperatures (35–60 °C) in phosphate-citrate buffer (pH 7.0) was measured according to the method described in the “Enzyme assay” section. The temperature with the highest enzyme activity was defined as the optimum temperature and its enzyme activity was defined as 100%. To evaluate its temperature stability, Bgl1973 was incubated at different temperatures (35–60 °C) for 60 min and the residual relative enzyme activity was determined at optimum temperature in phosphate-citrate buffer (pH 7.0). The enzyme activity of the untreated enzyme solution was regarded as 100%.
Determination of optimum pH and pH stability of Bgl1973
The enzyme activity was measured in different pH (pH 3.0–10.0) at optimum temperature. The pH with the highest enzyme activity was defined as the optimum pH, and its enzyme activity was defined as 100%. To test pH stability, Bgl1973 was incubated in phosphate-citrate buffer (pH 3.0–8.0) or 0.1 M Gly-NaOH buffer (pH 8.0–10.0) for 12 h at 4 °C, and then the residual relative enzyme activity was determined at optimum temperature in phosphate-citrate buffer (pH 7.0). The enzyme activity of the untreated enzyme solution was defined as 100%.
Determination of the effect of additives on relative enzyme activity
To evaluate the effect of reagents on enzyme activity, Bgl1973 was mixed with organic solvents including ethanol, isopropanol, ethylene glycol, methanol, acetonitrile, and pyridine, to final concentrations of 5%, 10%, 15%, and 20% v/v, or NaCl at final concentrations of 5%, 10%, 15%, 20%, and 25%, as well as metal salts including NiCl2, MgCl2, FeCl3, CaCl2, MnCl4, CoCl2, ZnCl2, KCl, CuSO4, and AlCl3, to final concentrations of 0.5, 5, and 12.5 mM. After incubating at optimum pH and temperature for 20 min, the enzyme activity was assessed. Likewise, Bgl1973 was mixed with SDS (0.1% w/v), imidazole (10 mM), Tween-80 (1% w/v), EDTA (50 mM), or urea (1% w/v) for 20 min in the same conditions and the enzyme activity was then determined. All enzyme activity assays were performed in triplicate for each analysis. The activity of the original enzyme was defined as 100%, and the residual activity of the enzyme after treatment with different reagents was calculated using the following equation:
Enzyme kinetic parameters
To calculate the kinetic parameters of Bgl1973, 0.2 μg of Bgl was mixed with pNPGlc dissolved in phosphate-citrate buffer, pH 7.0 to final concentrations of 0.2, 0.4, 0.6, 0.8, 0.1, 1.5, and 2.0 mM. After incubating at 50 °C for 10 min, the substrate hydrolysis was determined. The Michaelis–Menten constant (Km) and maximum velocity (Vmax) of Bgl1973 were determined using a non-linear fit in OriginPro 8.0 software. The turnover constant (kcat) and the catalytic coefficient (kcat/Km) were then calculated.
Substrate specificity
A total of 0.2 μg of specified Bgl1973 was mixed with individual substrates including pNPGlc, pNPX, pNPGal, α-pNPG, and pNPNag, to a final concentration of 2 mM, and the reactions were incubated in optimum conditions for 20 min, after which the enzyme activity was determined. For oligosaccharide and polysaccharide hydrolysis, substrate (cellobiose, sucrose, salicin, and carboxymethyl cellulose) was dissolved in phosphate-citrate buffer (pH 7.0). A total of 2 μg of Bgl1973 was then mixed with a substrate solution to indicated final concentration to start each reaction. After being incubated in optimal conditions for 20 min, the reactions were stopped by heating at 80 °C for 5 min and the glucose release was measured using a GOP-POD kit (Fusco et al. 2018).
Effect of glucose on enzyme activity
The effect of different concentrations (0–3.5 M) of glucose on the enzyme activity of Bgl1973 was investigated using pNPGlc as a substrate based on the standard enzymatic reaction system under optimal conditions. In addition, the glucose inhibitory constant (Ki) for competitive inhibition was calculated using GraphPad Prism 8.0. The enzyme activity without glucose addition was defined as 100%, and the relative enzyme activity with different concentrations of glucose was calculated.
Molecular docking
A BLAST search was performed against PDB (Protein Data Bank) using the Bgl1973 amino acid sequence as a query to obtain a template for homology modeling. Based on the Swiss-Model online modeling server (https://www.swissmodel.expasy.org/), a Bgl1973 protein structure was predicted and an automatic model for Swiss-Model was constructed (Waterhouse et al. 2018) using BaBgl3 from Bifidobacterium adolescentis (PDB: 5WAB) as a template. The save tool (https://servicesn.Mbi.ucla.edu/saves) was used to evaluate the quality of the predicted tertiary structure and PyMOL mapping software was used to draw the three-dimensional structure of this protein.
To obtain an insight into the potential mechanism of the glucose tolerance of Bgl1973, Bgl1973 (glucose tolerant) and BaBgl3 (glucose intolerant) were docked with ligands (pNPGlc and glucose) using AutodockTools1.5.6. The chemical structures of pNPGlc and glucose were downloaded in MOL2 format from ZINC (http://zinc.docking.org). The model structure of the protein was used as a receptor protein, and the active site was wrapped in a docking box. The number of grid points in the X, Y, Z directions was set to 74 × 90 × 78, the grid spacing was 0.375 Å, the number of docking times was 100, and the remaining parameters were set to default values. The ligand was then moved into this grid to obtain the lowest energy conformation for further analysis using PyMol and Discovery Studio Client molecular visualizing software.
Statistical analyses
All statistical data is presented as the mean ± standard deviation of three determinations using Origin 8.0 (Originlab, Massachusetts, USA) and SPSS 20.0 (IBM, NY, USA). The Student’s t test was employed to evaluate the significant difference between two groups. *p < 0.05.
Results
Sequence analysis of Bgl1973
DNA sequence data showed that Bgl1973 consisted of 748 amino acids and was annotated as a putative GH3 family glycosyl hydrolase. It had 70.82% similarity to the GH3 family β-glucosidase from Rhodococcus sp. WS4 (GenBank: TQC50928.1). To clarify the relationship of Bgl1973 with other GH3 family glycosyl hydrolases, twenty-three GH3 family genes, including four xylosidases, 14 β-glucosidases, and five β-N-acetylglucosaminidase, were retrieved from the NCBI database and a phylogenetic tree was constructed. The results showed that Bgl1973 was clustered in the β-glucosidase branch, while it had a distant relationship with the β-N-acetylglucosaminidase and β-xylosidases in the GH3 family (Fig. 1).
Multiple sequence alignment was then performed between Bgl1973 and three structure determined GH3 β-glucosidases from Bifidobacterium adolescentis (PDB: 5WAB) (Florindo et al. 2018), Kluyveromyces marxianus (PDB: 3ABZ) (Yoshida et al. 2010), and Streptomyces venezuelae (PDB: 4I3G) (Zmudka et al. 2013). As shown in Fig. 2, Bgl1973 had multiple highly conserved amino-acid motifs, including SDW and SEGF domains, and importantly, a D230 in the SDW motif and an E419 in the SEGF motif were identified as the key residues for nucleophilic attack and as a proton donor in GH3 Bgl, respectively (Bause and Legler 1974; Varghese et al. 1999).
Multiple sequence alignment of Bgl1973 against three characterized GH3 family β-glucosidases. Bifidobacterium adolescentis (PDB: 5WAB) (Florindo et al. 2018a), Kluyveromyces marxianus (PDB: 3ABZ) (Yoshida et al. 2010), and Streptomyces venezuelae (PDB: 4I3G) (Zmudka et al. 2013). The catalytic sites are represented by an asterisk (*) on the bottom of the alignment. The conserved catalytic aspartic acid residue act as active nucleophile sites (D230) and conserved glutamic acid acts as catalytic proton donors (E419)
Cloning and expression of Bgl1973
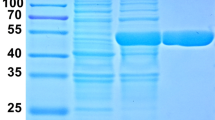
The cloned Bgl1973 gene was next inserted into the pET-28-HMT vector with an N-terminal HMT tag composed of 6 × His, maltose binding protein (MBP), and a TEV protease cut site (Fig. 3a). The recombination plasmid was verified by sequencing and expressed as soluble protein in E. coli BL21 (DE3). This recombinant HMT-Bgl1973 protein was purified using Ni–NTA affinity chromatography. After being cut with TEV protease, the HMT Tag, uncleaved HMT-Bgl1973, and TEV protease were removed using Ni–NTA resin, and homogeneous untagged Bgl1973 protein was finally obtained. The purified Bgl1973 protein showed a single band with a molecular weight of appropriately 75 kDa by SDS-PAGE, which was consistent with its theoretical predicted molecular weight (78 kDa) (Fig. 3b).
The purification of Bgl1973 and the effects of pH and temperature on its activity. a Schematic diagram of the pET-28-HMT-Bgl1973 expression vector. b SDS-PAGE of purified Bgl1973. Samples were subjected to 10% SDS-PAGE and gels were stained with Coomassie brilliant blue R250. Lane M: Protein marker; Lane 1: Purified HMT-Bgl1973; Lane 2: HMT-Bgl1973 digested with TEV; Lane 3: Purified Bgl1973 (without HMT tag). c Optimum temperature determination. The activity of Bgl1973 was measured over a range of temperatures (35–60 °C) at pH 7.0. d Thermostability assessment. Bgl1973 was incubated at different temperatures (35-–60 °C) for 60 min and the residual enzyme activity was determined. e Optimum pH determination. The activity was measured over a range of pH values (3.0–8.0). f pH stability. Bgl1973 was incubated at pH 7.0 with different pH (pH 3.0–10.0) for 12 h at 4 °C, and the activity was determined at the optimum temperature
Effects of pH and temperature on the enzymatic activity and stability of Bgl1973
Temperature and pH are key parameters for enzymatic activity and stability. As shown in Fig. 3c, the optimum temperature of Bgl1973 was 50 °C at pH 7.0. Most mesophilic β-glucosidases show maximal activity in the temperature range from 35 to 65 °C (Ketudat Cairns and Esen 2010). The optimum temperature of Bgl1973 was similar to most GH3 family β-glucosidases from other mesophilic microorganisms, such as Pseudoalteromonas sp. (Qu et al. 2020) and Serratia sp.TN49 (Zhou et al. 2011). Thus, Bgl1973 was pre-incubated in different temperatures (35–60 °C) for 1 h to determine its thermostability. Bgl1973 retained more than 80% of its maximal activity under 40 °C. However, its enzyme activity rapidly declined with temperature increase, and the activity almost could not be detected at 60 °C (Fig. 3d).
The effect of pH on the enzymatic activity of Bgl1973 is shown in Fig. 3e. The optimum pH of Bgl1973 was 7.0. It retained more than 75% relative activity at pH 6.0 and pH 7.5, while the residual activity was 24.94% and 53.65% of the maximal activity at pH 5.0 and pH 8.0, respectively. The results indicated that Bgl1973 preferred neutral pH conditions. Interestingly, Bgl1973 displayed an interesting pH-stability characteristic (Fig. 3f). The activity of Bgl1973 was maintained at approximately 80% of its initial activity in the pH range from 7.0 to 8.0 after pre-incubation at 4 °C for 12 h, while its pH stability was decreased in pH values below 6.0 or above 9.0. The results indicated that Bgl1973 could function in neutral conditions and maintain it stability in weakly alkaline environments. Interestingly, a β-glucosidase from Serratia sp. TN49, a symbiotic bacterium isolated from the gut of long horned beetle (Batocera horsfieldi) larvae, was also reported to tolerate to alkaline conditions (Zhou et al. 2011).
Effects of organic solvents, NaCl, metal ions, and chemical reagents on the enzyme activity of Bgl1973
The effects of organic solvents including ethanol, isopropanol, ethylene glycol, methanol, acetonitrile, and pyridine on the enzyme activity of Bgl1973 are presented in Fig. 4a. We found that organic solvents had significant inhibitory effects on the enzymatic activity of Bgl1973 in a dose-dependent manner. At a concentration of 5% pyridine, the residual activity of Bgl1973 was less than 20%, indicating it was susceptible to pyridine. The retained activity was 65–95% in a concentration of 5% ethanol, isopropanol, methanol, or acetonitrile, while the activity decreased dramatically as the concentrations increased to 10%. In contrast, the enzyme activity of Bgl1973 was significantly enhanced by ethylene glycol, especially 15% ethylene glycol (130.0 ± 7.2%).
Salt is often employed to pretreat raw biomasses in industrial settings. To investigate the salt-tolerance of Bgl1973, the enzyme activity was evaluated in the presence of different concentrations of NaCl. As shown in Fig. 4b, the enzyme activity of Bgl1973 weakened as the NaCl concentration increased, but Bgl1973 still had more than 60% residual activity in 20% (3.40 M) NaCl and maintained 34% residual activity even at a concentration of NaCl up to 25% (4.27 M). The NaCl tolerance capability of Bgl1973 was comparable to that of β-glucosidases from marine bacterial Alteromonas sp. L82 (53.3% activity at 4 M NaCl) (Sun et al. 2018) and Thermococcus sp. (70% activity in the presence of 1.5 M NaCl) (Sinha and Datta 2016). The interplay of the hydrophobic and electrostatic interactions between the protein and the salt solution may contribute to the salt-tolerance of Bgl, similar to other halophilic proteins (Arakawa and Tokunaga 2004).
Table 1 summarizes the effects of different concentrations of metal ions on the enzyme activity of Bgl1973 at a final concentration of 0.5 mM, 2.5 mM, and 12.5 mM. Bgl1973 displayed excellent metal ion tolerance. Its enzyme activity was not impaired by Ca2+, K+, Na+, Co2+, Mn2+, Al3+, Mg2+, or Fe3+ ions, even when the final concentration reached 12.5 mM. In contrast, Zn2+ and Cu2+ showed some inhibitory effects on the Bgl1973 activity, which maintained 73.62% and 88.78% of its original enzyme activity in the presence of 12.5 mM Zn2+ and Cu2+, respectively.
Other chemical reagents, such as Tween-80, SDS, urea, imidazole, and EDTA, are usually used as pretreatment reagents for biomass processing. As shown in Table 2, EDTA (50 mM) did not significantly inhibit Bgl1973 activity, a result that was consistent with the influence of metal ions. Interestingly, the enzyme activity of Bgl1973 increased in the presence of 1% Tween-80 (w/v), 10 mM imidazole, 50 mM EDTA, and 1% urea (w/v). In contrast, the Bgl1973 activity was destroyed by 0.1% SDS, which could be partially explained by the unique properties of SDS to disrupt the hydrophobic interaction of proteins or cause the unfolding of their natural structures (Kaushal et al. 2021). Generally speaking, the overwhelming inhibitory effect of SDS on Bgl activity is expected (Méndez-Líter et al. 2017; Zhou et al. 2011) and only a few Bgl proteins can resist SDS (Sun et al. 2020).
Effect of glucose on Bgl1973 enzymatic activity
The effect of glucose on the enzymatic activity of Bgl1973 is shown in Fig. 4c, and the enzymatic activity of Bgl1973 gradually decreased with increasing glucose concentration (0–1.0 M). The Bgl1973 activity still maintained 83% of its original enzymatic activity in the presence of 1.0 M glucose. When the glucose concentration was higher than 1.0 M, the activity rapidly decreased with glucose concentration increase, even decreasing to 20% activity in the presence of 3.5 M glucose. Furthermore, the glucose inhibitory constant (Ki) of Bgl1973 was determined to be 1713 mM, higher than that of GH3 family Bgls from most sources (Table 3).
Kinetic parameters
The kinetics of enzyme-catalyzed reactions can reflect the rate of an enzymatic reaction and its influencing factors, and it can be used to infer the possible chemical changes from substrate to product. The kinetic parameters of Bgl1973 were then determined using pNPGlc as a substrate. As shown in Fig. 4d and Table 3, under optimal conditions, the Km and Vmax of the Bgl1973 were 0.22 mM and 44.44 μmol/min mg respectively. Additionally, the apparent kcat and catalytic coefficient (kcat/Km) was 57.78 s−1 and 262.6 s−1 mM−1, respectively. The kinetic parameters of many reported Bgls from GH3 family were summarized in Table 3. As can be seen, the Km value of Bgl1973 was similar to most of GH3 family Bgls but much lower than that of Bgls from Str. venezuelae (Zmudka et al. 2013), Serratia sp. TN49 (Zhou et al. 2011), and Scleractinian coral metagenomics (Su et al. 2021), indicating that Bgl1973 showed a high substrate affinity for pNPG. The ratio of kcat to Km is an important parameter for measuring the catalytic efficiency of enzymes. Accordingly, the kcat/Km value of Bgl1973 was measured, and it was lower than Bgls from Asp. oryzae (Langston et al. 2006) and K. marxianus (Yoshida et al. 2010), but it still has similar kinetic properties to most GH3 Bgls.
Substrate specificity of Bgl1973
Different substrates were next used to analyze the substrate specificity of Bgl1973, and the results are shown in Table 4. For aryl glycoside substrates, the specific activity of Bgl1973 against pNPGlc was 19.10 ± 0.59 U/mg, whereas the specific activity of Bgl1973 against pNPX was 3.83 ± 0.06 U/mg. In addition, we found that Bgl1973 could hydrolyze cellobiose containing β (1 → 4) glycoside bonds but could not hydrolyze sucrose-containing α (1 → 2) glycosidic bonds, indicating that Bgl1973 was a β (1 → 4) glycosidase. As expected, the glycoside of salicin could be effectively hydrolyzed by Bgl1973 (20.43 ± 0.92 U/mg) but carboxymethyl cellulose could not be hydrolyzed by Bgl1973.
Structural mechanism of glucose tolerance
The glucose tolerance mechanism of Bgl1973 was investigated by using homology modeling and molecular docking stimulation. By search against PDB (Protein Databank), it was found that the amino sequence of Bgl1973 had the highest similarity with that of B. adolescentis GH3 family β-glucosidase BaBgl3 (PDB ID 5wab) (Florindo et al. 2018) with the identity of 50.26%. The three-dimensional structure of Bgl1973 was modeled by homology modeling using the crystal structure of BaBgl3 (PDB ID 5wab) as a template. The potential pNPGlc and glucose binding sites were subsequently analyzed by molecular docking stimulation.
As shown in (Fig. 5a–b), Bgl1973 has a typical (β/α)8 barrel structure and (α/β)6 sandwich domains, Asp230, and Glu419 residues on these two domains that act as nucleophilic groups and proton donors, respectively (Ketudat Cairns and Esen 2010). A Ramachandran plot was obtained by analyzing the tertiary structure of Bgl1973, and this revealed that the proportion of amino acid residues in the allowable region exceeded 95% (Fig. 5c), indicating that the predicted tertiary structure of Bgl1973 was reliable and could be used as a template for molecular docking.
The molecular docking diagram between BaBgl3 (glucose intolerant) and pNPGlc and pNPGlc + glucose is shown in Fig. 6a–g. The Asp44 and Lys153 are the primary amino acids of active pocket of BaBgl3 which interact with sugar moiety (Fig. 6b). Asp44 makes hydrogen bonds with glucose hydroxyl groups OH3 and OH4, while Lys153 interacts with OH2 and OH3 of glucose (Fig. 6c). As shown in Fig. 6d, the binding site of glucose is almost overlapped with sugar moiety of pNPGlc. The hydrophilic amino acids of the active pocket interacts with glucose including Asp44, Arg120, Lys153, His154, Arg164, Tyr200, and Asp232 (Fig. 6d). Asp44 and Lys153 interact with glucose hydroxyl groups OH1 through hydrogen bonding, and Asp232 interacts with OH4, which was consistent as Florindo et al. (Florindo et al. 2018). Glucose prefers binding the bottom of the active pocket (Fig. 6g) and prevents pNPGlc from binding, which may be the reason for its glucose intolerance.
Molecular docking of BaBgl3 and Bgl1973 with glucose and pNPGlc. Interaction analysis of BaBgl3 (5wab) (a ~ b) and Bgl1973 ((a) ~ (b)) with pNPGlc: pNPGlc and binding sites are shown in yellow and red, respectively; two-dimensional (2D) view of BaBgl3 interactions with pNPGlc (c) and glucose (f), Bgl1973 interactions with pNPGlc ((c)) and glucose ((f)). Interaction analysis of BaBgl3 (d ~ e) and Bgl1973 ((d) ~ (e)) with both pNPGlc and glucose. pNPGlc, glucose, and binding sites are shown in yellow, magenta, and red, respectively. Electrostatic potential surface of BaBgl3 (g) and Bgl1973 ((g)). These images were created using PyMOL and Discovery Studio Client molecular visualizing software
Fig 6(a)–(g) shows molecular docking results between Bgl1973 and pNPGlc and pNPGlc + glucose. Arg162, Ala349, Ser418, and Phe421 are the critical amino acids for the interaction between Bgl1973 and pNPGlc (Fig (b)). Ser418 and Phe421 interact with glucose hydroxyl groups OH6, and Ala349 interacts with OH3 and OH4 via hydrogen bonds, respectively (Fig (c)). Arg162 could form hydrogen bond with nitro group of p-nitrophenol moiety. In contrast, the binding site of glucose was different from that of sugar moiety of pNPGlc in Bgl1973. Residues including Asp43, Arg49, Lys151, His152, Arg162, Glu221, and Asp230 played an essential role in the interaction between glucose and Bgl1973 (Fig. 6(d)–(e)). Arg162, Asp232, and Lys151 made hydrogen bonds with glucose hydroxyl groups OH1, OH2, and OH4, respectively. His152 and Met195 interacted with OH3, and Arg49 and Glu121 interact with OH6 (Fig. 6(f)). The binding location was partially overlapped with p-nitrophenol moiety but distinct from that of sugar moiety (Fig. 6(g)). In addition, the active pocket of Bgl1973 was found to be narrower and deeper than that of BaBgl3 (Fig. 6(g)).
Discussion
The β-glucosidases have abilities to both hydrolyze and synthesize β-D-glycosidic bonds with a variety of substrates, and have attracted much attention for a range of applications, including bioethanol production, fruit juice and wine aroma improvement, and bioactive aglycon and oligosaccharide synthesis (Salgado et al. 2018; Singh et al. 2016; Zhang et al. 2021b). However, the activity of β-glucosidases is easily affected by many physiochemical conditions, and many β-glucosidase are especially sensitive to their end product glucose, which significantly limits their applications in many industries (Salgado et al. 2018). The identification of glucose-tolerant enzymes is of great importance for improving their industrial applications and provides the possibility to create more robust enzymes via genetic modification (Singhania et al. 2017). In the present study, a glucose-tolerant GH3-family β-glucosidase (Bgl1973) from Leifsonia sp. ZF2019 was expressed in E. coli and characterized.
Most of the characterized Bgls have shown strong enzymatic activity in weakly acidic conditions (pH 4.0–6.5) (Meng et al. 2009; Teugjas and Väljamäe 2013). For example, the optimal pH of BGL0224 from O. oeni SD-2a was 5.0 (Zhang et al. 2021a), while the optimal pH of PABGL from Pseudoalteromonas was 7.0 (Qu et al. 2020). The optimum pH of Bgl1973 was found to be 7.0 and to be more stable in neutral and weakly alkaline conditions (Fig. 3). This property can be efficiently utilized in the textile industry due to more favorable polymer formation under weakly alkaline conditions in the presence of Bgl (Meng et al. 2009). Bgl1973 also showed maximal activity at 50 °C while the residual activity decreased to appropriate 38% of basal after being pre-incubating at 50 °C for 1 h. A similar result has been reported in other studies about Microbulbifer thermotolerans (Pyeon et al. 2019) and Thermoanaerobacterium saccharolyticum (Kim et al. 2022). The results indicated that, although Bgl1973 has the highest activity at 50 °C, the structure was not sufficiently stable to maintain its conformation and activity at temperatures above 40 °C.
During the bioconversion of lignocellulosic biomass to biofuels, various pretreatments (including dilute acid, alkaline, organic solvents, among others) or additives (such as detergents and metal ions) are often employed as pre-treatments (Chen et al. 2018). The residues of these substances may affect enzyme activity. Although Bgl1973 can only maintain its activity at low concentrations (5%, w/v) of organic solvents (ethanol, isopropanol, methanol, or acetonitrile), Tween-80 (1%, w/v), urea (1%, w/v), and imidazole (10 mM) were associated with a stimulatory effect on Bgl1973 activity (Table 2). As a detergent, Tween-80 may facilitate the release of products from enzymes via reduction of the interaction between Bgl1973 and the end product glucose (Zheng et al. 2008). Furthermore, Tween-80 has been proposed to increase cellulase stability by reducing thermal denaturation (Yang et al. 2011). 0.5 mM imidazole has been reported as a inhibitor of the sweet almond β-glucosidase (Field et al. 1991). However, it has also been reported to activate sweet almond β-glucosidase by exerting a conformational transition poising the enzyme towards more proficient catalysis in a concentration range of 0.125–0.250 mM (Caramia et al. 2017). Imidazole may stimulate the activity of Bgl1973 through a similar mechanism. Urea is a strong denaturant and most of Bgls are sensitive to urea (Jabbour et al. 2012; Kar et al. 2017). The activity of Bgl1973 increased slightly in the present of 1% urea. The urea resistance of Bgl1973 was comparable with that of SfBGL1 from Saccharomycopsis fibuligera and TrBGL1 from Trichoderma reesei, which retain 63.2 ± 0.1% and 91.5 ± 1.5% of their respective activity in the presence of 0.4 M urea (Ma et al. 2015). These results indicated that Bgl1973 was stable and compatible to hydrolyze various pretreated lignocellulose types.
Although most metal ions do not result in any significant inhibition effect on the activity of Bgls, Ag+, Hg+2, Cu2+, Zn2+, and Fe3+ have been reported to inhibit Bgl activity to some extent. The activity of BglA from Paenibacillus xylanilyticus KJ-03 was decreased to 2% and 30% in the presence of 5 mM Cu2+ and Zn2+, respectively (Park et al. 2013). BglA49 from Serratia sp.TN49 maintained 16.03% of its original enzyme activity at 10 mM Cu2+ and only 15.07% was presented in 10 mM Zn2+ (Zhou et al. 2011). The activity of BglNB11 from Saccharomonospora sp. NB11 was completely inhibited by 2 mM Cu2+ or Zn2+ (Saleh Zada et al. 2021). The current study found that Bgl1973 was stable in the presence of 12.5 mM of various metal ions and showed excellent NaCl tolerance, indicating that it might be suitable for application in multiple metal ion conditions.
The kinetic parameters of Bgls from various resources are compared in Table 3. The kinetic parameters varied in response to the resources used as substrates. The Km, Vmax, and kcat of Bgl1973 were 0.22 mM, 44.44 μmol/min mg, and of 57.78 s−1, respectively. Bgl1973 has a low Km for pNPGlc and a relatively high kcat. Similarly, Bgl1973 has a higher specific activity for pNPGlc (19.10 ± 0.59 U/mg) and salicin (23.73 ± 0.82 U/mg), but it showed lower activity on pNPX or cellobiose and could hardly hydrolyze other substrates. Therefore, it can be classified as an aryl-BGL (Zhang et al. 2021a).
It was interesting to note that the activity of Bgl1973 only decreased 20% in 1 M glucose. The glucose tolerance capability of Bgl1973 was comparable to many glucose tolerance β-glucosidases from the GH1 family, such as Bglm from a hot-spring metagenome (retains 80% activity in 1 M glucose) or AaBGL1 Actinomadura amylolytica YIM 77,502 (retains 40% activity in 2 M glucose) (Yin et al. 2018), but lower than DT-Bgl from Anoxybacillus sp. DT3-1 (Chan et al. 2016), or BglNB11 from Saccharomonospora sp. NB11 (Saleh Zada et al. 2021). This was higher than almost all other GH3 Bgls (Table 3), with the exception of MpBgl3 from fungal M. pulchella, which had a similar glucose tolerance capability (tolerant to 1 M glucose) (Monteiro et al. 2020) as Bgl1973. To the best of our knowledge, the majority of the glucose-tolerant Bgls belong to the GH1 family. Hence, Bgl1973 may provide an alternative model for the analysis of the glucose tolerance mechanism of Bgls.
Homology modeling and molecular docking were also carried out to gain insights into the glucose tolerance mechanism of Bgl1973 by comparing it with a glucose-intolerant GH3 Bgl from B. adolescentis (BaBgl3). Asp44 and Arg120 were the binding sites for both sugar moiety of pNPGlc and glucose in BaBgl3 (Fig. 6a–f), while only one site (Arg162) contacted with p-nitrophenol moiety of pNPGlc and glucose in Bgll973 (Fig. 6(a)–(f)). In this case, glucose and pNPGlc compete for binding sites that may result in BaBgl3 glucose intolerance, similar to HiBG (de Giuseppe et al. 2014). In contrast, glucose and pNPGlc hardly compete for interaction with Bgl1973. Furthermore, the glucose binding sites of Bgl1973 were found to be located in the middle of the active site channel, which was similar to the glucose-stimulated GH1 Bgl (BglNB11) from Saccharomonospora sp. NB11 (Saleh Zada et al. 2021). The glucose located in the middle of the active site channel was also found to activate the cleavage of the substrate through transglycosylation or other mechanisms (such as allosteric effect) in glucose-tolerance Bgls from the GH1 family (Yang et al. 2015). In addition, the active channel of Bgl1973 (Fig. 6(g)) was shown to be narrower and deeper than that of BaBgl3 (Fig. 6g). The deeper catalytic cavity and less accessible catalytic site entrance for substrate were proposed to contribute to the glucose tolerance of GH1 (de Giuseppe et al. 2014) and MpBgl3 (Monteiro et al. 2020). Despite further experiments being needed to verify this, the glucose binding location and the narrow and deep active channel geometry may contribute to the glucose tolerance of Bgl1973.
In summary, a novel glucose-tolerant GH3 family β-glucosidase Bgl1973 was cloned from Leifsonia sp. ZF2019, a bacterium isolated from the larvae of a pest of Gardenia jasminoides Ellis fruits. It was heterologously expressed in E. coli, and the biochemical properties were characterized here for the first time. Bgl1973 was active in neutral and weakly alkaline conditions at moderate temperatures. Bgl1973 showed excellent salt and metal ion tolerance and maintained 80% of its activity in the presence of 1 M glucose. A molecular docking study suggested that the glucose binding location and the narrow and deep active channel geometry may contribute to the glucose tolerance of Bgl1973. These results provide a foundation for the analysis of glucose-tolerant molecular mechanisms in other Bgls and have future applications to Bgls in other industries.
Data availability
The authors will make all data underlying the described findings available without restriction upon request.
Code availability
Not applicable.
References
Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK (2005) Protein database searches using compositionally adjusted substitution matrices. FEBS J 272(20):5101–5109. https://doi.org/10.1111/j.1742-4658.2005.04945.x
Arakawa T, Tokunaga M (2004) Electrostatic and hydrophobic interactions play a major role in the stability and refolding of halophilic proteins. Protein Pept Lett 11(2):125–132. https://doi.org/10.2174/0929866043478220
Austin BP, Nallamsetty S, Waugh DS (2009) Hexahistidine-tagged maltose-binding protein as a fusion partner for the production of soluble recombinant proteins in Escherichia coli. Methods Mol Biol 498:157–172. https://doi.org/10.1007/978-1-59745-196-3_11
Bause E, Legler G (1974) Isolation and amino acid sequence of a hexadecapeptide from the active site of beta-glucosidase A3 from Aspergillus wentii. Hoppe Seylers Z Physiol Chem 355(4):438–442. https://doi.org/10.1515/bchm2.1974.355.1.438
Bhatia Y, Mishra S, Bisaria VS (2002) Microbial beta-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol 22(4):375–407. https://doi.org/10.1080/07388550290789568
Bohlin C, Praestgaard E, Baumann MJ, Borch K, Praestgaard J, Monrad RN, Westh P (2013) A comparative study of hydrolysis and transglycosylation activities of fungal β-glucosidases. Appl Microbiol Biotechnol 97(1):159–169. https://doi.org/10.1007/s00253-012-3875-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Cao L-c, Wang Z-j, Ren G-h, Kong W, Li L, Xie W, Liu Y-h (2015) Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol Biofuels 8(1):202. https://doi.org/10.1186/s13068-015-0383-z
Caramia S, Gatius AGM, Dal Piaz F, Gaja D, Hochkoeppler A (2017) Dual role of imidazole as activator/inhibitor of sweet almond (Prunus dulcis) beta-glucosidase. Biochem Biophys Rep 10:137–144. https://doi.org/10.1016/j.bbrep.2017.03.007
Chamoli S, Kumar P, Navani NK, Verma AK (2016) Secretory expression, characterization and docking study of glucose-tolerant β-glucosidase from B. subtilis. Int J Biol Macromol 85:425–433. https://doi.org/10.1016/j.ijbiomac.2016.01.001
Chan CS, Sin LL, Chan KG, Shamsir MS, Manan FA, Sani RK, Goh KM (2016) Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp DT3-1. Biotechnol Biofuels 9(1):174. https://doi.org/10.1186/s13068-016-0587-x
Chen YA, Zhou Y, Qin YL, Liu DH, Zhao XB (2018) Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: pretreatment, hydrolysis conditions and lignin structure. Bioresour Technol 269:329–338. https://doi.org/10.1016/j.biortech.2018.08.119
de Giuseppe PO, Souza TDCB, Souza FHM, Zanphorlin LM, Machado CB, Ward RJ, Jorge JA, Furriel RDM, Murakami MT (2014) Structural basis for glucose tolerance in GH1 beta-glucosidases. Acta Crystallogr D 70:1631–1639. https://doi.org/10.1107/S1399004714006920
Evtushenko LI, Dorofeeva LV, Subbotin SA, Cole JR, Tiedje JM (2000) Leifsonia poae gen nov sp nov isolated from nematode galls on Poa annua and reclassification of “Corynebacterium aquaticum” Leifson 1962 as Leifsonia aquatica (ex Leifson 1962) gen nov nom rev comb nov and Clavibacter xyli (Davis et al 1984) with two subspecies as Leifsonia xyli Davis et al 1984 gen nov comb nov. Int J Syst Evol Microbiol 50(1):371–380. https://doi.org/10.1099/00207713-50-1-371
Field RA, Haines AH, Chrystal EJT, Luszniak MC (1991) Histidines, histamines and imidazoles as glycosidase inhibitors. Biochem J 274(3):885–889. https://doi.org/10.1042/bj2740885
Florindo RN, Souza VP, Manzine LR, Camilo CM, Marana SR, Polikarpov I, Nascimento AS (2018) Structural and biochemical characterization of a GH3 β-glucosidase from the probiotic bacteria Bifidobacterium adolescentis. Biochimie 148:107–115. https://doi.org/10.1016/j.biochi.2018.03.007
Fusco FA, Fiorentino G, Pedone E, Contursi P, Bartolucci S, Limauro D (2018) Biochemical characterization of a novel thermostable β-glucosidase from Dictyoglomus turgidum. Int J Biol Macromol 113:783–791. https://doi.org/10.1016/j.ijbiomac.2018.03.018
Godse R, Bawane H, Tripathi J, Kulkarni R (2021) Unconventional β-glucosidases: a promising biocatalyst for industrial biotechnology. Appl Biochem Biotechnol 193(9):2993–3016. https://doi.org/10.1007/s12010-021-03568-y
Harnpicharnchai P, Champreda V, Sornlake W, Eurwilaichitr L (2009) A thermotolerant beta-glucosidase isolated from an endophytic fungi Periconia sp with a possible use for biomass conversion to sugars. Protein Expr Purif 67(2):61–69. https://doi.org/10.1016/j.pep.2008.05.022
Huang Y, Busk PK, Grell MN, Zhao H, Lange L (2014) Identification of a β-glucosidase from the Mucor circinelloides genome by peptide pattern recognition. Enzyme Microb Technol 67:47–52. https://doi.org/10.1016/j.enzmictec.2014.09.002
Jabbour D, Klippel B, Antranikian G (2012) A novel thermostable and glucose-tolerant beta-glucosidase from Fervidobacterium islandicum. Appl Microbiol Biotechnol 93(5):1947–1956. https://doi.org/10.1007/s00253-011-3406-0
Kar B, Verma P, den Haan R, Sharma AK (2017) Characterization of a recombinant thermostable β-glucosidase from Putranjiva roxburghii expressed in Saccharomyces cerevisiae and its use for efficient biomass conversion. Process Biochem 63:66–75. https://doi.org/10.1016/j.procbio.2017.08.005
Kaushal G, Rai AK, Singh SP (2021) A novel β-glucosidase from a hot-spring metagenome shows elevated thermal stability and tolerance to glucose and ethanol. Enzyme Microb Technol 145:109764. https://doi.org/10.1016/j.enzmictec.2021.109764
Ketudat Cairns JR, Esen A (2010) beta-Glucosidases. Cell Mol Life Sci 67(20):3389–33405. https://doi.org/10.1007/s00018-010-0399-2
Kim BN, Yeom SJ, Kim YS, Oh DK (2012) Characterization of a β-glucosidase from Sulfolobus solfataricus for isoflavone glycosides. Biotechnol Lett 34(1):125–129. https://doi.org/10.1007/s10529-011-0739-9
Kim IJ, Bornscheuer UT, Nam KH (2022) Biochemical and structural analysis of a glucose-tolerant beta-glucosidase from the hemicellulose-degrading Thermoanaerobacterium saccharolyticum. Molecules 27(1):290. https://doi.org/10.3390/molecules27010290
Kumar S, Stecher G, Tamura K (2016) MEGA7 molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Langston J, Sheehy N, Xu F (2006) Substrate specificity of Aspergillus oryzae family 3 beta-glucosidase. Biochim Biophys Acta 1764(5):972–978. https://doi.org/10.1016/j.bbapap.2006.03.009
Li G, Jiang Y, Fan XJ, Liu YH (2012) Molecular cloning and characterization of a novel β-glucosidase with high hydrolyzing ability for soybean isoflavone glycosides and glucose-tolerance from soil metagenomic library. Bioresour Technol 123:15–22. https://doi.org/10.1016/j.biortech.2012.07.083
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B 2014 The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42(Database issue):D490-D495.https://doi.org/10.1093/nar/gkt1178
Ma Y, Liu X, Yin Y, Zou C, Wang W, Zou S, Hong J, Zhang M (2015) Expression optimization and biochemical properties of two glycosyl hydrolase family 3 beta-glucosidases. J Biotechnol 206:79–88. https://doi.org/10.1016/j.jbiotec.2015.04.016
Méndez-Líter JA, Gil-Muñoz J, Nieto-Domínguez M, Barriuso J, de Eugenio LI, Martínez MJ (2017) A novel, highly efficient β-glucosidase with a cellulose-binding domain: characterization and properties of native and recombinant proteins. Biotechnol Biofuels 10:256. https://doi.org/10.1186/s13068-017-0946-2
Méndez-Líter JA, Nieto-Domínguez M, Fernández de Toro B, González Santana A, Prieto A, Asensio JL, Cañada FJ, de Eugenio LI, Martínez MJ (2020) A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microb Cell Fact 19(1):127. https://doi.org/10.1186/s12934-020-01386-1
Meng X, Shao Z, Hong Y, Lin L, Li C, Liu Z (2009) A novel pH-stable bifunctional xylanase isolated from a deep-sea microorganism Demequina sp JK4. J Microbiol Biotechnol 19(10):1077–1084
Monteiro LMO, Pereira MG, Vici AC, Heinen PR, Buckeridge MS, Polizeli M (2019) Efficient hydrolysis of wine and grape juice anthocyanins by Malbranchea pulchella β-glucosidase immobilized on MANAE-agarose and ConA-Sepharose supports. Int J Biol Macromol 136:1133–1141. https://doi.org/10.1016/j.ijbiomac.2019.06.106
Monteiro LMO, Vici AC, Pinheiro MP, Heinen PR, de Oliveira AHC, Ward RJ, Prade RA, Buckeridge MS, Polizeli M (2020) A highly glucose tolerant ss-glucosidase from Malbranchea pulchella (MpBg3) enables cellulose saccharification. Sci Rep 10(1):6998. https://doi.org/10.1038/s41598-020-63972-y
Mariano DCB, Leite C, Santos LHS, Marins LF, Machado KS, Werhli AV, Lima LHF, de Melo-Minardi RC (2014) Characterization of glucose-tolerant β-glucosidases used in biofuel production under the bioinformatics perspective: a systematic review. Genet Mol Res 16:(3). https://doi.org/10.4238/gmr16039740
Nielsen H, Tsirigos KD, Brunak S, von Heijne G (2019) A brief history of protein sorting prediction. Protein J 38(3):200–216. https://doi.org/10.1007/s10930-019-09838-3
Park DJ, Lee YS, Choi YL (2013) Characterization of a cold-active β-glucosidase from Paenibacillus xylanilyticus KJ-03 capable of hydrolyzing isoflavones daidzin and genistin. Protein J 32(7):579–584. https://doi.org/10.1007/s10930-013-9520-3
Pyeon HM, Lee YS, Choi YL (2019) Cloning, purification, and characterization of GH3 beta-glucosidase, MtBgl85, from Microbulbifer thermotolerans DAU221. Peer J 7:e7106. https://doi.org/10.7717/peerj.7106
Qu X, Ding B, Li J, Liang M, Du L, Wei Y, Huang R, Pang H (2020) Characterization of a GH3 halophilic β-glucosidase from Pseudoalteromonas and its NaCl-induced activity toward isoflavones. Int J Biol Macromol 164:1392–1398. https://doi.org/10.1016/j.ijbiomac.2020.07.300
Rossi M, Amaretti A, Leonardi A, Raimondi S, Simone M, Quartieri A (2013) Potential impact of probiotic consumption on the bioactivity of dietary phytochemicals. J Agric Food Chem 61(40):9551–9558. https://doi.org/10.1021/jf402722m
Robert X, Gouet P 2014 Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42(Web Server issue):W320-W324.https://doi.org/10.1093/nar/gku316
Salgado JCS, Meleiro LP, Carli S, Ward RJ (2018) Glucose tolerant and glucose stimulated beta-glucosidases - a review. Bioresour Technol 267:704–713. https://doi.org/10.1016/j.biortech.2018.07.137
Sathe SS, Soni S, Ranvir VP, Choudhari VG, Odaneth AA, Lali AM, Chandrayan SK (2017) Heterologous expression and biochemical studies of a thermostable glucose tolerant β-glucosidase from Methylococcus capsulatus (bath strain). Int J Biol Macromol 102:805–812. https://doi.org/10.1016/j.ijbiomac.2017.04.078
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Singh G, Verma AK, Kumar V (2016) Catalytic properties functional attributes and industrial applications of β-glucosidases. 3 Biotech 6(1):3. https://doi.org/10.1007/s13205-015-0328-z
Singhania RR, Patel AK, Pandey A, Ganansounou E (2017) Genetic modification: a tool for enhancing beta-glucosidase production for biofuel application. Bioresour Technol 245:1352–1361. https://doi.org/10.1016/j.biortech.2017.05.126
Sinha SK, Datta S (2016) beta-Glucosidase from the hyperthermophilic archaeon Thermococcus sp is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl Microbiol Biotechnol 100(19):8399–8409. https://doi.org/10.1007/s00253-016-7601-x
Su H, Xiao Z, Yu K, Zhang Q, Lu C, Wang G, Wang Y, Liang J, Huang W, Huang X, Wei F (2021) High diversity of β-glucosidase-producing bacteria and their genes associated with Scleractinian corals. Int J Mol Sci 22(7):3523. https://doi.org/10.3390/ijms22073523
Sun J, Wang W, Yao C, Dai F, Zhu X, Liu J, Hao J (2018) Overexpression and characterization of a novel cold-adapted and salt-tolerant GH1 β-glucosidase from the marine bacterium Alteromonas sp L82. J Microbiol 56(9):656–664. https://doi.org/10.1007/s12275-018-8018-2
Sun J, Wang W, Ying Y, Hao J (2020) A novel glucose-tolerant GH1 beta-glucosidase and improvement of its glucose tolerance using site-directed mutation. Appl Biochem Biotechnol 192(3):999–1015. https://doi.org/10.1007/s12010-020-03373-z
Saleh Zada N, Belduz AO, Güler HI, Khan A, Sahinkaya M, Kaçıran A, Ay H, Badshah M, Shah AA, Khan S 2021 Cloning, expression, biochemical characterization, and molecular docking studies of a novel glucose tolerant β-glucosidase from Saccharomonospora sp. NB11. Enzyme Microb Technol 148:109799 https://doi.org/10.1016/j.enzmictec.2021.109799
Teugjas H, Väljamäe P (2013) Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol Biofuels 6(1):105. https://doi.org/10.1186/1754-6834-6-105
Tiwari R, Kumar K, Singh S, Nain L, Shukla P (2016) Molecular detection and environment-specific diversity of glycosyl hydrolase family 1 β-glucosidase in different habitats. Front Microbiol 7:1597. https://doi.org/10.3389/fmicb.2016.01597
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley beta-D-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7(2):179–190. https://doi.org/10.1016/s0969-2126(99)80024-0
Volkov PV, Rozhkova AM, Zorov IN, Sinitsyn AP (2020) Cloning, purification and study of recombinant GH3 family β-glucosidase from Penicillium verruculosum. Biochimie 168:231–240. https://doi.org/10.1016/j.biochi.2019.11.009
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, deBeer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Wu Y, Chi S, Yun C, Shen Y, Tokuda G, Ni J (2012) Molecular cloning and characterization of an endogenous digestive β-glucosidase from the midgut of the fungus-growing termite Macrotermes barneyi. Insect Mol Biol 21(6):604–614. https://doi.org/10.1111/j.1365-2583.2012.01164.x
Wu J, Geng A, Xie R, Wang H, Sun J (2018) Characterization of cold adapted and ethanol tolerant β-glucosidase from Bacillus cellulosilyticus and its application for directed hydrolysis of cellobiose to ethanol. Int J Biol Macromol 109:872–879. https://doi.org/10.1016/j.ijbiomac.2017.11.072
Xia W, Xu X, Qian L, Shi P, Bai Y, Luo H, Ma R, Yao B (2016) Engineering a highly active thermophilic β-glucosidase to enhance its pH stability and saccharification performance. Biotechnol Biofuels 9:147. https://doi.org/10.1186/s13068-016-0560-8
Yang M, Zhang A, Liu B, Li W, Xing J (2011) Improvement of cellulose conversion caused by the protection of Tween-80 on the adsorbed cellulase. Biochem Eng J 56(3):125–129. https://doi.org/10.1016/j.bej.2011.04.009
Yang Y, Zhang X, Yin Q, Fang W, Fang Z, Wang X, Zhang X, Xiao Y (2015) A mechanism of glucose tolerance and stimulation of GH1 β-glucosidases. Sci Rep 5:17296. https://doi.org/10.1038/srep17296
Yin YR, Sang P, Xian WD, Li X, Jiao JY, Liu L, Hozzein WN, Xiao M, Li WJ (2018) Expression and characteristics of two glucose-tolerant GH1 β-glucosidases from Actinomadura amylolytica YIM 77502(T) for promoting cellulose degradation. Front Microbiol 9:3149. https://doi.org/10.3389/fmicb.2018.03149
Yoshida E, Hidaka M, Fushinobu S, Koyanagi T, Minami H, Tamaki H, Kitaoka M, Katayama T, Kumagai H (2010) Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem J 431(1):39–49. https://doi.org/10.1042/bj20100351
Zada NS, Belduz AO, Güler HI, Sahinkaya M, Khan SI, Saba M, Bektas KI, Kara Y, Kolaylı S, Badshah M, Shah AA, Khan S (2021) Cloning, biochemical characterization and molecular docking of novel thermostable β-glucosidase BglA9 from Anoxybacillus ayderensis A9 and its application in de-glycosylation of Polydatin. Int J Biol Macromol 193:1898–1909. https://doi.org/10.1016/j.ijbiomac.2021.11.021
Zhang C, Zhang L, Wang D, Ma H, Liu B, Shi Z, Ma X, Chen Y, Chen Q (2018) Evolutionary history of the glycoside hydrolase 3 (GH3) family based on the sequenced genomes of 48 plants and identification of jasmonic acid-related GH3 proteins in Solanum tuberosum. Int J Mol Sci 19(7):1850. https://doi.org/10.3390/ijms19071850
Zhang J, Zhao N, Xu J, Qi Y, Wei X, Fan M (2021a) Homology analysis of 35 β-glucosidases in Oenococcus oeni and biochemical characterization of a novel β-glucosidase BGL0224. Food Chem 334:127593. https://doi.org/10.1016/j.foodchem.2020.127593
Zhang P, Zhang R, Sirisena S, Gan R, Fang Z (2021b) Beta-glucosidase activity of wine yeasts and its impacts on wine volatiles and phenolics: a mini-review. Food Microbiol 100:103859. https://doi.org/10.1016/j.fm.2021.103859
Zheng Y, Pan Z, Zhang R, Wang D, Jenkins B (2008) Non-ionic surfactants and non-catalytic protein treatment on enzymatic hydrolysis of pretreated Creeping Wild Ryegrass. Appl Biochem Biotechnol 146(1–3):231–248. https://doi.org/10.1007/s12010-007-8035-9
Zhou J, Zhang R, Shi P, Huang H, Meng K, Yuan T, Yang P, Yao B (2011) A novel low-temperature-active β-glucosidase from symbiotic Serratia sp TN49 reveals four essential positions for substrate accommodation. Appl Microbiol Biotechnol 92(2):305. https://doi.org/10.1007/s00253-011-3323-2
Zmudka MW, Thoden JB, Holden HM (2013) The structure of DesR from Streptomyces venezuelae, a β-glucosidase involved in macrolide activation. Protein Sci 22(7):883–892. https://doi.org/10.1002/pro.2204
Acknowledgements
We thank Dr. David Waugh (National Cancer Institute) for the generous gift of the protein expression vector pET28-HMT and the TEV protease expression strain. In addition, we also thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31970042) and Zhejiang Provincial Natural Science Foundation of China (LY17C200019).
Author information
Authors and Affiliations
Contributions
Y.H. and G.Z.X. contributed to the conception and design of this study and the interpretation of data; Y.H., C.X.W., and R.H.J. performed experiments; Y.H., Q.X.N, Y.W., Q.X.G, and Y.Z.Z. analyzed the data; Y.H. and G.Z.X. wrote the manuscript. All authors contributed to critical revision of the manuscript and gave final approval of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Y., Wang, C., Jiao, R. et al. Biochemical characterization of a novel glucose-tolerant GH3 β-glucosidase (Bgl1973) from Leifsonia sp. ZF2019. Appl Microbiol Biotechnol 106, 5063–5079 (2022). https://doi.org/10.1007/s00253-022-12064-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12064-0