Abstract
As the energy demand is escalating tremendously and crude oil being the primary energy source for at least the next two decades, the production of crude oil should be enhanced to meet the global energy needs. This can be achieved by either exploration of new oil fields for crude oil extraction or employing enhanced oil recovery (EOR) technology to recover the residual oil from existing marginal oil fields. The former method requires more capital investment and time; therefore, this review focuses on the latter. In general, the abandoned oil fields still have 50% of crude left which is unrecovered due to lack of technology. Hence, EOR came into existence after the conventional methods of recovery (primary and secondary recovery) were found to be inefficient and less economical. Nineteen percent of the EOR projects are based upon cEOR methods worldwide, of which more than 80% of projects use economically feasible polymer flooding process for oil recovery. Both synthetic and naturally derived polymers have been used widely for this purpose; however, many recent studies have shown the lower stability of synthetic polymers under extreme reservoir conditions of high salinity and temperature. Additionally, naturally derived polymers face microbial degradation as the major limitation. Therefore, a number of novel polymers are currently studied for their suitability as an efficient EOR polymer. Latest findings have also revealed that biopolymers play an important role in wettability alteration, pore evolution by bioplugging, and reducing fingering effect. Injection of biopolymers can also lead to the selective plugging of thief zones which redirects water flood to the inaccessible oil pores. Therefore, the current study focuses on such principle and mechanism of polymer flooding along with the reservoir and field characteristics which affects the polymer flooding. It also discusses the scope of biopolymer along with the screening criteria for use of novel polymers and strategies to overcome the problems during polymer flooding.
Key points
• Discussion of macroscopic and microscopic mechanisms of polymer flooding.
• Screening criteria of polymers prior to flooding are essential.
• Biopolymers are eco-friendly and are applicable for a wide range of reservoir conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since the human civilization transitioned from the use of coal to oil as its primary energy source, the demand for oil has been steadily increasing and will follow the same path for at least the next two decades. Oil acts as a raw material for the functioning of almost all industries, irrespective of the field they operate in. Besides, petroleum products have found its way in a variety of applications based on a regular human lifestyle. Petroleum, thus, is often termed as “industrial blood.” In accordance with India Energy Outlook 2021, Fig. 1 shows that there is a direct co-relation between GDP and energy demand. In the given Indian model, as the energy demand increased over the decades, i.e., from 2000 to 2019, the economic growth (GDP) also increased. Consumption of crude oil by nations, both developed and developing, is higher, compared to their production rate which results in an increase in import of the commodity, and hence it plays a crucial role in maintaining international political relations between countries too. The USA being a developed nation shows the highest oil consumption in the Statistical Review of World Energy 2020 which is expected to decrease in upcoming decades, while developing countries like India will show an increase in their demand and consumption in such sector. In order to meet such demands and prevent the global energy crisis, the oil fields should be utilized properly. With the conventional recovery methods, only 25–40% of oil is being recovered while more than 50% of oil remain in the fields. Further recovery of such a significant volume of residual oil is extremely difficult and therefore such low producing wells are shut off or different novel tertiary oil extraction strategies are employed.
Graphical representation of energy demand vs GDP—India Model (Source: India energy outlook 2021)
Recovery of oil from the reservoir is a sequential process involving primary, secondary. and tertiary processes. The primary process constitutes the oil recovery due to the differential pressure between the reservoir and production well and recovers nearly 10% of original oil in place (OOIP) during this process (Planckaert 2005). Following this process, the reservoir pressure depletes and an external fluid such as gas or water is injected to maintain the differential pressure within the reservoir. This injection stage is known as secondary recovery and contributes to 15–60% of OOIP retrieval. After primary and secondary recoveries have been done, the further recovery process becomes economically unfeasible (Lake et al. 2014), and often oil companies abandon the oil fields. Such fields are called mature or marginal fields. As the marginal fields contain more than 50% of oil, abandonment of it is not the best choice. So, to counter-attack the problem and to increase the recovery of oil from such fields, enhanced oil recovery (EOR) method is adopted which has gained the limelight over the years. EORs are expensive (synthetic polymers as compared to novel biopolymers) but show a significant increment in recovery. Therefore, much care has to be taken in selecting optimal recovery method for the given reservoir condition; otherwise, the operation would not be viable.
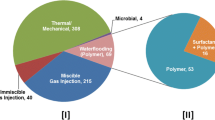
Generally, EOR is classified into 4 different types which are chemical flooding, thermal, miscible, and immiscible gas flooding, and microbial (Terry 2001). These EOR methods can be employed on both onshore and offshore flooding. The summary of EOR methods applied is given in Fig. 2. As per a recent review, Vishnumolakala et al. (2020) projected the distribution of different EOR projects for different countries. They reported that the USA and Canada are the leading countries which predominantly apply EOR techniques for crude oil production compared to other countries. Additionally, since 2010, the number of EOR projects implemented has significantly increased with the chemical EOR (cEOR) process showing a steep rise from 10.8 to 19.1% of the total EOR projects (Rellegadla et al. 2017, 2021; Vishnumolakala et al. 2020). Implementation of chemical EOR (cEOR) has also shown promising results with additional oil recovery from few marginal oil wells in an economical way (Firozjaii and Saghafi 2020). cEOR includes the use of polymer, surfactant, alkali, emulsions, and often their mixture based on the reservoir condition it is used on. Among which, more than 80% use the polymer flooding method while the rest of the 20% of the projects apply surfactant or a combination of polymer-surfactants (ASP) (https://www.iea.org/reports/world-energy-outlook-2018). Figure S1 shows the number of polymer flooding pilot projects that took place in different countries. Since reservoir shows a heterogeneous property, various oil fields (Figure S2) showing a spectrum of properties have been flooded widely by using different polymers (Agrawal et al. 2011; Lucas et al. 2009). Therefore, it requires a proper understanding of the characteristics of both polymer and oil fields for ensuring the success of the project. The main principle behind using a polymer is to efficiently push oil towards the production well by improving the sweep efficiency of the driving fluid (Stegemeier 1997). Different types of water-soluble polymers hence are used for this work. Figure S2 shows widely used polymers in polymer flooding with HPAM used in more than 60% of the projects.
A summary of enhanced oil recovery projects worldwide focusing on polymer flooding. The pie chart [I] shows a number of different enhanced oil recovery projects reported between the years 1996 and 2010 (Rellegadla et al 2017). The [II] pie chart representing different EOR projects till present (Vishnumolakala and Zhang 2020)
Water-soluble polymer agents for polymer-based EOR are broadly classified into two types: (i) synthetic polymer; (ii) biopolymer (Chang 1978). Synthetic polymers are artificially synthesized by the polymerization of acrylamide monomers and are often modified based on the requirement of the reservoir. Hydrolyzed polyacrylamide (HPAM) is the most common and widely used synthetic polymer both at the field and experimental level due to its cost-effective nature (Sheng 2013). Most synthetic polymers are PAM derivatives and show specific properties like salt tolerance (KYPAM), hydrophobically associating polymers [P (AM/AA/BEM)], and 2-acrylamide 2-methyl propane sulfonate (AMPS) (Sheng et al. 2015). They can be sub-divided into hydrophobic associating polymer [P (AM/AA/BEM)], cross-linked polymer, comb polymer [KYPAM], and star polymer (Li et al. 2021).
Biopolymers are biomacromolecules that are derived from natural resources. Major sources of biopolymers are plants (cellulose), microbes (xanthan gum), fungi (chitosan), or algae. Biopolymers have been widely used in almost all commercial industries which include the oil industry as well. The most common biopolymer used by the petroleum industry as a flooding agent is xanthan gum, which is produced by Pseudoxanthomonas. Xanthan gum is often chosen over other polymers for its higher tolerance to a wide range of salinity and temperature ranges. It was found that under high salt and temperature condition, it does not lose its properties and maintains about 80% of its original viscosity (Rellegadla et al. 2018). Hence, for the reservoirs, having temperatures around 120℃, xanthan gum was the most suitable choice. Biopolymer like that of synthetic ones not only improves mobility ratio but also shows selective plugging of thief zones and redirecting the waterflood towards the inaccessible oil pores (Sen 2008). Other than xanthan gum, biopolymers like guar gum, scleroglucan, cellulose, chitosan, and carboxymethyl cellulose (CMC) also show promising results in terms of temperature, pH, salinity, shear strength, etc. with respect to oil recovery. Though biopolymers have significant advantages over synthetic polymers, however, they are less applied in field-wide applications during flooding processes due to major issues such as related to aging and microbial degradation. Microbial degradation takes place for both types of polymers, synthetic and biopolymer, when injected in liquid form but degradation is higher for biopolymers compared to synthetic ones. Such degradation can lead to formation damage by pore plugging. While biopolymers also face injection issues due to the accumulation of biomaterial debris at the wall of wellbore (Firozjaii and Saghafi 2020).
Thus, polymer flooding overall has several unraveled advantages over other EOR methods which include decreasing the water to oil mobility ratio, improving the mobility of injected fluid with subsequent increase in sweep efficiencies both vertical and areal. Moreover, it uses less water compared to the method which uses only water as an injectable fluid and is cost-effective compared to that of other EOR techniques (Mohsenatabar Firozjaii et al. 2018). Besides, using polymers also include few limitations such as a fraction of injected polymer is retained in the porous media due to adsorption, mechanical entrapment, or precipitation which can lead to formation damage. Adsorption of polymer, if severe, is irreversible and occupies a large pore volume which causes a decrease in formation permeability and in turn lowers recovery. Another major limitation of polymer flooding includes degradation due to mechanical or biological stress, high rock retention of polymers because of different reservoir conditions, and filtration damage (Xiao and Qiao 2017). Thus, the current review focuses on the changes occurred in the polymer flooding technology over the years with the advancement in understanding new principles and mechanism affecting polymer role, its screening criteria, the characteristics of the reservoirs affecting the flooding process, and the novel polymers developed addressing all these factors playing a role during the polymer flooding process.
Principle and mechanism of polymer flooding
The foremost choice when it comes to increasing oil production is secondary flooding. During secondary flooding, water is injected to sweep the oil towards the production well (Tang and Morrow 1997); however, this is not always possible. Oil and water are immiscible fluids and because of the low viscosity of water, it often penetrates into the oil and comes onto the production well while the oil remains in the reservoir. The penetrating effect of water into the oil due to the viscosity difference is termed as fingering phenomenon (Needham and Doe 1987). This problem was overcome by adding polymer to the injection water to increase its viscosity and in turn lowering the water to oil mobility ratio (Chang 1978). Polymer flooding is the most economical method among the EOR and its mechanism of actions is still being researched. The polymer flooding mechanisms can be studied in two different aspects; (i) macroscopic sweep efficiency improvement and (ii) microscopic displacement efficiency improvement (Wei 2016).
Macroscopic sweep efficiency improvement
Mobility ratio
The main principle of using polymer is to reduce the water to oil mobility ratio. Mobility ratio (M) quantifies the mobility difference between the water and oil phases. During water flooding, the mobility ratio is calculated using Eq. 1 (Fan et al. 2018).
where kr refers to the relative permeability, µ is denoting the fluid viscosity; λ represents the fluid mobility and the subscript w stands for the water phase, whereas o symbolizes the oil phase. Similar to the above equation, M is applicable for polymer flooding too. After adding polymer in water, the water mobility decreases compared to that of oil and this high viscous water now pushes oil front like a piston (Chang 1978). Buckley-Leverett Eq. 2 shows this polymer flooding mobility ratio as
where fw is the fractional flow.
Based on Eq. (2), when M < 1, the fractional flow curve shows a piston-like flow and the average water saturation has a larger value; in turn, the residual oil in the reservoir is reduced (Lake et al. 2014). Hence, M ≤ 1 is favorable while M > 1 results in an unfavorable mobility condition resulting in a viscous fingering effect (Figure S3). The same results were observed in terms of viscosity of displacing (water) and displaced (oil) fluid. When the displacing fluid viscosity is lower than oil, i.e., at 0.1 viscosity ratio, the remaining oil after flooding is 45% of the OOIP which is a significant decrease in recovery efficiency. But when the viscosity ratio is 1 which is attained when polymer is added to water, the remaining oil after flooding is reduced to 20% of the OOIP. Overall, the highest viscosity ratio is the highest oil recovery (Mansour et al. 2016).
Disproportionate permeability reduction (DPR)
Disproportionate permeability reduction is also termed relative permeability modification. It is defined as the property of a polymer to reduce excessive water production while enhancing the recovery of oil (Taha and Amani 2019). In other words, it is the reduction in water relative permeability krw by adding polymer in the form of a gel. Polymer is converted into a gelant by the use of organic or inorganic cross-linkers added with it. At a specific time and temperature, the gelant is transformed to semi-solid gel which blocks the water permeability in that region (Singh and Mahto 2017). DPR either will not or will minimally affect the oil relative permeability (Niu et al. 2006). DPR occurs in polymer flooding due to wettability alteration, segregation of oil and water flow pathways, swelling, polymer shrinkage, and layer formation on pore walls by adsorbed polymer (Zaitoun and Kohler 1988). Swelling of polymer gel impedes water flow while dehydration of polymer gel occurs in oil presence which promotes oil flow (Willhite et al. 2002). However, it was confirmed that the dominant reason behind DPR is adsorption of polymer and segregation of flow pathways, i.e., by averting the drive water towards vacant under swept pores bottomhole in the producing zone (Singh and Mahto 2017). Plugging of these high-permeable layers diverts the drive fluid in the oil-rich zone which further enhances the recovery of oil. This blockade effect of the polymer gel does not affect residual oil recovery since it is easily removed compared to cement plugging that affects permanently (Singh Yadav and Mahto 2013). The use of cross-linkers like chromium acetate and phenol formaldehyde with widely used polymers such as HPAM and xanthan gum increases this effect (Lenji et al. 2018).
Flow resistance induced by polymer elasticity
In 2009, Dehghanpour and Kuru (2009) reported polymer having a higher elasticity showed a significant pressure drop during flow in porous media. Moreover, even when the shear viscosities of both polymers are the same, polymers with higher elasticity show higher flow resistance compared to that of polymer with lower elasticity when passed through a porous medium (Veerabhadrappa 2012). Doda et al. (2016) have also mentioned the elastic nature of polymer affecting the residual resistance factor. According to his results, a higher RRF value significantly contributes to a reduction in water phase permeability due to the blockage of porous media. It results in more stable viscous front propagation (Doda et al.2016). These elastic effects can be further improved by increasing the molecular weight distribution without altering shear viscosity (Dehghanpour and Kuru 2009). Thus, it confirms that elasticity directly effects the flow resistance in improving the macroscopic sweep efficiency.
Microscopic displacement efficiency improvement
As mentioned above, elasticity plays an important role in minimizing the fingering effect while stabilizing propagating front (Figure S4) and improving microscopic displacement efficiency (Wei 2016). Many different researchers performed visual experiments to confirm the elasticity effect of polymer on microscopic recovery (Table S1) (Veerabhadrappa et al. 2013). Using an industrial CT system, Hou et al. (2009) reported that with the increase in microscopic displacement efficiency, the water to oil mobility ratio is also increased during polymer flooding averting the flow pathway of displacing fluid leading to the redistribution of oil saturation (Hou et al. 2009). Therefore, both polymer viscoelasticity and diversion of the water paths play a role in mobilizing and displacing the residual oil. The viscoelastic nature of the polymer is further discussed in the screening criteria. In 2000, Wang et al. (2000) confirmed that polymer flooding promotes displacement of residual oil which was initially immobilized due to capillary forces and rock structure, thereby increasing the oil recovery. The mechanisms that promote such displacement include (i) pulling effect, (ii) stripping effect, (iii) oil thread, and (iv) shear thickening effect.
Pulling effect
Wang and coworkers (2007) studied the action of elastic fluids when passed over dead ends. They showed that when elastic fluid flows over dead ends, there is a generation of stresses between oil and polymer solution along with the shear stress present due to longer molecular chains of the polymer. Therefore, when a larger force is imposed on oil droplets, polymer molecules will pull them out of dead ends thus increasing the sweep efficiency (Wang et al. 2007). Besides, it was found that the pulling effect is relative to the elasticity of the driving fluid. Thus, the viscoelastic polymers like HPAM are widely used as they exhibit pushing ability as well pulling ability when the oil is immobilized in dead ends. Figure S5 shows the elasticity effect on the movement of residual oil in dead ends. Luo et al. (2016) studied how polymer elasticity affects the microscopic oil displacement efficiency and found that a rise in the elasticity of displacing fluid declines the oil trapped in the dead-end pore. The trapped oil can also be present in a configuration with both open ends available for flow. Under such cases, the trapping occurs due to capillary forces and nearly 50% of this oil can be recovered due to the pulling effect.
Oil thread
The second possible mechanism involved in increasing microscopic sweep efficiency is oil thread which also utilizes the elastic property. Polymers during flow aggregate with residual oil downstream and tend to pull out the oil into oil columns forming oil threads. Though, there is a high interfacial tension (IFT) between oil and polymer layer which destabilizes the column breaking down into smaller droplets, and in turn, oil gets re-entrapped by capillary forces. This condition can be avoided by the use of elastic polymer with which normal stress will stabilize the oil threads (Delshad et al. 2008). In 2013, Hossein Sedaghat et al. (2013) observed that the larger normal force on the convex surface of oil thread as to its concave surface helps to stabilize oil threads and prevents them from deforming and thus increasing the displacement efficiency (Hossein Sedaghat et al. 2013). This force also describes the elasticity of polymer solution by showing direct relation with the Deborah number (NDeh). Deborah number describes the viscoelastic nature of the polymer. It defines the rheology of the polymer. It was introduced by Reiner (1964) who defined it as the ratio of relaxation time of a material to the observation or experimental time (Reiner 1964) (Eq. 3).
where tobs is a characteristic time of the deformation process and λ(T) is the relaxation time. Deborah number (De) is directly proportional to the viscoelastic response of polymer during the observation time. The higher the value of De indicates solid nature while lower values indicate more fluid nature of the material (Wang et al. 2001).
Stripping effect
For an oil-wet porous medium, a continuous oil film is present on the rock surface due to the adsorbed residual oil. Khalilinezhad et al. (2019) who observed the activity of polymers compared the velocity profile in a capillary for a Newtonian and a non-Newtonian fluid. They examined that the velocity gradient near the capillary wall for an elastic fluid was comparatively higher compared to a Newtonian fluid (Khalilinezhad et al. 2019). Therefore, compared to water, polymer solution generates stronger force during its flow facilitating movement (stripping) of adsorbed oil layer off the surface and promoting recovery of residual oil (Yakimchuk et al. 2020). Thus, this stripping effect during polymer movement could lead to wettability alteration of oil-wet surfaces, thereby enhancing recovery of residual oil (Wei et al.2014).
Shear thinning and thickening
Unlike water, the polymer solution is not a Newtonian fluid and thus relationship of viscosity with that of shear stress and shear rate is not linear. A polymer solution shows three types of rheological behavior as a function of shear rate in a porous medium: Newtonian, shear thinning, and shear thickening. Though, EOR polymers generally have a shear-thinning property. The shear-thinning is occasionally considered comparable to the pseudoplastic behavior of fluid during rheological studies. Since, shear-thinning behavior is defined as a fluid behavior when exposed to applied stress (Afolabi et al. 2019). The relationship of polymer solution is given by the power law equation, as it is a shear-thinning fluid (Eq. 4).
where K denotes the consistency index, γ symbolizes the shear rate,τ is the shear stress, and n refers to the flow behavior index. The parameters K and n characterize the rheology of power law fluids in which flow behavior index n is dimensionless while the dimension of K depends on n. Pseudoplastic behavior is also termed as shear thinning and seen when n < 1which means that apparent viscosity decreases with the increase in shear rate. n > 1 is for another type of non-Newtonian fluid which follows dilatant behavior and not pseudoplastic (Doran 1995).
Moreover, when a polymer solution moves through a series of pores, its molecules come across elongation and contraction stresses. According to this, with different compositions of the polymers, the apparent viscosity of the polymer is decreased with the increase in shear rate (Fig. 3). The reason behind this phenomenon is the arrangement of polymeric molecules within a shear rate field where there is reduced internal friction. Adding to this, the polymer solution exhibits a homogeneous sequence (lower Newtonian) at a lower shear rate, whereas with an increase in shear rate, polymer apparent viscosity gets reduced giving rise to power law (Fig. 3). This region (shear thinning) follows the power law index, and expressed by the Ostwald de Waele model, given in Eq. 5. The polymer solution functions as an upper Newtonian fluid when there is a constant increase of shear rate.
This figure shows a representation of the rheology of shear thinning fluid adapted from Firozjaii and Saghafi (2020)
where τ represents the shear stress, γ is the shear rate, µ describes the dynamic viscosity, K is the consistency index, and n is the power law index.
Shear thickening behavior is shown by polymer solution during a high velocity flow which does not provide its molecules sufficient relaxation time for re-coiling and to adapt the flow geometry. The effect thus helps in rapidly displacing the mobile with the driving fluid but it is still hard-to-displace oil more effectively from small-scale heterogeneities (Wei 2016). Additionally, Garrouch and Gharbi (2006) showed that De is not a suitable parameter for viscoelastic characterization. Instead, “Viscoelasticity number (Nv)” which is a dimensional number can properly distinguish viscous flow from viscoelastic flow and is much more adequate. Overall, new models were tested to include polymer viscosity in terms of shear thinning and shear thickening phenomenon. Thus, viscosity promotes both macroscopic and microscopic sweep efficiency (Garrouch and Gharbi 2006).
Reservoir properties
Reservoir heterogeneity
The success of oil recovery during the polymer flooding is greatly influenced by reservoir heterogeneity as the variation in the quality of the reservoir causes the accurate prediction of oil saturation but also has an influence on fluid flow. The reservoir quality variation is explained by porosity, permeability, capillary pressure, and water saturation (Yıldız and Yılmaz 2020). The high permeability and the low permeability zones of reservoir occurring during the oil formation and deposition are the main reason for reservoir heterogeneity (Xie et al. 2016). Polymer when interacts with fluid and porous medium alters some of the rock properties. Along with the increase in viscosity and mobility ratio, disproportionate permeability reduction (DPR) is also introduced to decrease the amount of produced water. Choice of an inapt polymer coupled with plugging causes the reduction in permeability which has an unfavorable effect on productivity specifically in reservoirs with low permeability. The reduction in permeability causes severe damage to the reservoir which is not repairable leading to an increase in the cost of oil production with a decrease in productivity. Due to the capillary effect, polymers plugging the pore walls decrease the relative permeability of water in contrast to the relative permeability of oil as adsorption-entanglement polymer layers are formed (Fig. 4).
This figure shows the effects of polymer adsorption on relative permeabilities of oil and water. Adapted from (Yoo et al. 2020)
The main factors responsible for declining of reservoir quality by pore throat plugging is the presence of detrital and diagenetic rooted clay-coating minerals (illite, kaolinite, illite/smectite mixed clay layer) together with cementation by quartz overgrowth and carbonate concretions in the matrix of the sandstone layers hence decrease production rates and oil recovery. The porosity distribution (mean 14.99%) with a CV value of 0.29 indicates a homogenous distribution whereas permeability values (mean 1.469mD) with a CV of 2.28 predicts a very heterogenous medium of the sandstones. The porosity and permeability is quite variable on a microscale and exerts great control on production rates, oil recovery, and water saturation. The water saturation in the sandstone reservoir levels varies significantly, even in the zone above oil–water contact due to the change in permeability. Variation of permeability values of the sandstone successions in a wide range accentuates the reservoir heterogeneity (Yıldız and Yılmaz 2020). The presence of shale barrier in heavy oil reservoirs has a higher cumulative oil volume whereas residual oil saturation is low, thus having a positive effect on heavy oil recovery (Zhang et al. 2021).
The permeability reduction due to retention in a porous medium is defined by the residual resistance factor (RRF) whereas the amplification in viscosity and mobility control of the polymer is defined by the term resistance factor (RF). Manichand and Seright (2014) in an experiment with xanthan gum and HPAM revealed that polymer retention occurs due to adsorption and mechanical entrapment. According to their results, 35.2% of HPAM and half of xanthan retention was attributed to adsorption, whereas the other half of xanthan and 64.8% of HPAM retention was due to mechanical entrapment (Manichand and Seright 2014). At Yariguí-Cantagallo Field in Columbia (sandstone reservoir with permeability of 1.279mD), a test was conducted for 24 months with HPAM which resulted in a residual resistance factor (RRF) of 3 and water cut of up to 5%. In this field experiment, the polymer flooding was considered as a practical approach both technically and economically (Lucas et al. 2009). According to the results of Knobloch et al. (2018) on flooding experiments with HPAM (Flopaam) and a biopolymer Scleroglucan, the major retention mechanism was mechanical entrapment in the case of synthetic polymer and adsorption in the case of Scleroglucan. A higher RRF was observed in the case of Scleroglucan despite the fact that HPAM (Flopaam) was earlier tested for higher RRF. Even when the concentrations of both the polymers were increased, there was an increase in the resistance factor (RF) for both the polymers but RRF increased only for HPAM (Flopaam) (Knobloch et al. 2018). The qualitative analysis provided that most polymer solutions showed a filtration-like process at the injection site. Alkaline surfactant polymer (ASP) formulations have been also successfully used in both sandstone and carbonate reservoirs in China and Oman. The plugging phenomenon in the case of Flopaam was observed when its concentration increased from 1000 to 1500 ppm, with no noticeable plugging at 1000 ppm and a sharp increase at 1500 ppm. This proves that above a particular concentration of Flopaam, there is an abrupt increase in permeability reduction. Contrastingly, the adsorption of Scleroglucan appears to be the same at lower concentrations as well. According to the above-mentioned research, it seems that RRF has a larger influence on the adsorption of the polymer than mechanical entrapment. At such low flow rates, none of the polymers used showed hydrodynamic retention. Qin et al. (2021) while working with silylated-polyacrylamide on carbonate rocks founded that it worked as a relative permeability modifier (RPM) which reduces the water permeability in a hydrocarbon reservoir thus resulting in a lower amount of produced water while maintaining the crude oil production (Qin et al. 2021).
Inaccessible pore volume (IPV)
The fraction of the rock pore volume which is not accessible to the polymer during flooding is referred to as IPV (Torrealba and Hoteit 2019). As explained by Sheng (2010), in a porous medium when the molecule size of the polymer is larger in size than the pores, then it is not possible for the polymer to pass via such pores. Therefore, that volume of pores which is not reached by the polymeric molecules is referred to as inaccessible pore volume (Sheng 2010). IPV along with the polymer size and its concentration also depends upon the charge on the polymer, divalent ion concentration, salinity, rock surface effect, temperature, and the pore size of the rock (including dead-end pores) (Torrealba and Hoteit 2019). The effect of different associating polymers was studied on a sand-packed column of absolute permeability 21.6 D to determine the IPV by Pancharoen et al. (2010). The molecular weight of the polymers was thus figured as an important factor to have an impact on the IPV. Polymer chains with more hydrophobic regions and larger molecular volumes are characterized as high molecular weight associating polymers which have larger molecular clew dimension in contrast to pore throat sizes. The work done by Pancharoen et al. (2010) showed that in low molecular weight associating polymers, the range of IPV observed was 12–20% in contrast to 33–49% in high molecular weight associating polymers depending on the approach used. This was explained in reference to hydrophobic interactions which are directly proportional to the molecular weight of the polymer. Thus, out of many mechanisms, IPV is also responsible for polymer transport in porous media, and in case if it is the prime factor involved, it may lead to polymer acceleration (Rellegadla et al. 2019). This would occur when polymer solution is injected at salinity lower than the reservoir salinity (Afolabi et al. 2019).
Temperature and salinity
To understand the rheological behavior of polymer solutions at different concentrations, temperature and salinity conditions play a major role. According to the studies, with an average temperature of 46.1 °C, a polymer can stand up to 100 °C of temperature. Romero et al. (2002) reported that oxygen contamination and hardness may enhance the hydrolysis of PAM at a temperature as low as 60 °C. Muhammed et al. (2020) reported despite the fact that temperature affects both the polymers xanthan gum and HPAM, the former is much more stable under harsh temperature and saline conditions. The results of single-phase core flooding experiments performed by Unsal et al. (2018) suggested that polymer retention is directly proportional to the salinity of the reservoir. Under the challenging conditions of reservoirs, conventional HPAM has a lot of limitations. To overcome its limitations, many other polymers were proposed such as associative polymers, co- or terpolymers combining acrylamide with monomers such as ATBS or NVP, or biopolymers like xanthan gum or scleroglucan. Associative polymers are hence more desirable as biopolymers are sensitive to biodegradation which limits their use in the fields. Some of the new co- and terpolymers are already successfully being field-tested. Delamaide (2018) claimed that as some of the field projects are already working in high saline conditions (200 g/L), the high temperature seems to be the major concern. Alfazazi et al. (2018) carried out the screening of three NVP HPAM base polymers and states that they show promising results in heterogenous carbonate reservoirs with a temperature of 120 °C and 167,000 ppm salinity. A copolymer of HPAM (KYPAM) is resistant to divalent cations as its structure consists of an ionic functional group (Zhu et al. 2012). Hence, the viscosity of KYPAM is higher as compared to HPAM in more salinity due to flexible chains are stretching. According to laboratory experiments also performed by Luo et al. (2002), KYPAM has good shear and thermal stability along with more temperature resistance. Zwitterionic polymers also perform multiple functions that are with asphaltene inhibition-dispersion activity; they also alter the wettability of rock and further affecting the permeability (Alcazar-Vara et al. 2015) demonstrating that ZP can be used to modify rock wettability to increase the oil recovery in high salinity environments.
Wettability alteration
Askarinezhad and colleagues through their experiments showed that the wettability of a surface plays a vital role in disproportionate permeability reduction. Non-ionic PAM on sandstone cores reported the “wall effect” as the main mechanism for the DPR ability of a polymer. Furthermore, wall effects include three parts mentioned as (a) steric effect, (b) lubrication effects, and (c) formation wettability alteration. Polymers get adsorbed on oil-wet material leading to wettability alteration to water-wet system. The water-wet surface thus increases the oil mobility contributing to the polymer DPR effect. Experiments carried out in an oil-wet core gave the stabilized RRFo (residual resistance factor of oil) values achieved at considerably lower pore volumes of injected oil, whereas lower RRFo levels were achieved in the oil-wet formations compared to water-wet ones, with the RRFo displaying a clear separation from the achieved RRFw (Askarinezhad et al 2021). ZP has the potential to alter the rock wettability which further enhances the crude oil recovery in a high saline reservoir (Araujo and Araujo 2018). Wettability alteration plays an important role in the enhancement of the oil recovery process as it is a property of the porous surface; it affects the adsorption and oil/water separation. Wettability and its applications are based on the approaches of hydrophilicity, hydrophobicity, and super-wettability. Out of these three processes, alteration due to hydrophilicity holds a vital role in oil recovery. Adsorption of the organic pollutant is effectively affected by the hydrophobic nature of the rock surface. Conclusively, the super-wetting property of the matrix confirms the highly effective separation of oil from water.
Fluid properties
Interfacial tension and foam stability
The separation of water from crude oil is an adversity to the oil recovery process associated with polymer flooding which is tracked to the interfacial tension (IFT) characteristics of polymers to further stabilize the crude oil emulsions (Al-Sabagh et al. 2016). The authors emphasized that when compared to low molecular weight surfactants, the ability of associative polymers to reduce the IFT is not critical. It was observed in the experiments performed by Meiqin et al. (2011) that the increase in the concentration of associative polymers is responsible for the increase in interfacial shear viscosity of the oil–water film which thereupon stabilizes the emulsion.
A foam is basically a gas phase dispersed in a liquid phase. This property could be utilized to improve the mobility of gas to enhance oil recovery (Ahmed et al. 2017). The productivity of foam is predicted by how long it remains stable in the vicinity of oil. HPAM when compared with an associative polymer (PEFs) with a polymer concentration of 2000 ppm was found to be unstable at temperature 80 °C and pressure 14.5 psi as associative polymers have enhanced thickening ability (arising from hydrophobic interactions). This enhanced thickening effect of the associative polymer tends to limit gas diffusion thereby enhancing foam stability through a gradual reduction in foam volume.
Polymer mobility
Improvement in sweep efficiency with increase in mobility of injection fluid is done with the help of polymer whose concentration relies upon reservoir conditions. However, due to the higher cost of the polymer involved, an increase in its concentration is not advisable as it may also create injectivity issues (high pressure). As an alternative, Delshad and coworkers (2008) had come with an economical and more effective way of using a high molecular weight polymer at a lower concentration to attain a higher viscosity rate that will hence enhance the sweeping efficiency. Despite the fact, the above-mentioned method is still bounded because as discussed above a high molecular weight polymer also increases inaccessible pore volume (IPV). The reservoir pores have been classified as micropores, i.e., 50 nm pore size by the International Union of Pure and Applied Chemistry (IUPAC). Subsequently, an increase in molecular weight might cease the polymeric molecule from flowing through the pores. An illustration by Green and Willhite (1998) significantly proves that HPAM is more suitable when compared to xanthan gum on the basis of smaller void spaces in the porous rock as IPV of xanthan gum ranges between 20 and 31% in contrast to 0.18 and 0.24% of HPAM. Zhang et al. (2011) have also concluded in his experiments that the higher the reduction factor of maximal permeability, the higher is the oil recovery. Polymer mobility could create a velocity gradient at the rock surface which causes the removal of adsorbed oil from the rock. At higher velocities, the polymer behaves as a shear thickening fluid which could limit the injectivity of the polymer.
Polymer retention and relaxation time
Due to the high flow rates, polymeric molecules could be pushed into the cavities where they have no space to flow, such a situation is referred to as hydrodynamic retention. Polymer retention majorly depends upon the polymer concentration, salinity, permeability, injection velocity, etc. as mentioned by AlSofi (Alsofi et al. 2018). Experimental studies determined that the HPAM retention increased from 140 to 155 µg/g with the increase in permeability from 500 to 2000mD. However, when the increase in permeability is minimal, the polymer retention also remains the same showing that permeability retention is almost insensitive to permeability in low permeable porous media, i.e., below 200mD (Yoo et al. 2020). Based on the experimental results of core flooding by Zhang and Seright (2014), polymer retention increases with the increase in polymer concentration upto 4000 ppm above which near-constant retention of the polymer was achieved. Polymer retention is directly proportional to salinity; in low salinity conditions, polymer retention is reduced than that of higher salinity conditions (Unsal et al. 2018). The polymer retention mechanism in the porous medium is based upon three mechanisms as shown in Fig. 5. The first one is the physical interaction between the polymer molecules and the rock surface due to hydrogen bonding or Van der Waal forces. The amount of the polymer adsorbed is proportional to the rock surface available. The second factor is based upon the entrapment of the polymer molecule in the pore whose outlet diameter is smaller than that of the diameter of the polymer molecule. The third mechanism which relies upon the flow velocity of the polymer is known as hydrodynamic retention in which an increase in the flow rate causes extra deposition in porous media.
This figure is a schematic representation of mechanism of polymer retention (adapted from Yoo et al. 2020)
According to experiments performed by Vela et al. (1976), the result showed that permeability is indirectly related to retention. As permeability is increased from 12 to 137 mD, there is a significant decrease in polymeric retention (Vela et al. 1976). In the experiments reported by Zaitoun and Kohler (1987), they claimed that there is no significant change in retention when permeability is already higher. Therefore, permeability is considered as an important property of the reservoir at the pore-scale and microscale level at values lower than 100 mD and becomes less important at higher permeabilities (Vela et al 1976). The relaxation time of the polymer is also an important factor to consider where oil recovery is measured. As the relaxation time is increased, the polymer molecules get to stabilize their structure which implies the effect of elasticity more prominently. So, polymers with the longer relaxation time are selected for EOR processes (Zhang et al. 2011).
Screening of polymer for EOR
Polymers are long-chain macromolecules which are derived synthetically or naturally, in raw or modified form. They exhibit a range of functions which includes thickening, cross-linking, and adsorption, because of which they are applied in various industries including petroleum. Different types of polymers which are used in the petroleum industry are mentioned in Table 1. The two major types of polymers involved in EOR are water and oil-based polymers. Different polymer solutions are made and chosen based on the oil and reservoir condition to increase efficiency. Polymer solution properties are affected by the number of elements like the viscosity of the solution, shear stress, core temperature of the reservoir, and some physical environmental conditions such as pH, metal ions, O2, and salinity (Chatterji and Borchardt 1981). So, to preselect the polymer for injection, we need to know the reservoir characteristics; its temperature and salinity to evaluate the chemistry of a stable polymer. The average permeability of the reservoir helps in the selection of polymer in terms of average molecular weight to ensure smooth propagation of injection fluid. Similarly, based on the field data, research articles, and surveys, screening strategies for polymer selection are explained below in detail:
Cost
The economic feasibility of the recovery is very important in the petroleum industry. Thus, the cost of the polymer is a major factor due to its large-scale input. Other factors like costs of chemicals, prices of oil, taxation, capital investment, and biocide usage also affect the overall economics of the polymer flooding (Chang 1978). For instance, the choice of the product whether the polymer chosen is powder or inverse emulsion also affects its cost. Thus, for choosing favorable polymer logistical studies are needed depending upon the field (Rellegadla et al. 2017).
Polymer chemistry
Linkage of monomer units plays a determining role in showcasing polymer properties. It helps to analyze the polymer status when subjected to different reservoir conditions. Xanthan gum being an anionic heteropolysaccharide can withstand temperature up to 80 °C, with 3 pH and high salinity conditions of up to 3% salt due to a linear β-(1–4)-d-glucopyranose glucan backbone providing stable viscosity (Garcıa-Ochoa et al. 2000). Furthermore, carbonate modification of its side chains has been shown to significantly increase its viscosity (Reddy 2011). Another biopolymer, scleroglucan, showed heat tolerance up to 135 °C and alkali tolerance of pH 12.3 at 25 °C due to the presence of a linear chain of β-d-(1–3)-glucopyranosyl (Kalpakci et al. 1990; Leonhardt et al. 2014). The guar gum molecule in solution form reaches its highest viscosity at pH 6–9 and maintains its stability at viscosity as low as 3.5 with a linear backbone consisting of (1–4)-β-d-mannopyranosyl unit. With the rise in temperature, a decrease in viscosity was observed as high temperature obstructs the interaction of water with polymer molecules. HPAM which under favorable conditions can stand up to 120 °C is hydrolyzed and precipitates due to the formation of acrylate groups above 60 °C at a critical concentration of divalent cations. Studies performed with ATBS combined HPAM show an increase in salinity and temperature tolerance till 95 °C as the presence of ATBS marks an increase in shear stability and a decrease in retention within the reservoirs.
Polymer molecular weight
The molecular weight of polymers varies between 2 and 35 million g (g mol)−1. Gel permeation chromatography (GPC) techniques are used to determine molecular weight distribution (polydispersity index of polyacrylamides) which is limited to high molecular weight polymer only and thus new techniques are being developed for such as calculating molecular weight distribution in low concentration solution by intrinsic viscosity measurement. Based on the laboratory and field cases, a suitable average weight is stated in Table S2 for good propagation of the injection fluid.
Salinity and ions
Salinity and ions’ presence in the reservoir has a negative impact on the viscosity of the polymer and thus leads to its loss. Salinity referred to as R+ is defined in Eq. 6:
where [Ccat div] stands for the number of moles of divalent cations whereas [Ccat mono] depicts the number of moles of monovalent cations present in the brine. Gaillard et al. (2017) discovered the interdependence of salinity and shear tolerance for different polymers and showed that when salinity is less than 50,000 ppm and R+ is below 0.05, the standard copolymer can be chosen, and in conditions where R+ is more than 0.05 with salinity less than 1,00,000 ppm, terpolymers with ATBS are effective. Copolymers of acrylamide with ATBS are efficient when salinity is below 1,00,000 ppm with R+ more than 0.1. Along with divalent ions, monovalent ions like Na+ and K+ found in the reservoir are also detrimental to polymer viscosity. As the Na+ and K+ increase, the viscosity of the polymer solution decreases. An increase in the monovalent ions results in the reduction of electrostatic attraction within molecules and between polymers and the molecules become curled in turn reducing viscosity. Compared to monovalent ions, divalent ions have a greater impact on the viscosity of polymer (Dong et al. 2019; Sun et al. 2018).
Viscosity
Viscosity is one of the major criteria to characterize polymer. Polymer generates viscosity in the medium only when it is able to interact with the solvent and is more energetically favorable than during its polymer–polymer interaction. A polymer expands due to electrostatic repulsion in a solvent like water. The larger the hydrodynamic volume, the higher the viscosity. In oil fields, the polymer that generates high viscosity at a minimum concentration and is able to maintain the viscosity for a longer period under reservoir conditions is most valuable.
The degree to which the polymer enhances the viscosity of a solvent can be given by the specific viscosity Eq. 7
where η represents the solution viscosity and ηs is the solvent viscosity. The equation gives an idea of how the polymer contributes to the viscosity of the solvent when there is no interpolymer interaction (Kawada et al. 2006). Viscoelastic effects of polymeric fluids when it flows through porous media were first recognized by Acharya 1986; Smith (1970). According to Zhang et al. (1994), viscoelasticity of polyacrylamide solution exhibited only when it was passed through porous media and therefore hypothesized that this effect is dependent upon shear rate. It was then concluded that the smaller the pore size, the greater is the “elastic viscosity” and hence greater is the flow resistance. However, viscoelastic polymer with longer molecular chains can tangle up and pull out the residual oil from the pores which relates the elasticity of polymer solutions directly to the residual oil pulled out of the “dead ends.” Polymer elasticity contributes to a 5% increase in enhanced oil recovery by increasing oil displacement efficiency. An experiment performed at laboratory scale in Canada’s Cactus Lake Reservoir for polymer flooding advised that with an increase in viscosity of polymer solution up to 25cp (7.3 s−1), recovery of oil (1610cp) from field cores (at 27 °C and 1 ft/D) was efficiently increased due to oil displacement as a function of polymer-solution. No considerable benefit was observed from injecting polymer solutions more viscous than 25 cp (Seright et al. 2018). So, the choice of the polymer with suitable viscosity and its consistency is much needed during flooding.
Chemical degradation
Chemical degradation otherwise called oxidative degradation is a free radical chain reaction occurring in the reservoirs containing oxygen (dissolved in water) which react (redox reaction) with either iron or H2S to produce oxygen-centered free radicals (example – OH) which reduces the hydrodynamic volume of the polymer thus degrading polymers into its monomeric unit and reducing its viscosity. So, in order to prevent oxidative degradation of the polymer, three main factors namely oxygen, H2S, and iron are kept in check. Due to the high sulfonating degree of copolymers of AM resulting in increased salt tolerance and also least affected with the presence of divalent ions. In the absence of dissolved oxygen and divalent cations, HPAM can maintain half of its viscosity for 7 years at 100 °C and for about 2 years at 120 °C (Araujo and Araujo Fresky 2018). Jensen et al. (2018) conducted a pilot scale test of scleroglucan (a biopolymer) and found that it is stable in the presence of hydrogen sulfide and ferrous species. Scleroglucan polymer also does not suffer any drop in viscosity in presence of a high concentration of divalent cations making possible the re-injection of produced water without any treatment (Jensen et al. 2018).
Thermal degradation
Hydrolysis of polymer often comes down to its resistance towards temperature and shear stress. The monomeric unit and structure of a polymer determine how effectively it can minimize the adverse effects of temperature. When reservoir temperature is above 250°F, acrylamide groups of acrylamide-based polymers hydrolyze (Chang 1978). The primary mechanism behind its degradation is amide group hydrolysis. The thermal stability of a polymer could be increased by incorporating monomer units like ATBS and NVP by divalent cations as they will help in preventing hydrolysis. The incorporation of sodium-2-acrylamido-2-methylpropanesulfonate, acrylamido-tertio-butyl sulfonate improves the polymer stability up to 120 °C (Araujo and Araujo Fresky 2018). When the salinity of brine is mild and temperature is above 75 °C, ATBS incorporation is done which is found to uphold the viscosity up to 95 °C. NVP is considered in very salty brines with a temperature above 100 °C. The development of thermo-responsive polymers has an upper hand over other conventional co- and terpolymers when it comes to high-temperature reservoirs. Thermo-responsive polymers with side groups of lower critical solution temperature (LCST) moieties having water-soluble chains allow association when the specific temperature is reached. As the temperature rises, the viscosity of the thermo-responsive polymers also rises unlike the conventional polymers (Thomas et al. 2017). Hence, the polymers whose viscosity is elevated with temperature are used as they show several advantages like (i) injecting solution with low viscosity which will lead to short-term injectivity and (ii) use in high salinity conditions.
Mechanical degradation
Herrera et al. (2020) has mentioned in their studies the variables that influence the mechanical degradation of the polymer. The factors are molecular weight, the diameter of the capillary, and the pressure differential being the most influential factor among all. The pressure drops, together with the abrupt reduction in the cross-sectional part of the capillary, give rise to a rupture in the molecules due to the extensional forces acting thereon. This is mainly because the capillary diameter and the pressure differential are highly related to the shear stresses to which the polymer is subjected, being fundamental factors in the fluids’ continuity. The smaller is the capillary diameter, the higher will be the rate of degradation (Herrera et al. 2020). Loss of viscosity occurs when a polymer is exposed to extreme shear rate or singular pressure drops in a pipe, a pump, or a choke, mechanical degradation occurs, which leads to loss of viscosity. Molecular weight and chain length show direct relation with that mechanical degradation. The higher the weight or longer the chain of the polymer, the more it is prone to mechanical degradation (Zaitoun et al. 2012). Therefore, the use of polymer with lower molecular weight and shorter chains is preferred which is determined by using tools like screen factor and core flooding. Chain flexibility is another factor that comes into play. Polymer with high flexibility is sensitive to shear degradation and so, groups like acrylate, N-vinyl pyrrolidone acrylamide copolymer, or ATBS that provide rigidity to a polymer backbone are incorporated in it (Zaitoun et al. 2012). According to a recent research, flexible chain polymers considered as a rigid rod in solution shows a stable shear behavior with high viscosity at the low molecular weight (Araujo and Araujo Fresky 2018). Shear degradation studies performed by Jensen et al. (2018) show that when Scleroglucan recycled through a centrifugal pump causes less than 5% drop in viscosity even after 100 passes. Capillary shear testing also showed the same results (Jensen et al.2018).
Solubility properties
A promising polymer is the one which readily dissolves, hydrates, develops desired viscosity, and is easily injected into the reservoir. Standard approaches are followed to ensure the appropriate injectability of polymer. For instance, dissolution of polyacrylamide is done using a mechanical stirrer at 500 rpm. Often, a stock solution of 0.5% or 1% active concentration is prepared to increase the dissolution efficiency. As suggested by API that while dissolving powder form of polymer, the stirring vortex should be maintained while adding each granule that is uniformly wetted (API 1990; API-RP-63). The addition of powder should not be so fast that fish eyes are created. It can be avoided by simultaneous addition of powder and vigorous agitation. For blending of both the emulsion and powder form of polymer, the solution is initially stirred for 30 min at 500 rpm and later stirred for 2 h by decreasing the velocity to 300 rpm. The final solution is then free of lumps and undissolved particles. Later required dilutions are made for the targeted viscosity and concentration.
Filtration properties
A filter ratio test of polymer is necessary before injection into the reservoir. It checks the full dissolution of polymer solutions. It is done to avoid wellbore plugging which is due to cross-linking or by cellular debris (Chang 1978). The test monitors the filtration rate of the solution under steady pressure. Different procedures are taken up by different companies which leads to difficulty in interpreting the resultant values. Based on the literature study, some methods are summarized in supplementary Table S3. Various filter types like polycarbonate and cellulosic are used. Enzyme clarification and diatomaceous earth filters are also preferred for the filter ratio test. Since different filters show different results, the test is repeated 2–3 times to ensure the credibility and consistency of the result. Therefore, polymers are being subjected to filter tests using micron-sized filters in order to prevent wellbore plugging.
Compatibility test
The usage of different additives like biocide and corrosion inhibitors in the field is first confirmed for its compatibility towards the polymer used. The same is done when surfactant cocktail is co-injected with the polymer in surfactant-polymer (SP) or alkaline surfactant polymer (ASP) processes. Surfactants are found to be successful in tertiary recovery as they reduce interfacial tension (IFT) and lower adsorption of polymers on the rock surface of the reservoir. They increase the capillary number to overcome capillary forces in action in turn increasing oil mobility. Oil recovery is enhanced using surfactants as compared to water flooding (Sheng et al. 2015). But when surfactant polymer incompatibility (SPI) is seen, detrimental effects in the recovery process are observed as the loss of the surfactant increases. Surfactant loss is due to its adsorption on the rock surface because of electrostatic attraction forces. When the surfactant is not compatible with polymer in SP or ASP processes, phase separation is seen resulting in surfactant loss. Such losses lead to an increase in demand for surfactants for ideal oil displacement efficiency. It was seen that at optimum salinity condition, there is a reduction in the entrapment process causing the minimum surfactant loss in porous medium. Thus, salinity plays an important role in SPI.
Therefore, to procure a steady solution of a polymer and a surfactant, their compatibility should be tested and analyzed prior to injection as stability of the injecting solution is an important factor. Sometimes co-solvents work as supplements to achieve such requirements. The compatibility test usually takes place by preparing the polymer solution of required concentration along with the addition of chemicals which are to be used during the flooding experiments in the field. While doing so, viscosity loss of solution can be minimized by not using cationic products with anionic ones. For example, biocides like tetrakis hydroxymethyl phosphonium sulfate (THPS) are not compatible with anionic HPAM. If added to HPAM, for example, at a concentration of 500 ppm, viscosity loss of 20–30% was observed within the time span of 2 h. Compounds like imidazole and methenamine molecules are found to be well suited with anionic HPAM.
Injectivity reduction and injection pressure
Ranjbar et al. (1992) discuss the flow behavior of viscoelastic polymer solutions in their experiments. They explained that there is an increase in effective viscosity above a critical injection rate, along with the influential parameters including concentration, molecular weight of the polymer, degree of hydrolyzation, core permeability, salinity, and temperature (Ranjbar et al. 1992). Shear degradation of the polymer due to the high flow rate in the porous medium proves to be beneficial as it is the prime factor for reduced injectivity in a way that it reduces the viscoelasticity of the polymer, but if there is a corresponding loss of shear viscosity along with the mechanical degradation, it could be detrimental for oil mobilization deep in the reservoir. Rock configuration is the main reason due to which the immovable residual oil is trapped in “dead end” in the form of droplets by capillary forces.
Zhang et al. (2011) have demonstrated the evolution of aqueous pressure as functions of time and space. It was found that the pressure drop between the production and injection well is the chief reason for the extraction of oil. In cEOR, the viscosity of the aqueous phase is increased by a polymer which further increases the flow resistance of displacement fluid. Hence, to displace the polymer resolution, higher injection pressure is required which leads to the pressure drop spreading around the production well and decrease in surrounding pressure gradually. Gogarty et al. (1972) also demonstrated that the pressure drop for viscoelastic fluid should be greater than the pure viscous fluids around the core.
Novel polymers
Dupuis illustrates a whole new form of synthetic polymers which were tested in core flooding experiments having heat tolerance of up to 140 °C. These are NVP-free polymers consisting of variable content of ATBS designed for use in harsh conditions such as Middle East reservoirs (TDS > 220 g/L) (Dupuis et al. 2017). They gave a valuable result in both types of reservoirs, i.e., carbonate and sandstone (permeability range 100–700mD), and were also more stable than ATBS or NVP individually. Under anaerobic conditions, homoglucan (a water-soluble biopolymer) exhibited great salinity tolerance (up to 220 g/L) along with high thermal stability (up to 120 °C) for more than 8 months with nominal loss in viscosity (Quadri et al. 2015). In favor of this, with the increase in temperature and salinity, the biopolymer adsorption on the rock surface decreases. Lyu et al. (2019) used AM, sodium styrene sulfonate (SSS), and acryloxyethyl trimethylammonium chloride (DAC) to prepare a thermo-resistant and shear-stable amphoteric polyacrylamide (PASD), by free-radical polymerization in high salinity solution. The novel thermal and salinity resistant polymer so formed maintained viscosity retention of nearly 40% at 120 °C (Lyu et al. 2019). A hydrophobically modified HEC using bromo dodecane (BD-HMHEC) was tested for rheological properties, and oil displacement efficiency in the reservoirs of Daqing had salt tolerance up to 100 g/L and was found stable at a temperature of 90 °C, improving the oil recovery by 7–14% in contrast to HEC flooding at concentrations of 4 g/L under similar conditions (Araujo and Araujo Fresky 2019).
Conclusion(s)
An efficient and effective recovery process is needed in order to meet the booming demands of energy. Even though the majority of the fields apply the thermal process for recovery under favorable conditions, polymer flooding is also a suitable candidate for fields where thermal recovery cannot be done and there is a need for a cost-effective method. Polymer flooding can be applied in reservoirs having oil API gravity up to 40, average permeability as less as 10 md, viscosity up to 1000, and depth up to 9000. Also, compared to water flooding, polymer flooding has a greater success of recovery with the less usage of water (Mohsenatabar Firozjaii et al. 2018), and therefore polymer flooding has been gaining the limelight in the petroleum industry. Polymers are finding its application as an injection fluid which decreases the water to oil ratio and improves recovery. There have been hundreds of known polymers, both synthetic and naturally derived. They are being tested and are found to be successful in field trials in enhancing the recovery of oil. Among the polymers, biopolymers are gaining attention as they along with improving recovery are found to be cost-effective in the long run and are eco-friendly. Even though they provide more efficiency at a lower cost, there are certain drawbacks of it as they are strongly affected by the geological conditions and operation process. They are also prone to microbial degradation compared to synthetic polymers which can be eradicated by the use of biocides in the reservoir. Thus, screening of the polymers and the reservoir conditions is much needed and essential prior to flooding. Furthermore, on the basis of this review, the future challenge to improve recovery efficiencies by polymer flooding can be addressed by using associated polymers with that of alkali/surfactant or by combining EOR methods based on the evolution of reservoir properties during flooding.
References
Abraham S, Rajamanickam D, Srinivasan B (2018) Research article preparation, characterization and cross-linking of chitosan by microwave assisted synthesis. Sci Int 6:18–30
Acharya AR (1986) Particle transport in viscous and viscoelastic fracturing fluids. SPE Prod Eng 1(02):104–110
Afolabi RO, Oluyemi GF, Officer S, Ugwu JO (2019) Hydrophobically associating polymers for enhanced oil recovery–part a: a review on the effects of some key reservoir conditions. J Pet Sci Eng 180:681–698
Agrawal A, Chatterjee I, Voordouw G, Lomans B, Kuijvenhoven C, Henderson J (2011) Determining microbial activities in fracture fluids compromising water reuse or disposal. SPE Int Symp Oilfield Chem. https://doi.org/10.2118/141352-MS
Ahmed S, Elraies KA, Tan IM, Hashmet MR (2017) Experimental investigation of associative polymer performance for CO2, foam enhanced oil recovery. J Pet Sci Eng 157:971–979
Ai H, Liu M, Yu P, Zhang S, Suo Y, Luo P, Li S, Wang J (2015) Improved welan gum production by Alcaligenes sp. ATCC31555 from pretreated cane molasses. Carbohydr Polym 129:35–43
Alcazar-Vara LA, Zamudio-Rivera LS, Buenrostro-Gonzalez E (2015) Multifunctional evaluation of a new supramolecular complex in enhanced oil recovery, removal/control of organic damage, and heavy crude oil viscosity reduction. Ind Eng Chem Res 54(32):7766–7776
Alfazazi U, AlAmeri W, Hashmet MR (2018) Screening of new HPaM base polymers for applications in high temperature and high salinity carbonate reservoirs. In: Abu Dhabi International Petroleum Exhibition & Conference. Soc Pet Eng J. https://doi.org/10.2118/192805-MS
Al-Sabagh A, Betiha M, Osman D, Hashim A, El-Sukkary M, Mahmoud T (2016) Preparation and evaluation of poly (methyl methacrylate)-graphene oxide nanohybrid polymers as pour point depressants and flow improvers for waxy crude oil. Energy Fuels 30(9):7610–7621
AlSofi AM, Wang J, Kaidar ZF (2018) SmartWater synergy with chemical EOR: effects on polymer injectivity, retention and acceleration. J Pet Sci Eng 166:274–282
Araujo YC, Araujo Fresky M (2018) Polymers for application in high temperature and high salinity reservoirs-critical review of properties and aspects to consider for laboratory screening. Rev Fuentes 16(2):55–71
Araujo YC, Araujo Fresky M (2019) Polymers for EOR in offshore reservoirs: recommended practices for laboratory screening. In: Offshore Technology Conference, 2019. In: Offshore Technology Conference. Soc Pet Eng J. doi: https://doi.org/10.4043/29260-MS
Askarinezhad R, Hatzignatiou DG, & Stavland A (2021) Associative polymers for disproportionate permeability reduction–formation wettability effects. J Petrol Sci Eng 109060
Bakhshi M, Ozeiri M, Sharif A, Aalaie J (2017) Effect of hydrophobic modification on the structure and rheology of aqueous and brine solutions of scleroglucan polymer. Korean J Chem Eng 34(3):903–912
Chang HL (1978) Polymer flooding technology yesterday, today, and tomorrow. J Pet Technol 30(08):1113–1128
Chatterji J, Borchardt J (1981) Applications of water-soluble polymers in the oil field. J Pet Technol 33(11):2042–2056
Dehghanpour H, Kuru E (2009) A new look at the viscoelastic fluid flow in porous media: a possible mechanism of internal cake formation and formation-damage control. In: SPE International Symposium on Oilfield Chemistry, 2009. Soc Pet Eng J. https://doi.org/10.2118/121640-MS
Delamaide E (2018) Polymers and their limits in temperature, salinity and hardness: theory and practice. In: SPE Asia Pacific Oil and Gas Conference and Exhibition. Soc Pet Eng J SPE-192110-MS. https://doi.org/10.2118/192110-MS
Delshad M, Kim DH, Magbagbeola OA, Huh C, Pope GA, Tarahhom F (2008) Mechanistic interpretation and utilization of viscoelastic behavior of polymer solutions for improved polymer-flood efficiency. In: SPE Symposium on Improved Oil Recovery. Soc Pet Eng J SPE-113620-MS. https://doi.org/10.2118/113620-MS
Doda A, Sahib M, Trivedi J (2016) Effect of water saturation on the role of polymer elasticity during heavy oil recovery. J Dispersion Sci Technol 41(9):1265–1273. https://doi.org/10.1080/01932691.2016.1188710
Dong W, Wang K, Qiu Y, Peng W (2019) Effect of polymerized sewage on viscosity of polymer solution and countermeasures. In: IOP Conference Series: Mater Sci Eng 634: 012045
Doran PM (1995) Bioprocess engineering principles. Elsevier ISBN 0080528120
Dupuis G, Antignard S, Giovannetti B, Gaillard N, Jouenne S, Bourdarot G, Morel D, Zaitoun A (2017) A new thermally stable synthetic polymer for harsh conditions of Middle East reservoirs. Part I. Thermal Stability and Injection in Carbonate Cores. In: Abu Dhabi International Petroleum Exhibition & Conference. Soc Pet Eng J SPE-188479-MS. https://doi.org/10.2118/188479-MS
Fan JC, Wang FC, Chen J, Zhu YB, Lu DT, Liu H, Wu HA (2018) Molecular mechanism of viscoelastic polymer enhanced oil recovery in nanopores. R Soc Open Sci 5(6):180076
Firozjaii AM, Saghafi HR (2020) Review on chemical enhanced oil recovery using polymer flooding: fundamentals, experimental and numerical simulation. Petroleum 6(2):115–122
Gaillard N, Thomas A, Bataille S, Dupuis G, Daguerre F, Favéro C (2017) Advanced selection of polymers for EOR considering shear and hardness tolerance properties. In: Conference Proceedings, IOR 2017 - 19th European Symposium on Improved Oil Recovery (1):1–18. https://doi.org/10.3997/2214-4609.201700333
Garcıa-Ochoa F, Santos V, Casas J, Gómez E (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18(7):549–579. https://doi.org/10.1016/s0734-9750(00)00050-1
Garrouch AA, Gharbi RC (2006) A novel model for viscoelastic fluid flow in porous media. In: SPE Annual Technical Conference and Exhibition. Soc Pet Eng J SPE-102015-MS. https://doi.org/10.2118/102015-MS
Gogarty W, Levy G, Fox V (1972) Viscoelastic effects in polymer flow through porous media. In: Fall Meeting of the SPE of AIME. Soc Pet Eng J. SPE-4025-MS. https://doi.org/10.2118/4025-MS
Green DW, Willhite GP (1998) Enhanced oil recovery. Henry L. Doherty Memorial Fund of AIME, SPE 6
Gupta PK, Raghunath SS, Prasanna DV, Venkat P, Shree V, Chithananthan C, Choudhary S, Surender K and Geetha K (2019) An update on overview of cellulose, its structure and applications. Cellulose 846–1297
Herrera J, Maya G, Prada LC, Maldonado L, Castro R, Quintero H, Barbosa D, Pérez E (2020) Experimental evaluation of the mechanical degradation of HPAM polymeric solutions used in enhanced oil recovery. CT&F-Ciencia Tecnol Futuro 10(2):131–141
Hossein Sedaghat M, Hossein Ghazanfari M, Parvazdavani M, Morshedi S (2013) Experimental investigation of microscopic/macroscopic efficiency of polymer flooding in fractured heavy oil five-spot systems. J Energy Resour Technol 135(3)
Hou J, Li Z, Zhang S, Cao X, Du Q, Song X (2009) Computerized tomography study of the microscopic flow mechanism of polymer flooding. Transp Porous Media 79(3):407–418
https://iea.blob.core.windows.net/assets//77ecf96c-5f4b-4d0d-9d93d81b938217cb/World_Energy_Outlok_2018.pdf. Accessed 5 Jul 2021
https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statisticalreview/bp-stats-review-2020-full-report.pdf. Accessed 13 Jul 2021
https://www.iea.org/reports/india-energy-outlook-2021. Accessed 13 Jul 2021
Jensen T, Kadhum M, Kozlowicz B, Sumner ES, Malsam J, Muhammed F and Ravikiran R (2018) Chemical EOR under harsh conditions: scleroglucan as a viable commercial solution. In: SPE Improved Oil Recovery Conference, Tulsa, Oklahoma, USA, April 2018. https://doi.org/10.2118/190216-MS
Kalpakci B, Jeans Y, Magri N, Padolewski J (1990) Thermal stability of scleroglucan at realistic reservoir conditions. In: SPE/DOE Enhanced Oil Recovery Symposium, 1990. Soc Pet Eng J. SPE- 20237-MS. https://doi.org/10.2118/20237-MS
Kawada Y, Nagahama H, Hara H (2006) Irreversible thermodynamic and viscoelastic model for power-law relaxation and attenuation of rocks. Tectonophysics 427(1–4):255–263
Khalilinezhad SS, Hashemi A, Mobaraki S, Zakavi M, Jarrahian K (2019) Experimental analysis and numerical modeling of polymer flooding in heavy oil recovery enhancement: a pore-level investigation. Arabian J Sci Eng 44(12):10447–10465
Knobloch LO, Reina REH, Födisch H, Ganzer L (2018) Qualitative and quantitative evaluation of permeability changes during EOR polymer flooding using micromodels. World J Eng Technol 6(02):332
Lake LW, Johns R, Rossen B, Pope GA (2014) Fundamentals of Enhanced Oil Recovery. SPE 1:496
Lenji MA, Haghshenasfard M, Sefti MV, Salehi MB, Moghadam AM (2018) Numerical modeling and experimental investigation of inorganic and organic crosslinkers effects on polymer gel properties. J Pet Sci Eng 160:160–169
Leonhardt B, Ernst B, Reimann S, Steigerwald A, Lehr F (2014) Field testing the polysaccharide schizophyllan: results of the first year. In: SPE Improved Oil Recovery Symposium. Soc Pet Eng J. SPE-169032-MS. https://doi.org/10.2118/169032-MS
Li X, Zhang F, Liu G (2021) Review on polymer flooding technology. In: IOP Conference Series: Earth and Environmental Science 675(1):012199
Lucas EF, Mansur CR, Spinelli L, Queirós YG (2009) Polymer science applied to petroleum production. Pure Appl Chem 81(3):473–494
Luo J-H, Pu R-Y, Wang P-M, Bai F-L, Zhang Y, Yang J-B, Liu Y-Z (2002) Performance properties of salt tolerant polymer KYPAM for EOR. Oilfield Chem 1:64–67
Luo D, Wang F, Zhu J, Cao F, Liu Y, Li X, Willson RC, Yang Z, Chu C-W, Ren Z (2016) Nanofluid of graphene-based amphiphilic Janus nanosheets for tertiary or enhanced oil recovery: high performance at low concentration. Proc Natl Acad Sci 113(28):7711–7716
Lyu Y, Gu C, Tao J, Yao X, Zhao G, Dai C (2019) Thermal-resistant, shear-stable and salt-tolerant polyacrylamide/surface-modified graphene oxide composite. J Mater Sci 54(24):14752–14762
Manichand RN, Seright R (2014) Field vs. laboratory polymer-retention values for a polymer flood in the Tambaredjo field. SPE Reservoir Eval Eng 17(03):314–325
Mansour E, Al-Sabagh A, Desouky S, Zawawy F, Ramzi M (2016) Experimental approach of minimum miscibility pressure for CO2 miscible flooding: application to Egyptian oil fields. Int J New Technol Res 2(5):105–112 ISSN:2454–4116
Meiqin L, Hao W, Dan T, Leiting M, Hua Z, Mingyuan L (2011) Oil-water interfacial properties and emulsion stability of produced water from an oilfield with polymer-surfactant combination flooding. J Petrochem Technol 7
MohsenatabarFirozjaii A, Derakhshan A, Shadizadeh SR (2018) An investigation into surfactant flooding and alkaline-surfactant-polymer flooding for enhancing oil recovery from carbonate reservoirs: experimental study and simulation. Energy Sour A Recov Utiliz Environ Effects 40(24):2974–2985. https://doi.org/10.1080/15567036.2018.1514439
Muhammed NS, Haq M, Al-Shehri D, Rahaman MM, Keshavarz A, Hossain S (2020) Comparative study of green and synthetic polymers for enhanced oil recovery. Polymers 12(10):2429
Needham RB, Doe PH (1987) Polymer flooding review. J Pet Technol 39(12):1503–1507
Niu J, Chen P, Shao Z, Wang D, Sun G, Li Y (2006) Research and development of polymer enhanced oil recovery. In: Cao H-Q (ed) Research and development of Enhanced oil recovery in Daqing. Petroleum Industry Press, Beijing, pp 227–325
Pancharoen M, Thiele MR, Kovscek AR (2010) Inaccessible pore volume of associative polymer floods. In: SPE Improved Oil Recovery Symposium. SPE
Planckaert M (2005) Oil reservoirs and oil production. In: Ollivier B, Magot M (eds) Petroleum Microbiology. ASM Press, Washington DC, pp 3–19
Qin L, Myers MB, Otto C, Verrall M, Zhong Z, Arjomand E, Saeedi A, Wood CD (2021) Further insights into the performance of silylated polyacrylamide-based relative permeability modifiers in carbonate reservoirs and influencing factors. ACS Omega 6(21):13671–13683. https://doi.org/10.1021/acsomega.1c00820
Quadri SMR, Shoaib M, AlSumaiti AM, Alhassan SM (2015) Screening of polymers for EOR in high temperature, high salinity and carbonate reservoir conditions. In: International petroleum technology conference. Soc Pet Eng J. IPTC-18436-MS. https://doi.org/10.2523/IPTC-18436-MS
Ranjbar M, Rupp J, Pusch G, Meyn R (1992) Quantification and optimization of viscoelastic effects of polymer solutions for enhanced oil recovery. In: SPE/DOE Enhanced Oil Recovery Symposium. Soc Pet Eng J. SPE-24154-MS. https://doi.org/10.2118/24154-MS
Reddy B (2011) Viscosification-on-demand: chemical modification of biopolymers to control their activation by triggers in aqueous solutions. In: SPE International Symposium on Oilfield Chemistry. Soc Pet Eng J. SPE-141007-MS. https://doi.org/10.2118/141007-MS
Reiner M (1964) The Deborah number. Phys Today 17(1):62
Rellegadla S, Prajapat G, Agrawal A (2017) Polymers for enhanced oil recovery: fundamentals and selection criteria. Appl Microbiol Biotechnol 101(11):4387–4402
Rellegadla S, Bairwa H, Kumari M, Prajapat G, Nimesh S, Pareek N, Jain S, Agrawal A (2018) An effective approach for enhanced oil recovery using nickel nanoparticles assisted polymer flooding. Energy Fuels 32:11212–11221
Rellegadla S, Jain S, Agrawal A (2019) Oil reservoir simulating bioreactors: tools for understanding petroleum microbiology. Appl Microbiol Biotechnol 104:1035–1053
Rellegadla S, Jain S, Sangwai JS, Lavania M, Lal B, Gieg L, Rajasekar A, Bera A, Agrawal A (2021) Wettability alteration of the oil-wet carbonate by viscosity-augmented guar galactomannan for enhanced oil recovery. ACS Appl Polym Mater 3(4):1983–1994
Romero C, Alvarez J, Müller A (2002) Micromodel studies of polymer-enhanced foam flow through porous media. In: SPE/DOE improved oil recovery symposium. Soc Pet Eng J SPE-75179-MS. https://doi.org/10.2118/75179-MS
Sen R (2008) Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust Sci 34(6):714–724
Seright R, Skjevrak I (2014) Effect of dissolved iron and oxygen on stability of HPAM polymers. Paper SPE 169030 presented at the SPE Improved Oil Recovery Symposium held in Tulsa, Oklahoma, USA, 12–16 April
Seright R, Skjevrak I (2015) Effect of dissolved iron and oxygen on stability of hydrolyzed polyacrylamide polymers. SPE J 20(03):433–441
Seright RS, Campbell A, Mozley P, Han P (2010) Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J 15(02):341–348
Seright RS, Wang D, Lerner N, Nguyen A, Sabid J, Tochor R (2018) Can 25-cp polymer solution efficiently displace 1,600-cp oil during polymer flooding? SPE J 23(06):2260–2278
Sheng JJ (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing
Sheng JJ (2013) Enhanced oil recovery field case studies. Gulf Professional Publishing
Sheng JJ, Leonhardt B, Azri N (2015) Status of polymer-flooding technology. J Can Pet Technol 54(02):116–126
Singh R, Mahto V (2017) Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. Pet Sci 14(4):765–779
Singh Yadav U, Mahto V (2013) Modeling of partially hydrolyzed polyacrylamide-hexamine-hydroquinone gel system used for profile modification jobs in the oil field. J Pet Eng 2013:709248. https://doi.org/10.1155/2013/709248
Smith FW (1970) The behavior of partially hydrolyzed polyacrylamide solutions in porous media. J Pet Technol 22(02):148–156
Stegemeier G (1997) Mechanisms of entrapment and mobilization of oil in porous media. Improved Oil Recovery by Surfactant and Polymer Flooding. Academic Press, New York, pp 55–92
Sun G, Li D, Zhang D, Xu T (2018) Study on improving viscosity of polymer solution based on complex reaction. In: IOP Conf Ser: Mater Sci Eng 369: 012045
Taha A, Amani M (2019) Overview of water shutoff operations in oil and gas wells; chemical and mechanical solutions. Chem Eng 3(2):51
Tang G, Morrow NR (1997) Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reservoir Eng 12(04):269–276
Terry RE (2001) Enhanced oil recovery. Encyclo Physical Sci Technol 18:503–518
Thomas A, Braun O, Dutilleul J, Gathier F, Gaillard N, Leblanc T, Favero C (2017) Design, characterization and implementation of emulsion-based polyacrylamides for chemical enhanced oil recovery. In: IOR 2017–19th European Symposium on Improved Oil Recovery 2017(1):1–29. https://doi.org/10.3997/2214-4609.201700286
Torrealba V, Hoteit H (2019) Improved polymer flooding injectivity and displacement by considering compositionally-tuned slugs. J Pet Sci Eng 178:14–26
Unsal E, Ten Berge A, Wever D (2018) Low salinity polymer flooding: lower polymer retention and improved injectivity. J Pet Sci Eng 163:671–682
Veerabhadrappa SK (2012) Study of effects of polymer elasticity on enhanced oil recovery by core flooding and visualization experiments. MS thesis, University of Alberta. https://doi.org/10.7939/R39M64
Veerabhadrappa SK, Trivedi JJ, Kuru E (2013) Visual confirmation of the elasticity dependence of unstable secondary polymer floods. Ind Eng Chem Res 52(18):6234–6241
Vela S, Peaceman D, Sandvik EI (1976) Evaluation of polymer flooding in a layered reservoir with crossflow, retention, and degradation. SPE J 16(02):82–96
Vishnumolakala, Narendra, Zhang, Norasyikin Bte Ismail (2020) A comprehensive review of enhanced oil recovery projects in Canada and recommendations for planning successful future EOR projects. In: SPE Canada Heavy Oil Conference, Virtual, September. Soc Pet Eng J. SPE-199951-MS. https://doi.org/10.2118/199951-MS
Wang D, Cheng J, Yang Q, Wenchao G, Qun L, Chen F (2000) Viscous-elastic polymer can increase microscale displacement efficiency in cores. In: SPE annual technical conference and exhibition. Soc Pet Eng J. SPE-63227-MS. https://doi.org/10.2118/63227-MS
Wang D, Xia H, Liu Z, Yang Q (2001) Study of the mechanism of polymer solution with visco-elastic behavior increasing microscopic oil displacement efficiency and the forming of steady oil thread flow channels. In: SPE Asia Pacific oil and gas conference and exhibition. Soc Pet Eng J SPE-68723-MS. https://doi.org/10.2118/68723-MS
Wang D, Wang G, Wu W, Xia H, Yin H (2007) The influence of viscoelasticity on displacement efficiency--from micro to macro scale. In: SPE Annual Technical Conference and Exhibition, Soc Pet Eng J. SPE-109016-MS. https://doi.org/10.2118/109016-MS
Wei B (2016) Advances in polymer flooding. Viscoelastic and viscoplastic materials. 1st edition InTech Publication. https://doi.org/10.5772/64069
Wei B, Romero-Zerón L, Rodrigue D (2014) Oil displacement mechanisms of viscoelastic polymers in enhanced oil recovery (EOR): a review. J Pet Explor Prod Technol 4(2):113–121
Willhite GP, Zhu H, Natarajan D, McCool C, Green D (2002) Mechanisms causing disproportionate permeability reduction in porous media treated with chromium acetate/HPAM gels. SPE J 7(01):100–108
Xia S, Zhang L, Davletshin A, Li Z, You J, Tan S (2020) Application of polysaccharide biopolymer in petroleum recovery. Polymers 12(9):1860. https://doi.org/10.3390/polym12091860
Xiao J, Qiao G (2017) Understanding the problems and challenges of polymer flooding technique. Oil Gas Res 3(126):2472–2518. https://doi.org/10.4172/2472-0518.1000126
Xie K, Lu X, Li Q, Jiang W, Yu Q (2016) Analysis of reservoir applicability of hydrophobically associating polymer. SPE J 21(01):1–9
Yakimchuk I, Evseev N, Korobkov D, Ridzel O, Pletneva V, Yaryshev M, Ilyasov I, Glushchenko N, Orlov A (2020) Study of polymer flooding at pore scale by digital core analysis for East-Messoyakhskoe oil field. In: SPE Russian Petroleum Technology Conference, Soc Pet Eng J SPE-202013-MS. https://doi.org/10.2118/202013-MS
Yıldız G, Yılmaz İÖ (2020) Reservoir heterogeneity of Ordovician sandstone reservoir (Bedinan Formation, SE Turkey): diagenetic and sedimentological approachs. Mar Pet Geol 118:104444
Yoo H, Kim H, Sung W, Lee J (2020) An experimental study on retention characteristics under two-phase flow considering oil saturation in polymer flooding. J Ind Eng Chem 87:120–129
Zaitoun A, Kohler N (1987) The role of adsorption in polymer propagation through reservoir rocks. In: SPE International Symposium on Oilfield Chemistry, Soc Pet Eng J SPE-16274-MS. https://doi.org/10.2118/16274-MS
Zaitoun A and Kohler N (1988) Two-phase flow through porous media: effect of an adsorbed polymer layer. In: SPE Annual Technical Conference and Exhibition. Soc Pet Eng J SPE-18085-MS. https://doi.org/10.2118/18085-MS
Zaitoun A, Makakou P, Blin N, Al-Maamari RS, Al-Hashmi A, Abdel-Goad M, Al-Sharji HH (2012) Shear stability of EOR polymers. SPE J 17(02):335–339
Zhang G, Seright R (2014) Effect of concentration on HPAM retention in porous media. SPE J 19(03):373–380
Zhang Y, Li C, Wang X, Hu J (1994) The viscoelastic effects of polymer solutions in porous media. J Daqing Pet Inst 18(2):139–143
Zhang Z, Li J, Zhou J (2011) Microscopic roles of “viscoelasticity” in HPMA polymer flooding for EOR. Transp Porous Media 86(1):199–214
Zhang L, Li J, Sun L, Yang F (2021) An influence mechanism of shale barrier on heavy oil recovery using SAGD based on theoretical and numerical analysis. Energy 216:119099
Zhu YY, Luo WL, Jian GQ, Wang CA, Hou QF, Niu JL (2012) Development and performance of water soluble salt-resistant polymers for chemical flooding. Adv Mater Res 476:227–235
Funding
This work was supported by NER TWINNING Project (Award no: BT/PR25132/NER/95/1034/2017) project to Dr. Akhil Agrawal provided by the Department of Biotechnology, Ministry of Science and Technology, India. SM acknowledges the support of the JRF fellowship from DBT India. This work was also supported by departmental facilities that were generated by the DST FIST grant (SR/FST/LSI-676/2016) to the Department of Microbiology, Central University of Rajasthan.
Author information
Authors and Affiliations
Contributions
SM and HY were involved in literature review, writing, and designing the figures. RS and AA finalized the manuscript. All authors contributed to the critical preparation and final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahajan, S., Yadav, H., Rellegadla, S. et al. Polymers for enhanced oil recovery: fundamentals and selection criteria revisited. Appl Microbiol Biotechnol 105, 8073–8090 (2021). https://doi.org/10.1007/s00253-021-11618-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11618-y