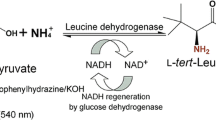
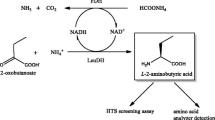
Abstract
L-tert-leucine (L-Tle) is widely used as vital chiral intermediate for pharmaceuticals and as chiral auxiliarie for organocatalysis. L-Tle is generally prepared via the asymmetric reduction of trimethylpyruvate (TMP) catalyzed by NAD+-dependent leucine dehydrogenase (LeuDH). To improve the catalytic efficiency and coenzyme affinity of LeuDH from Bacillus cereus, mutation libraries constructed by error-prone PCR and iterative saturation mutation were screened by two kinds of high-throughput methods. Compared with the wild type, the affinity of the selected mutant E24V/E116V for TMP and NADH increased by 7.7- and 2.8-fold, respectively. And the kcat/Km of E24V/E116V on TMP was 5.4-fold higher than that of the wild type. A coupled reaction comprising LeuDH with glucose dehydrogenase of Bacillus amyloliquefaciens resulted in substrate inhibition at high TMP concentrations (0.5 M), which was overcome by batch-feeding of the TMP substrate. The total turnover number and specific space-time conversion of 0.57 M substrate increased to 11,400 and 22.8 mmol·h−1·L−1·g−1, respectively.
Key points
• The constructed new high-throughput screening strategy takes into account the two indicators of catalytic efficiency and coenzyme affinity.
• A more efficient leucine dehydrogenase (LeuDH) mutant (E24V/E116V) was identified.
• E24V/E116V has potential for the industrial synthesis of L-tert-leucine.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
L-tert-leucine (L-Tle) is a vital chiral intermediate for the synthesis of anti-cancer or anti-HIV protease inhibitors (Xu and Singh 2002; Li et al. 2014). And it is also widely used as a chiral auxiliarie for organocatalyst in asymmetric synthesis because of its inflexible and hydrophobic tert-butyl group (Josephsohn et al. 2001; Bommarius et al. 1995). Compared with the chemical production of L-Tle, enzymatic methods are regarded as alternative processes as they are environmentally friendly and highly enantioselective (Laumen et al. 2002; Kim et al. 2013; Xue et al. 2018). Therefore, the NAD+-dependent leucine dehydrogenase (LeuDH, EC 1.4.1.9), which catalyzes the conversion of trimethylpyruvate (TMP) to L-Tle, is the most promising tool for its high theoretical conversion rate and atom efficiency (Li et al. 2014; Lu et al. 2016; Jiang et al. 2017).
LeuDHs have been discovered in Sporosarcina psychrophile (Zhao et al. 2012), Alcanivorax dieselolei (Jiang et al. 2016), Exiguobacterium sibiricum (Li et al. 2014), and the halophilic thermophile Laceyella sacchari (Zhu et al. 2016b), and each shows a broad substrate spectrum. However, native LeuDHs are unsuitable for large-scale L-Tle production due to its low affinity for coenzymes and low catalytic efficiency for artificial substrates. Enzyme engineering has become the most important strategy for improving kinetic properties of enzymes for industrial applications (Turner 2009). Zhu et al. (Zhu et al. 2016a) modified LeuDH from Lysinibacillus sphaericus using error-prone PCR (ep-PCR) and obtained several mutant enzymes with improved activity. The half-life and catalytic efficiency of LeuDHs from Bacillus cereus for unnatural amino acid production was increased based on site-directed mutation by Rao’s group (Zhou et al. 2018). These studies focused more on the catalytic efficiency of substrate rather than the affinity of the enzyme for its coenzyme. The reaction efficiency of coenzyme-dependent enzymes can be increased by using higher coenzyme concentration. However, this is not practical since it increases production costs. Therefore, finding or modifying enzymes with increased catalytic efficiency coupled with stronger affinity to their coenzymes is imperative for their industrial application.
Here, we evaluated the catalytic efficiency of enzymes for substrates and their affinity for coenzymes. To obtain variants with improved activity and coenzyme affinity, a two-step mutational assay was developed to create and screen libraries of mutant enzymes. Initially, a random mutagenesis library was generated by ep-PCR and screened by high-throughput screening based on TMP concentrations. The second step was to use iterative saturation mutations (ISM) to combine the dominant mutation sites previously screened to construct a new library. The library was screened by the low coenzyme concentrations high-throughput method. This two-step mutation and screening process allowed us to obtain mutants with higher catalytic activity at low coenzyme concentration than the wild type. Furthermore, when coupled with glucose dehydrogenase (Fig. 1), mutant (E24V/E116V) of LeuDH greatly improved the total turnover number (TTN) and space-time conversion efficiency than the wild type.
Materials and methods
Strains, vectors, and chemicals
Leucine dehydrogenase from Bacillus cereus Xb6 (BcLeuDH, GenBank no. KJ702046.1) and glucose dehydrogenase of Bacillus amyloliquefaciens DSM 7 (BaGDH, GenBank no. CP054415.1) were preserved in our laboratory. The pET-28a and the ClonExpress II One Step Cloning Kit were purchased from Novagen. The primers were synthesized by Sangon Biotech. Luria-Bertani (LB) media was used for the growth of E. coli. Restriction enzymes, rTaq polymerase, and PCR reagents were purchased from TaKaRa. The Plasmid Mini Kit I (100), BioSpin PCR Purification Kit, and DNA Gel Extraction Kit (100) were purchased from Omega. TMP and L-Tle were purchased from Xiya Co. Ltd. (Chengdu, China) and Sigma-Aldrich Chemical Co (St. Louis, MO, USA), respectively. All other chemicals were analytical grade and commercially available.
Construction of mutant library
The random mutagenesis library was generated by the ep-PCR method as described (Cirino et al. 2003; Zhu et al. 2016a). The leudh gene library containing random mutations was amplified using primers LeuDH-BamHI-F and LeuDH-XhoI-R with rTaq DNA polymerase. (All primers used in this study were listed in Table S1) The PCR mixture (50 μL) contained 5 μL of ep-PCR buffer (500 mM KCl, 70 mM MgCl2, 100 mM Tris-HCl; pH 8.3); 0.2 mM each of dATP and dGTP; 1.0 mM each of dCTP and dTTP; 5.5 mM MgCl2, 75 μM MnCl2, and 10 ng plasmid pET28a-leudh as template; 20 μM forward primer/reverse primer; 2.5 U rTaq; and distilled H2O. The PCR program was run for 30 cycles under the following conditions: 30 s at 98 °C, 10 s at 98 °C, 30 s at 53 °C, 70 s at 72 °C, and 10 min at 72 °C, after which it was kept at 10 °C. The ligation products were transformed into E. coli BL21 (DE3), and around 3000 transformants were recovered. The PCR product was digested with BamHI and XhoI and ligated into plasmid pET28a. Ten randomly picked clones from this library were sequenced and contained an average of two nucleotide mutations per clone.
In order to obtain better mutants, we used ISM to combine the sites selected by error-prone PCR (Reetz and Carballeira 2007). The plasmid pET28a-BcLeuDH was used as the template. The primers used were listed in Table S1. The screening volume of the clones reached 95% library coverage (Reetz et al. 2008).
High-throughput screening
Single colonies created by ep-PCR and ISM were picked into 96-well microtiter plates containing 1 mL of LB media with 50 μg/mL kanamycin using a QPix420 colony picker. In each plate, eight wells were reserved as the controls. The plates were shaken at 37 °C for 10 h, then shifted 50 μL of culture to 48-well microtiter plates containing 2 mL of LB media with 50 μg/mL kanamycin for an additional 2.5 h at 37 °C, and finally induced with 0.15 mM 2,4-dinitrophenylhydrazine (IPTG). After 12 h of induction at 17 °C, cells were harvested by centrifugation (12,857×g, 5 min, 4 °C), resuspended in 200 μL lysis buffer (100 mM Tris-HCl buffer, 4 mg/mL lysozyme, pH 7.5), and disrupted by freezing at − 80 °C for 30 min and by heat shocking at 45 °C for 3 min, and then incubated for 10 min at 37 °C. After centrifugation (3724×g, 20 min, 4 °C), the crude enzyme extracts were used for downstream enzymatic reactions.
Random mutagenesis library was screened by 2,4-dinitrophenylhydrazine (DNPH) method (Zhou et al. 2016; Zhu et al. 2016a). A dose of 25 μL of the crude extract of LeuDH mutant was added into 96-well microtiter plates containing 975 μL of the substrate solution (100 mM Tris-HCl, pH 8.5, 1 mM NAD+, 20 mM TMP, 200 mM glucose, 25 μL crude GDH extract, 500 mM NH4Cl). After incubation at 30 °C for 40 min, 100 μL of the reaction solution in each well was transferred to a new 96-well microtiter plates containing 50 μL of 2 mM DNPH (dissolved in 1.2 M HCl) to terminate the reaction. After incubation at 25 °C for 5 min, 50 μL of 4 M NaOH was added. The absorbance at 535 nm was measured, and the mutants with lower absorbance than wild-type enzyme were selected (Fig. S1).
ISM library was screened using an NAD+ auto fluorescence assay (Abrahamson et al. 2012). The assay involved reading the well’s absorbance at two wavelengths, 340 nm and 600 nm. The decreased absorbance at 340 nm corresponds to the consumption of NADH, while the 600 nm reading estimates the biomass present in the well. Differences over background in absorbance at 340 nm are normalized by the 600 nm absorbance readings. The change in absorbance at 340 nm for 1 min was divided by absorbance at 600 nm. The mutants with higher values than wild-type enzyme were selected. The reaction mixture (200 μL) contained 10 μL of crude enzyme extracts, 0.2 mM NADH, 10 mM TMP, 1 M NH4Cl, and 0.1 M Tris-HCl buffer (pH 8.5).
Expression and purification of enzymes
Mutants and wild type were chosen for purification. Cells were grown in LB medium at 37 °C until the OD600 reached about 0.6, and then they were induced by 0.1 mM IPTG. The culture was collected by centrifugation and washed three times using normal saline. Then, cell breaking was performed with an ultrasonic cell disruption system (SCIENTZ-IID). After centrifugation at 12,857×g for 30 min, the clarified supernatant was purified by Ni2+-NTA chromatography. The molecular mass of the purified LeuDH was examined by SDS-PAGE (Schägger 2006).
LeuDH-activity assay
The activity of LeuDH was determined at 30 °C by monitoring the absorbance change at 340 nm which was corresponding to the concentration variation of NADH (Li et al. 2014). For reductive amination, the reaction mixture (200 μL) contained 10 mM TMP, 0.2 mM NADH, 1 M NH4Cl, Tri-HCl buffer (100 mM, pH 8.5), and 20 μL crude or purified enzyme. One unit of enzyme activity was defined as the amount of enzyme catalyzing the reduction of 1 μmol TMP or oxidation of 1 μmol L-Tle per minute.
Kinetic parameters determination
Kinetic parameters of the wild-type BcLeuDH and variants were determined in Tri-HCl buffer (100 mM, pH 8.5) at 30 °C with varied concentration of TMP (with concentration range from 0.08 to 160 mM) or NADH (with concentration range from 0.01 to 0.5 mM). The Michaelis-Menten constants (Km and kcat) were calculated using the nonlinear curve in Origin 8.5.3.
Optimum pH, temperature, and stability
The enzyme activity of the wild-type BcLeuDH and variants was assayed at various pH (7.0–10.0) in NH4Cl-NH3·H2O buffer (30 °C). The pH stability of the wild-type BcLeuDH and variants was determined by enzyme incubation at 37 °C for 14 h at various pH (7.0–11.0) in NH4Cl-NH3·H2O buffer.
The enzyme activity of the wild-type BcLeuDH and variants was measured at various temperatures (30–70 °C) in 100 mM Tris-HCl buffer (pH 8.5). The half-lives of thermal inactivation (t1/2) of wild-type BcLeuDH and variants were calculated by incubation for different time at 55 °C in Tris-HCl buffer (100 mM pH 7.5). The values of t1/2 at 55 °C were calculated using the following formula: t1/2 = ln2 k−1 (k is first-order rate constant which is deduced from the semilogarithmic plot of incubation time versus residual activity) (Le et al. 2012). Activities of wild-type BcLeuDH and variants at 30 °C in Tri-HCl buffer (100 mM, pH 7.5) without incubation were defined as 100%.
Construction of co-expression system of LeuDH and GDH
The co-expression system containing LeuDH and GDH was constructed using a Shine-Dalgarno and aligned spacing sequence (SD-AS, GAAGGAGATATACC) as a linker between them (Zhou et al. 2015). Primers SD-AS-GDH-F and GDH-R were used to amplify short fragments containing SD-AS and GDH using pET28a-BaGDH as template. The long fragments containing pET28a, LeuDH and SD-AS were amplified by PCR as well (template: pET28a-BcLeuDH; primers: V-LeuDH-F/V-SD-AS-LeuDH-R). Next, the short and long fragments were linked by homologous recombination using the ClonExpress II One Step Cloning Kit to form pET28a-BcLeuDH-SD-AS-BaGDH (Wang et al. 2018).
Analytical methods
The substrate (TMP) was analyzed on a RP18ODS (5 μm) column, eluted with methanol/water/trifluoroacetic acid in a volume ratio of 55:45:0.001 (v/v) as mobile phase at a flow rate of 0.8 mL min−1 and detected at 230 nm, injection volume 10 μL (Li et al. 2014).
L-Tle in the reaction mixtures were labeled using 1-fluoro-2,4-dinitrophenyl-5-L-alanineamide (FDAA) and then analyzed on a Develosil ODS-UG-5 column (150 mm × 4.6 mm). A 10-μL sample of the amino acid, 8 μL of 1 M NaHCO3, and 40 μL of 1% (w/v) FDAA in acetone were mixed and heated for 1 h at 40 °C. When the sample was cooled to room temperature, 8 μL of 1 N HCl and 934 mL of 40% (v/v) aqueous acetonitrile were added to the mixture, after which it was vortexed and filtered (0.22 mm) for HPLC (Hanson et al. 2008). The HPLC conditions were as follows: mobile phase A 5% acetonitrile (0.05% trifluoroacetic acid, 1% methanol), mobile phase B 60% acetonitrile (0.05% trifluoroacetic acid, 1% methanol), and linear gradient from 0% to 100% B over 45 min at a flow rate 1 mL min−1 and detected at 340 nm, injection volume 20 μL (Bhushan and Brückner 2004; Zhang et al. 2019).
Homology modeling
The homologous structure of wild-type (WT) LeuDH was modeled using SWISS-MODEL (https://www. swissmodel.expasy.org). The X-ray structure of LeuDH from Geobacillus stearothermophilus (PDB ID: 6ACH), which has 82% sequence identity with BcLeuDH, was used as the structural template (Yamaguchi et al. 2018).
Results
Construction of the mutation library by ep-PCR and screening for high TMP catalytic efficiency
The random mutagenesis library was constructed based on wild-type LeuDH and screened by high-throughput screening method based on the spectrophotometric assay of substrate TMP (Zhou et al. 2016). Mutants with OD535 values below that of the wild type were selected from about 3000 colonies. After protein purification and determination of specific activity (Fig. S2), mutants with higher specific activity than the wild type were sequenced. Three mutations (E116Q, D126E, and E24A/D126E) were identified. Their specific activities were shown in Fig. 2a. In this section, we obtained 3 mutants through error-prone PCR, covering 3 mutation sites. Changes in these sites have a significant impact on specific activity of BcLeuDH.
Combination of mutation sites. a Comparison of specific activity of optimal mutation sites combination. b Comparison of specific activity of ISM. A represents a saturation mutation at position 24; B represents a saturation mutation at position 116; C represents a saturation mutation at position 126; the dotted line indicates that no better mutants have been screened. Specific activity detection conditions: TMP (10 mM), NH4Cl (1 M), NADH (0.2 mM), 0.1 M Tris-HCl buffer (pH 8.5), 30 °C
Construction of the mutation library by ISM and screening for high coenzyme affinity
Results of random mutagenesis showed that the mutant enzyme with the amino acid substitutions E24V, E116V, and D126E produced higher specific activity. Thus, we combined these three sites to obtain the optimal mutant. The three-point saturation combination mutation required about 8000 mutations. If NNK was used as the mutation codon, then 98,163 mutants would need to be screened to achieve 95% coverage. To minimize the size of the library of variants and improve screening efficiency, we applied ISM to combine the three points (E24, E116, and D126), thereby reducing the number of relevant mutants to be screened to 564. The high-throughput screening method was modified to detect NADH instead of TMP, with the coenzyme concentration reduced from 1 to 0.2 mM. Mutants with higher catalytic activity at low coenzyme concentrations were selected. As shown in Fig. 2b, the specific activities of E116V and D126E were 2.1- and 1.9-fold higher than that of the wild type, respectively, in the first round of saturation mutation. However, the saturation mutation of E24V did not result in a more effective mutant. Subsequently, a second round of saturation mutation was performed based on E116V and D126E, and two positive mutants were obtained. The specific activities of E24V/E116V and E24A/D126E were 2.5- and 2.6-fold higher than that of the wild type, respectively. No better mutant was found in the third round of saturation mutation. During the entire process, we screened four mutants (E116V, D126E, E24V/E116V, and E24A/D126E) with potential applications.
Catalytic characterization comparison of wild-type and mutant LeuDH
We compared enzymatic properties of the wild type and the four selected mutants (E116V, D126E, E24V/E116V, and E24A/D126E). As shown in Fig. S3, no obvious difference was observed in the optimal reaction temperature, pH, and pH stability between the mutants and the wild type. However, the thermal stability of the mutants improved significantly. As shown in Fig. S3b, the t1/2 of E116V, D126E, E24V/E116V, and E24A/D126E at 55 °C increased to 20.4 h, 23.9 h, 13.1 h, and 26.1 h, respectively, compared with that of the wild type (10.2 h). Tables 1 and 2 show the kinetic parameters of the wild type and mutants compared. The affinity of the engineered enzymes for NADH and TMP gradually increased in ISM. Compared with that of the native enzyme, the kcat/Km values of the most active mutant E24V/E116V for TMP and NADH increased 5.3- and 3-fold, respectively. This shows that the obtained mutants had improved coenzyme affinity and catalytic efficiency after ISM screening.
Conversion of TMP to L-Tle using coupled enzyme
We coupled BcLeuDH native enzyme or mutant with BaGDH to determine the influence of the coenzyme concentration on the transformation experiment. Reactions were performed with a fixed concentration of TMP (0.2 M), and variable NAD+ concentrations (0, 0.05, 0.10, 0.15 mM). As shown in Fig. 3, none of the reactions completed without added coenzyme. At 0.15 mM NAD+, the enzyme efficiency of the native LeuDH and the mutants was comparable. However, as the concentration of coenzyme decreased, the difference in efficiency between the native enzyme and the mutants gradually increased. At 0.05 mM NAD+, both native and mutant LeuDH completely converted TMP to L-Tle, but their efficiencies were significantly different. The catalytic efficiency of E24V/E116V was 10 times higher than that of the native enzyme. Experiments performed using a fixed concentration of TMP (0.5 M) and NAD+ (0.05 mM) revealed significantly lower efficiency than that at 0.2 M TMP, which suggested a feedback inhibition by the substrate (Fig. 4a). To alleviate this problem, we applied a batch-feeding strategy and successfully completed the conversion of 0.57 M substrate in 75 min (Fig. 4b), which was 2.4-fold faster than the conversion in unfed reaction. Comparisons with other studies (Table 3) revealed that the mutant E24V/E116V generated in this work had the highest specific time-space conversion rate (22.8 mmol·h−1·L−1·g−1) and TTN (11,400).
Effect of NAD+ concentration on the reductive amination of TMP. a 0 mM NADH; b 0.05 mM NADH; c 0.10 mM NADH; d 0.15 mM NADH. Reaction conditions: TMP (0.2 M), NH4Cl-NH3H2O (1 M), glucose (0.24 M) and cell-free extracts of E. coli cells (20 gL−1 wet cells) in Tri-HCl buffer (0.1 M, pH 8.5), 30 °C, 200 rpm, 2.5 h
Effect of substrate feeding strategy on the reductive amination of TMP. a 0.5 M TMP, 0.6 M glucose, ammonia-ammonium chloride (1 M), NAD+ (0.05 mM) and cell-free extracts of E. coli cells (20 g·L−1) in Tri-HCl buffer (0.1 M), ammonia for pH adjustment; b reaction conditions: three additions of TMP solution (1.25, 1.25, 1.25 mL three times at 15 min, 30 min, 45 min, final concentration 0.57 M), 0.6 M glucose, ammonia-ammonium chloride (1 M), NAD+ (0.05 mM) and cell-free extracts of E. coli cells (20 g·L−1) in Tri-HCl buffer (0.1 M), ammonia for pH adjustment
Discussion
Leucine dehydrogenase plays an important role in catalyzing the synthesis of L-Tle. Its low affinity for coenzymes and low catalytic efficiency for the unnatural substrate limits its industrial application. However, few studies have comprehensively considered the directed evolution of catalytic efficiency and coenzyme affinity. In this study, we used two high-throughput screening methods to evolve the catalytic efficiency and coenzyme affinity of the enzyme. In the process, two methods were used to constructing the libraries. Among them, epPCR expands the search range of mutation sites, and ISM reduces the screening effort drastically. This comprehensive screening strategy can be adapted for the screening of other dehydrogenase that catalyzes carbonyl compound conversion.
During the ISM screening of LeuDH activity, both E116V and D126E showed higher catalytic activity at low coenzyme concentration. However, a better mutant was not obtained when these two sites were combined, which indicated introduction of E116V and D126E in BcLeuDH did not have any synergistic effect. The kinetic parameters revealed improved catalytic efficiency and coenzyme affinity of the mutants. Results of enzyme stability experiments showed that the mutants had a longer half-life compared to the native enzyme. Simultaneously improvement of catalytic efficiency and thermostabilization is challenging due to the well-known stability-activity trade-off (Gong et al. 2018). Our results indicated that the mutation sites selected in this study may be used as a reference for any future modification of leucine dehydrogenases from other sources.
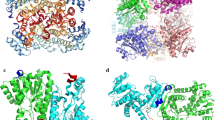
We analyzed the positions of the three mutation points (E24, E116, D126) in the secondary structure to understand the differences in the enzymatic properties of the mutants. LeuDHs are homooctamers composed of eight monomers (Baker et al. 1995). The secondary structure of native BcLeuDH is shown in Fig.5a, and Fig. 5b shows two adjacent monomers. Sites E24 is located between βA and βB, and D126 is located on the α4 helix. Both these two sites are distant from the catalytic pocket; 116E is located between βA and α4 in the substrate pocket channel, and therefore, it does not directly interact with the substrate and coenzyme in the pocket. Both E24 and D126 can form salt bridges, respectively, with N350 (located on α15) and K310 (located on α14) of the adjacent monomer. The kinetic analysis showed that the LeuDH mutants had higher catalytic efficiency (kcat/Km) than the wild type, which was attributed to the increased affinity of the mutants for TMP and NADH. It is interesting to observe that mutations distal to the substrate binding sites affect enzymatic activity, likely affecting conformation variation or subunits interactions (Lee and Goodey 2011). Several interactions such as hydrogen bonding, salt bridges, or hydrophobic interactions may contribute to improving thermal stability (Xu et al. 2019). In the E116V mutant of LeuDH, thermal stability improved possibly via improved substrate channel hydrophobicity (Zhou et al. 2018). As shown in Fig. S4, the D126E mutation of LeuDH shortened the distance between E126 and K310 and strengthened their ionic bonds, which may explain why the thermal stabilities of the D126E and E24A/D126E mutants were greatly improved.
Location analysis of mutation sites. a The secondary structure of BcLeuDH. The red box indicates the position of the mutation site in the sequence, and the black box indicates the associated site. b The model structure of wild type LeuDH. The structure obtained by homology modeling is a homooctamer. It shows two adjacent monomers of the homooctamer. E24 and D126 are at the interface of the monomers and form a salt bridge with the amino acid residues of the adjacent monomers
To further increase the coenzyme TTN, we increased the substrate concentration of the transformation experiment. The experiment with coupled LeuDH and BaGDH at 0.5 M TMP showed substrate inhibition, which was not alleviated during the reaction time-course. We speculate that the reaction of high substrate concentration may have an irreversible effect on the enzyme. To solve this problem, we used the strategy of adding the substrate in batches to reduce the influence of substrate inhibition. This strategy effectively reduced the impact of high substrate concentration, and the final concentration of the reaction reached 0.57 M.
In conclusion, the mutant E24V/E116V selected by two rounds of high-throughput screening showed improved catalytic efficiency, thermal stability, and a higher coenzyme affinity compared with the native enzyme. Coupling the E24V/E116V with BaGDH, resulted in a TTN of 11,400 with a specific time-space conversion rate of 22.8 mmol·h−1·L−1·g−1, which was significantly higher than that of the native enzyme (TTN: 4000; specific time-space conversion rate: 4 mmol·h−1·L−1·g−1). These results indicate that E24V/E116V is a useful enzyme with potential for the industrial production of valuable L-Tle.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abrahamson MJ, Vázquez-Figueroa E, Woodall NB, Moore JC, Bommarius AS (2012) Development of an amine dehydrogenase for synthesis of chiral amines. Angew Chem Int Ed Eng 51:3969–3972. https://doi.org/10.1002/anie.201107813
Baker PJ, Turnbull AP, Sedelnikova SE, Stillman TJ, Rice DW (1995) A role for quaternary structure in the substrate specificity of leucine dehydrogenase. Structure 3:693–705. https://doi.org/10.1016/S0969-2126(01)00204-0
Bhushan R, Brückner H (2004) Marfey's reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247. https://doi.org/10.1007/s00726-004-0118-0
Bommarius AS, Schwarm M, Stingl K, Kottenhahn M, Huthmacher K, Drauz K (1995) Synthesis and use of enantiomerically pure tert-leucine. Tetrahedron Asymmetry 6:2851–2888. https://doi.org/10.1016/0957-4166(95)00377-0
Cirino PC, Mayer KM, Umeno D (2003) Generating mutant libraries using error-prone PCR. In: Arnold FH, Georgiou G (eds) Directed evolution library creation. Methods in Molecular Biology. Humana Press, New Jersey, pp 3–9. https://doi.org/10.1385/1-59259-395-X:3
Gong X-M, Qin Z, Li F-L, Zeng B-B, Zheng G-W, Xu J-H (2018) Development of an engineered ketoreductase with simultaneously improved thermostability and activity for making a bulky atorvastatin precursor. ACS Catal 9:147–153. https://doi.org/10.1021/acscatal.8b03382
Hanson RL, Davis BL, Goldberg SL, Johnston RM, Parker WL, Tully TP, Montana MA, Patel RN (2008) Enzymatic preparation of a D-amino acid from a racemic amino acid or keto acid. Org Process Res Dev 12:1119–1129. https://doi.org/10.1021/op800149q
Jiang W, Sun D, Lu J, Wang Y, Wang S, Zhang Y, Fang B (2016) A cold-adapted leucine dehydrogenase from marine bacterium Alcanivorax dieselolei: characterization and L-tert-leucine production. Eng Life Sci 16:283–289. https://doi.org/10.1002/elsc.201500092
Jiang W, Xu C-Z, Jiang S-Z, Zhang T-D, Wang S-Z, Fang B-S (2017) Establishing a mathematical equations and improving the production of L-tert-leucine by uniform design and regression analysis. Appl Biochem Biotechnol 181:1454–1464. https://doi.org/10.1007/s12010-016-2295-1
Josephsohn NS, Kuntz KW, Snapper ML, Hoveyda AH (2001) Mechanism of enantioselective Ti-catalyzed strecker reaction: peptide-based metal complexes as bifunctional catalysts. J Am Chem Soc 123:11594–11599. https://doi.org/10.1021/ja011875w
Kim J-Y, Lee Y-A, Wittmann C, Park J-B (2013) Production of non-proteinogenic amino acids from α-keto acid precursors with recombinant Corynebacterium glutamicum. Biotechnol Bioeng 110:2846–2855. https://doi.org/10.1002/bit.24962
Laumen K, Kittelmann M, Ghisalba O (2002) Chemo-enzymatic approaches for the creation of novel chiral building blocks and reagents for pharmaceutical applications. J Mol Catal B Enzym 19-20:55–66. https://doi.org/10.1016/S1381-1177(02)00151-0
Le QAT, Joo JC, Yoo YJ, Kim YH (2012) Development of thermostable Candida antarctica lipase B through novel in silico design of disulfide bridge. Biotechnol Bioeng 109:867–876. https://doi.org/10.1002/bit.24371
Lee J, Goodey NM (2011) Catalytic contributions from remote regions of enzyme structure. Chem Rev 111:7595–7624. https://doi.org/10.1021/cr100042n
Li J, Pan J, Zhang J, Xu J-H (2014) Stereoselective synthesis of L-tert-leucine by a newly cloned leucine dehydrogenase from Exiguobacterium sibiricum. J Mol Catal B Enzym 105:11–17. https://doi.org/10.1016/j.molcatb.2014.03.010
Liu W, Ma H, Luo J, Shen W, Xu X, Li S, Hu Y, Huang H (2014) Efficient synthesis of L-tert-leucine through reductive amination using leucine dehydrogenase and formate dehydrogenase coexpressed in recombinant E. coli. Biochem Eng J 91:204–209. https://doi.org/10.1016/j.bej.2014.08.003
Lu J, Zhang Y, Sun D, Jiang W, Wang S, Fang B (2016) The Development of leucine dehydrogenase and formate dehydrogenase bifunctional enzyme cascade improves the biosynthsis of L-tert-leucine. Appl Biochem Biotechnol 180:1180–1195. https://doi.org/10.1007/s12010-016-2160-2
Luo W, Zhu J, Zhao Y, Zhang H, Yang X, Liu Y, Rao Z, Yu X (2020) Cloning and expression of a novel leucine dehydrogenase: characterization and L-tert-leucine production. Front Bioeng Biotechnol 8:1. https://doi.org/10.3389/fbioe.2020.00186
Menzel A, Werner H, Altenbuchner J, Gröger H (2004) From enzymes to “designer bugs” in reductive amination: a new process for the synthesis of L-tert-leucine using a whole cell-catalyst. Eng Life Sci 4:573–576. https://doi.org/10.1002/elsc.200402162
Reetz, Carballeira JD (2007) Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat Protoc 2:891–903. https://doi.org/10.1038/nprot.2007.72
Reetz, Kahakeaw D, Lohmer R (2008) Addressing the numbers problem in directed evolution. Chembiochem 9:1797–1804. https://doi.org/10.1002/cbic.200800298
Schägger H (2006) Tricine-SDS-PAGE. Nat Protoc 1:16–22. https://doi.org/10.1038/nprot.2006.4
Turner NJ (2009) Directed evolution drives the next generation of biocatalysts. Nat Chem Biol 5:567–573. https://doi.org/10.1038/nchembio.203
Wang L, Zhu W, Gao Z, Zhou H, Cao F, Jiang M, Li Y, Jia H, Wei P (2020) Biosynthetic L-tert-leucine using Escherichia coli co-expressing a novel NADH-dependent leucine dehydrogenase and a formate dehydrogenase. Electron J Biotechnol 47:83–88. https://doi.org/10.1016/j.ejbt.2020.07.001
Wang X, Nie Y, Xu Y (2018) Improvement of the activity and stability of starch-debranching pullulanase from Bacillus naganoensis via tailoring of the active sites lining the catalytic pocket. J Agric Food Chem 66:13236–13242. https://doi.org/10.1021/acs.jafc.8b06002
Xu Z, Cen Y-K, Zou S-P, Xue Y-P, Zheng Y-G (2019) Recent advances in the improvement of enzyme thermostability by structure modification. Crit Rev Biotechnol 40:83–98. https://doi.org/10.1080/07388551.2019.1682963
Xu Z, Singh J (2002) Process research and development for an efficient synthesis of the HIV protease inhibitor BMS-232632. Org Process Res Dev 6:323–328. https://doi.org/10.1021/op025504r
Xue Y-P, Cao C-H, Zheng Y-G (2018) Enzymatic asymmetric synthesis of chiral amino acids. Chem Soc Rev 47:1516–1561. https://doi.org/10.1039/c7cs00253j
Yamaguchi H, Kamegawa A, Nakata K, Kashiwagi T, Mizukoshi T, Fujiyoshi Y, Tani K (2018) Structural insights into thermostabilization of leucine dehydrogenase from its atomic structure by cryo-electron microscopy. J Struct Biol 205:11–21. https://doi.org/10.1016/j.jsb.2018.12.001
Zhang D, Jing X, Zhang W, Nie Y, Xu Y (2019) Highly selective synthesis of D-amino acids from readily available L-amino acids by a one-pot biocatalytic stereoinversion cascade. RSC Adv 9:29927–29935. https://doi.org/10.1039/C9RA06301C
Zhao Y, Wakamatsu T, Doi K, Sakuraba H, Ohshima T (2012) A psychrophilic leucine dehydrogenase from Sporosarcina psychrophila: purification, characterization, gene sequencing and crystal structure analysis. J Mol Catal B Enzym 83:65–72. https://doi.org/10.1016/j.molcatb.2012.06.018
Zhou J, Wang Y, Chen J, Xu M, Yang T, Zheng J, Zhang X, Rao Z (2018) Rational engineering of Bacillus cereus leucine dehydrogenase towards α-keto acid reduction for improving unnatural amino acid production. Biotechnol J 14:1800253. https://doi.org/10.1002/biot.201800253
Zhou J, Xu G, Han R, Dong J, Zhang W, Zhang R, Ni Y (2016) Carbonyl group-dependent high-throughput screening and enzymatic characterization of diaromatic ketone reductase. Catal Sci Technol 6:6320–6327. https://doi.org/10.1039/C6CY00922K
Zhou X, Zhang R, Xu Y, Liang H, Jiang J, Xiao R (2015) Coupled (R)-carbonyl reductase and glucose dehydrogenase catalyzes (R)-1-phenyl-1,2-ethanediol biosynthesis with excellent stereochemical selectivity. Process Biochem 50:1807–1813. https://doi.org/10.1016/j.procbio.2015.08.002
Zhu L, Wu Z, Jin J-M, Tang S-Y (2016a) Directed evolution of leucine dehydrogenase for improved efficiency of L-tert-leucine synthesis. Appl Microbiol Biotechnol 100:5805–5813. https://doi.org/10.1007/s00253-016-7371-5
Zhu W, Li Y, Jia H, Wei P, Zhou H, Jiang M (2016b) Expression, purification and characterization of a thermostable leucine dehydrogenase from the halophilic thermophile Laceyella sacchari. Biotechnol Lett 38:855–861. https://doi.org/10.1007/s10529-016-2053-z
Acknowledgements
We thank Editage (www.editage.cn) for English language editing.
Funding
This work was financially supported by the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX19_1831), National Natural Science Foundation of China (Nos. 21336009, 21176103), the National High Technology Research and Development Program of China (No. 2015AA021004), and the Overseas Expertise Introduction Project for Discipline Innovation, China (No. 111-2-06).
Author information
Authors and Affiliations
Contributions
F.Z., X.M., Y.N., and Y.X. conceived and designed the experiments. F.Z. performed the experiments. F.Z. and X.M. analyzed experimental data. F.Z., X.M., and Y.N. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 2863 kb)
Rights and permissions
About this article
Cite this article
Zhou, F., Mu, X., Nie, Y. et al. Enhanced catalytic efficiency and coenzyme affinity of leucine dehydrogenase by comprehensive screening strategy for L-tert-leucine synthesis. Appl Microbiol Biotechnol 105, 3625–3634 (2021). https://doi.org/10.1007/s00253-021-11323-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11323-w