Abstract
The low yield as bottleneck problem limits the application of microbial flocculant in water treatment. However, genetic information of microbial flocculant-producing strains can guide the regulation of microbial flocculant production, but it remains unknown. Agrobacterium tumefaciens F2 produced polysaccharide-based microbial flocculants in the fermentation medium but none in Luria Bertani medium; hence, the transcriptome was used to analyze the potentially associated genes with the production of microbial flocculants. Glucose, mannose, rhamnose, and galactose are the main sugar monomers, and genes (manA, glmM, manC, rfb genes, exo genes, etc.) with changed expression levels related to sugar monomers metabolism potentially participated in the biosynthesis of polysaccharide-based microbial flocculants. exoC, exoP, and manC were confirmed to participate in the biosynthesis via constructing the mutants F2-dexoC, F2-dexoP, and F2-dmanC. An exoF2 gene cluster was annotated due to the high percentage of matches between the genome sequences of strains F2 and C58, and exo genes in their genome sequences showed the similarity of 86~92%. The hypothetical pathway for the biosynthesis of polysaccharide-based microbial flocculants in strain F2 was proposed, laying the basis for the production yield regulation.
Key points
• An exo F2 gene cluster in the polysaccharide biosynthesis was annotated.
• exoC, exoP, and manC genes participated in the polysaccharide biosynthesis.
• A hypothetical biosynthesis pathway of polysaccharide in flocculant was proposed.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural microbial flocculants are attractive due to their ubiquitous and environment friendly nature, biodegradability, and high application value (Salehizadeh et al. 2018; Yang et al. 2016). In general, microbial flocculants can be divided into polysaccharide-, protein-, nucleic acid-, and lipid-based microbial flocculants. Notably, the extensive application of microbial flocculants produced by bacterial strains in aqueous environments renders it a potential biomaterial (Salehizadeh et al. 2018). However, the low yield of microbial flocculants is the bottleneck, limiting their large-scale industrial production. Genetic studies regarding microbial flocculant-producing bacteria and the biosynthetic mechanism of microbial flocculants can solve this problem. Reports suggest that genetic modification and alteration of biosynthetic or regulatory pathways improve exopolysaccharide yield (Ruffing and Chen 2006). Thus, investigations regarding the genetics of the biosynthetic pathway of microbial flocculant-producing bacteria may assist in improving the yield of polysaccharide-based microbial flocculants.
The microbial flocculant-producing bacterium, Agrobacterium tumefaciens F2, was isolated from the sludge of sewage treatment plants, and polysaccharide is the main component of its microbial flocculant products (Wu et al. 2015). Polysaccharide-based microbial flocculants produced by strain F2 have been used for pollutant treatment in the aqueous environment, but the genetics underlying their biosynthetic pathway are not yet understood. Fortunately, the relevant genes and pathways associated with the production of major bacterial exopolysaccharides by Agrobacterium have been widely studied. Succinoglycan is a common polysaccharide biosynthesized by bacteria of family from Rhizobiaceae, such as Rhizobium and Agrobacterium (Zevenhuizen 1997). Several studies have reported succinoglycan production by Agrobacterium (Cangelosi et al. 1987; Chouly et al. 1995; Evans et al. 2000; Stredansky and Conti 1999; Stredansky et al. 1998), while the succinoglycan biosynthetic pathway has been more widely proposed in Sinorhizobium meliloti (Becker 2015; Glucksmann et al. 1993; Janczarek 2011; Reuber and Walker 1993; Skorupska et al. 2006).
Wood et al. (2001) observed extensive similarity in the genome sequences of A. tumefaciens C58 and S. meliloti (Wood et al. 2001), and subsequent studies have also reported that strain C58 utilized an identical succinoglycan biosynthesis pathway (Wu et al. 2016). In general, the polysaccharide biosynthetic pathway can be classified into three main steps, including biosynthesis of nucleotide sugars, assembly of the repeat unit and polymerization, and export (Rehm 2010; Schmid et al. 2015). Succinoglycan is a branched polysaccharide consisting of glucose and galactose in the ratio of 7:1, with substituent additions of succinate, pyruvate, and acetate (Chouly et al. 1995; Reinhold et al. 1994; Schmid et al. 2015; Wang et al. 1999). In the succinoglycan biosynthetic pathway, exoC, exoB, and exoN are essential for sugar precursor biosynthesis, whereas exoY and exoF are responsible for galactose addition to the lipid carrier. Glucose residues are added by glycosyltransferases (GTs) encoded by exoALMOUW genes, which are also responsible for glycosidic bond formation. exoZ, exoH, and exoV are involved in the substituent modifications of the growing exopolysaccharides, adding acetyl, succinyl, and pyruvyl groups to the sugar chain. Polymerization of repeating units and secretion of exopolysaccharides are accomplished by proteins encoded by exoPQT (Becker 2015; Reuber and Walker 1993; Schmid et al. 2015; Skorupska et al. 2006; Wu et al. 2016). These studies have laid the foundation for an in-depth investigation regarding the biosynthetic pathway of polysaccharide-based microbial flocculants in strain F2.
However, certain information regarding polysaccharide-based microbial flocculant biosynthesis is still lacking, including (a) the homology in the genome sequence and the similarity between the exo genes of strains F2 and C58, (b) the sugar monomer composition of polysaccharide-based microbial flocculants, and (c) the key genes that participated in the biosynthesis of polysaccharide-based microbial flocculants. This has impeded uncovering of the biosynthetic pathway of polysaccharide-based microbial flocculants. Thus, the present study aims at investigating the above-mentioned unknown aspects to guide constructing the biosynthesis pathway of polysaccharide-based microbial flocculants in strain F2, and thereby guiding yield improvement of polysaccharide-based microbial flocculants and promoting their industrial application.
Materials and methods
Strain and medium
The A. tumefaciens F2 was isolated by our laboratory and deposited in the China General Microbiological Culture Collection Center (CGMCC no. 10131). This strain can produce polysaccharide-based microbial flocculants (Li et al. 2011; Wu et al. 2015). The fermentation medium (g/L) contained glucose, 10; urea, 0.5; MgSO4·7H2O, 0.2; KH2PO4, 2; K2HPO4, 5; NaCl, 0.1, and yeast extract, 0.5, with the initial pH of 7.2~7.5; it was sterilized at 112 °C for 30 min. All components in medium were purchased from the Sinopharm Chemical Reagent Co., Ltd.
Comparative analysis of the genome
The MUMmer software was used to compare the ortholog distribution among the genomes in A. tumefaciens F2 (GenBank No. AFSD00000000.1), Agrobacterium radiobacter K84 (CP000628), Agrobacterium vitis S4 (CP000633), and A. tumefaciens C58 (AE007869). Genome clustering analysis and collinear analysis were completed using the SiLiX and BSR methods. Protein functions were analyzed using Gene Ontology (GO), Clusters of Orthologous Group, Kyoto Encyclopedia of Genes and Genomes (KEGG), and InterPro Database.
Analysis of gene expression by RNA-seq and qRT-PCR
Strain F2 was first pre-cultured in Luria Bertani (LB) medium and was then inoculated into LB and fermentation medium, respectively. The samples used for transcriptome analysis were collected after the bacterial strain was cultured until 24 h. Total RNA was extracted from the strain F2 using the RNeasy mini kit (Qiagen, Germany) together with DNA digestion using the RNase-free DNase set (Qiagen, Germany), per the manufacturer’s instructions. RNA concentration and quality were estimated using a NanoDrop 8000 spectrophotometer (Thermo Scientific, USA). Ribosomal RNA removal was performed with the Ribo-Zero rRNA kit removal kit. Upon mixing with the fragmentation buffer, the mRNA was fragmented into short fragments. Then, cDNA was synthesized using the mRNA fragments as templates. Short fragments were purified and dissolved with EB buffer for end reparation and single nucleotide A (adenine) addition. Afterwards, the short fragments were connected with the adapters. After agarose gel electrophoresis, the suitable fragments were selected for PCR amplification as templates. During the QC steps, the Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-Time PCR System were used for the quantification and qualification of the sample library.
The primary sequencing data produced by Illumina HiSeq™ 2000, which are called raw reads, were subjected to QC to determine if a resequencing step is needed. After QC, the raw reads were filtered into clean reads, which were then aligned to the reference sequences with SOAPaligner/SOAP2. The alignment was subjected to QC to determine if resequencing is needed. The alignment data were utilized to calculate the distribution of reads on reference genes and perform coverage analysis. Gene expression was analyzed after the alignment result passed the QC. Differentially expressed genes (DEGs) were screened using statistical cutoff of |log2 ratio| ≥ 1 and FDR ≤ 0.001. All raw RNA sequencing data are deposited and are accessible in NCBI (PRJNA557413).
The samples were also used for quantitative real-time PCR (qRT-PCR) analysis. Total cDNA was obtained using the PrimeScript™ RT reagent kit with gDNA eraser (Takara Biotechnology, Co., Ltd., Dalian, China). The obtained cDNA was used for further qRT-PCR analysis using SYBR® Premix Ex Taq™ (Tli RNaseH Plus) on an ABI7500 (Applied Biosystems, USA), per the manufacturer’s instructions in a 50 μL reaction mixture. PCR was performed in triplicate under the following conditions: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 56 °C for 45 s, and 72 °C for 40 s, with a fixed melting curve analysis at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 15 s. Reactions without the cDNA template were used as negative controls. The mRNA levels were analyzed by comparing strain F2 cultured in the fermentation medium and in LB medium after normalization to the reference gene trpE using the 2−∆∆Ct method. All samples were measured in triplicate, and the average values of these measurements were used to calculate mRNA expression. In addition, the samples in the two media were also collected and analyzed using qRT-PCR under the above-mentioned conditions except that the collection time was at 9 h instead of at 24 h. All primers are listed in Table S1.
Gene disruption and complementation in A. tumefaciens F2
A 500-bp DNA fragment containing the middle part of exoC was amplified from the genomic DNA of strain F2 with the restriction sites of SacI and PstI. The purified PCR product was excised via restriction digestion with both SacI and PstI and inserted into the same sites in the pJQ200SK vector, yielding the plasmid pJQ-dexoC, with gentamicin resistance as a selectable marker. The plasmid pJQ-dexoC was introduced into Escherichia coli Top10 and then transferred into strain F2 cells via helper strain E. coli HB101 (pRK600). The exoC disruption was confirmed via PCR using the dexoC-F and dexoC-R primers, followed by sequencing. Meanwhile, the expression of the disrupted gene in the recombinant bacteria was analyzed using reverse transcription PCR (RT-PCR). RNA and cDNA were obtained using the same procedure as mentioned previously. The cDNA products were amplified in 50 μL RT-PCR mixtures with 1 μL cDNA as the template using PrimeSTAR® Max DNA polymerase (Takara Biotechnology, Co., Ltd., Dalian, China), followed by PCR and sequencing.
Two oligonucleotide primers were designed to amplify exoC containing the native promoter from the genomic DNA of strain F2 via PCR. The DNA fragment was amplified with 20 bp homologous arms and inserted into the broad-host-range plasmid pBBR1-MCS2, yielding the plasmid pBB-exoC with neomycin resistance as a selectable marker. The plasmid was transferred into E. coli DH5α cells and then introduced into F2-dexoC with the help of E. coli HB101 (pRK600). The complementation strain containing the pBB-exoC plasmid was designated as F2-dexoC-C. The respective recombinant strains with exoP and manC gene disruption (F2-dexoP and F2-dmanC) and complementation (F2-dexoP-C and F2-dmanC-C) were constructed using the same procedures as mentioned previously. The strains and plasmids used for genetic engineering are shown in Table 1 (Kovach et al. 1995; Quandt and Hynes 1993).
Microbial flocculant production
The wild type and recombinant strains were first pre-cultured in the fermentation medium at 30 °C and 160 rpm for 24 h. Subsequently, the seed culture was inoculated into the fermentation medium and cultured under the same conditions. The fermentation liquid was centrifuged at 10,000 ×g for 20 min to remove the cell pellets. Two volumes of cold ethanol was added to the supernatant for extracting microbial flocculants, and the microbial flocculant-producing capacity of the strains was first evaluated based on the ability to produce white flocs. The microbial flocculant-producing capacity of strains was further evaluated based on the polysaccharide content. The collected flocs were dialyzed, freeze-dried, and then redissolved in the original volume using ultrapure water. Polysaccharide concentration was determined using the phenol-sulfuric acid assay with glucose as the standard. In addition, OD660 was determined after every 3 h to analyze bacterial growth status.
Sugar monomer components
Polysaccharide-based microbial flocculants (2 mg) was dissolved into 1 mL of anhydrous methanol solution containing 1 M HCl, followed by sealed using N2, hydrolyzed at 80 °C for 16 h, and dried by air pump. Then, 2 M trifluoroacetic acid (TFA, 1 mL) was added and hydrolyzed at 120 °C for 1 h. A small amount of ethanol was added and dried by water bath at 60 °C, which was repeated for 3–5 times until complete evaporation of TFA.
1-Phenyl-3-methyl-5-pyrazolone (PMP) solution (0.5 mL) and NaOH (0.3 M, 0.5 mL) were added into the treated sample until fully dissolved. Next, 0.1 mL of the sample was performed with water bath at 70 °C for 30 min. Then, 0.3 M HCl (0.05 mL) and distilled water (0.05 mL) were added and thoroughly mixed after centrifugation at 10,000 rpm for 5 min. Trichloromethane (1 mL) was added and mixed well to extract the remaining PMP reagent. The obtained sample in the water layer was filtered through 0.22 μm filter membrane and diluted by distilled water for HPLC measurement with mannose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, arabinose, and fucose as standards.
Parameters included Shimadzu HPLC system with LC-10ATvp pump and SPD-10AVD ultraviolet detector and DIKMA InertsiL ODS-3 (4.6 × 150 mm). The mobile phase is PBS (0.1 M, pH 7.0) and acetonitrile with 82:18 (v/v). The flow rate was 1.0 mL min−1 with the sample volume of 20 μL, and the wavelength was 245 nm.
Results
General features and genome comparison of Agrobacterium
Comparison of the genome of strains F2, C58, S4, and K84 showed that the four strains shared a common core genome of approximately 939 genes, while strains F2 and C58 were highly similar as they shared the most coding sequences (Fig. 1a). The genome size of the strain F2 is roughly equal to that of strain C58 but smaller than those of strains S4 and K84. The collinear analysis showed high match between the genome sequences of strains F2 and C58 (Fig. 1b and Fig. 1c). Thus, strain F2 was widely collinear with strain C58, exhibiting good sequence conservation. Strain C58 can biosynthesize succinoglycan using exo genes, and thus an exoF2 gene cluster responsible in strain F2 was annotated on the basis of genome information (Fig. 2). Homologous sequence alignment showed that the exo genes in strain F2 were similar (86~92%) to that of strain C58 (Table S2). Thus, more information regarding the biosynthesis of polysaccharide-based microbial flocculants in strain F2 can be obtained based on the succinoglycan biosynthesis pathway.
Production of polysaccharide-based microbial flocculants
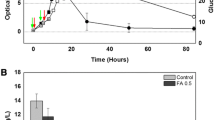
Bacterial growth and bioflocculant production of strain F2 cultured in LB and fermentation medium were analyzed, respectively. Strain F2 showed higher biomass in LB medium than when cultured in fermentation medium (Fig. 3a), but it could not produce bioflocculants in LB medium. In the fermentation medium, bioflocculant production paralleled cell growth in strain F2 during logarithmic and stationary phase (Fig. 3b). The polysaccharide content was approximately 303.93 mg/L in microbial flocculants produced by strain F2 cultured in the fermentation medium until 24 h. Furthermore, component analysis showed glucose (48.24%), mannose (27.60%), rhamnose (13.53%), and galactose (6.66%) to be the main sugar monomer components of polysaccharide-based microbial flocculants. Thus, transcriptome was used to analyze the gene expression difference of strain F2 when cultured in respective LB and fermentation medium at 24 h, on which time point the microbial flocculants were largely produced by strain F2 when cultured in the fermentation medium, whereas no microbial flocculants were produced in the LB medium.
Transcriptional analysis related to polysaccharide biosynthesis
RNA-seq was used to determine the changes in the expression of genes associated with polysaccharide-based microbial flocculant production under flocculating and non-flocculating conditions in strain F2. Gene expression analysis revealed significant changes in several genes involved in glycolysis and TCA cycle (Table S3), and the change profiles are shown in Fig. S1. Thus, perturbed energy metabolism was observed during polysaccharide-based microbial flocculant biosynthesis. Compared with that during no flocculation production, significant changes in the expression level of genes related to sugar metabolism were observed during microbial flocculant biosynthesis in strain F2 (Table S4). Along with the significant changes in the expression levels of exo genes in the exoF2 gene cluster, the expression levels of manA, glmM, and manC (related to mannose metabolism) and rfbA and rfbB (related to rhamnose metabolism) were also significantly up-regulated. Furthermore, the expression levels of some other genes, such as exoB, exoO, exoU, exoW, and exoT, also changed, but not as significantly as those of the above-mentioned genes. qRT-PCR confirmed the changes in the expression level of certain genes, including exoP, exoA, exoL, exoM, exoO, exoU, exoH, exoN, exoC, exoY, exoF, and manA (Fig. 4a). The trend in the changes in gene expression level at 24 h was in accordance with the RNA-seq results, thereby confirming the accuracy of the RNA-Seq analysis.
In addition, qRT-PCR showed that these genes in strain F2 cultured in the fermentation medium for 9 h were up-regulated compared with when cultured in the LB medium for 9 h (Fig. 4b). Results demonstrated that the production of microbial flocculants paralleled cell growth in strain F2 during the logarithmic and stationary phases, as well as the expression level of exo genes changed along with the production of microbial flocculants in strain F2.
Verification of the potential key genes
To verify whether exoC was involved in polysaccharide-based microbial flocculant production in strain F2, exoC was disrupted via a single-crossover event. Thus, the exoC-disrupted mutant F2-dexoC was constructed, in which exoC was not transcribed, as observed using RT-PCR analysis. The microbial flocculant-producing ability of the mutant F2-dexoC was determined and compared with that of the wild-type strain F2 as the control. Results showed that the wild-type strain F2 secreted microbial flocculants with polysaccharide content of 303.93 mg/L, whereas F2-dexoC did not produce microbial flocculants. Thus, the exoC mutation hindered microbial flocculant production by strain F2. In addition, the complementation strain F2-dexoC-C was constructed, which showed restoration of the wild-type phenotype and exhibited nearly identical microbial flocculant-producing capacity (Fig. S2a). Growth curves showed that the disruption and complementation of exoC in strain F2 did not hinder bacterial growth (Fig. S2b). Thus, the participation of exoC in the biosynthesis of polysaccharide-based microbial flocculants in strain F2 was confirmed.
Similarly, the mutant F2-dexoP was also obtained, in which exoP was not transcribed. No microbial flocculant was produced by the mutant F2-dexoP, although the complementation strain F2-dexoP-C restored microbial flocculant-producing capacity to the level of the wild-type strain F2 (Fig. S2c). Furthermore, the disruption and complementation of exoP in strain F2 did not hinder bacterial growth (Fig. S2d). Thus, the participation of exoP in the biosynthesis of polysaccharide-based microbial flocculants in strain F2 was confirmed.
Furthermore, the recombinant strain F2-dmanC was constructed, in which manC was not transcribed. Unlike the mutants F2-dexoC and F2-dexoP, the mutant F2-dmanC produced polysaccharide-based microbial flocculants at levels identical to that of the wild-type strain F2 (Fig. S2e). Further structure analysis showed the main sugar units of the microbial flocculants produced by the mutant F2-dmanC to be glucose (65.8%), mannose (11.3%), rhamnose (6.7%), and galactose (10.2%). Compared with glucose (48.24%), mannose (27.60%), rhamnose (13.53%), and galactose (6.66%) in microbial flocculants produced by wild strain F2, the proportion of mannose and rhamnose unit decreased, whereas the increased ratio of glucose and galactose unit was also observed. manC disruption and complementation in strain F2 did not hinder bacterial growth (Fig. S2f). Thus, manC was also confirmed to participate in the biosynthesis of polysaccharide-based microbial flocculant by strain F2.
Discussion
Difference of exopolysaccharide production in strain F2 when cultured in the LB medium and fermentation medium was also observed in other reports, namely, microorganisms may over-produce exopolysaccharides in glucose-rich medium but were unable to produce them in the LB medium (Ferreira et al. 2011; Zlosnik et al. 2008). A possible explanation is that high concentration of glucose and high carbon/nitrogen ratio in the fermentation medium may activate and regulate intracellular metabolic pathways in strain F2 to divert them into polysaccharide biosynthesis (Yu et al. 2015; Yu et al. 2017). RNA-Seq analysis revealed active expression of genes related to sugar metabolism during biosynthesis of microbial flocculants, such as the exo genes. Of them, either exoC or exoP disruption resulted in the no production of microbial flocculants in strain F2, so both exoC and exoP were confirmed to participate in the biosynthesis of microbial flocculants. Combined with the results of genome match and homologous sequence alignment between strains F2 and C58, results could indicate the similarity in the polysaccharide biosynthesis pathways using exo genes between strains F2 and C58. Thus, the functions of some of the exo genes were inferred based on their function in succinoglycan biosynthesis (Table 2).
The main sugar monomers of polysaccharide-based microbial flocculants are glucose, mannose, rhamnose, and galactose. Changes in the expression levels of certain genes related to mannose and rhamnose metabolism were also detected during the production of polysaccharide-based microbial flocculants, such as manA, glmM, manC, rfbA, and rfbB. Among them, manC was verified to be involved in the polysaccharide-based microbial flocculant biosynthesis. However, the effect of manC gene disruption on the biosynthesis of microbial flocculants in strain F2 is different from exoC and exoP gene disruption. Mutant with manC gene disruption showed unchanged production level of microbial flocculants but changed the proportion of sugar unit. The reason for the effects of manC gene disruption needs to be further clarified. But in any case, manC also participated in the biosynthesis of microbial flocculants. Except exo genes, the functions of other genes related to sugar metabolism were proposed according to genome annotation and KEGG database analysis (Table 2).
Furthermore, glycolysis/gluconeogenesis and tricarboxylic acid (TCA) cycle mainly provided the monomer and energy for polysaccharide biosynthesis as observed by the changes in the levels of gene expression. Glycolysis and TCA cycle are the main pathways related to energy metabolism, which can produce a large amount of energy-rich compounds (such as ATP, NADH, and FADH2) and precursors for useful compound biosynthesis, such as polysaccharides. Energy metabolism is also closely related to microbial activity and biosynthesis of biopolymer, such as polysaccharide. Significant changes of several genes involved in glycolysis and TCA cycle accelerated energy metabolism are observed along with polysaccharide biosynthesis, due to bacterial cells requiring more energy to ensure growth and metabolism (Guan et al. 2018). These observations may enable construction of the biosynthetic pathway of microbial flocculant.
On the basis of the reported succinoglycan biosynthesis pathway (Becker 2015; Skorupska et al. 2006; Wu et al. 2016) and the common pathway for biosynthesis of the nucleoside sugar of rhamnose and mannose (Freitas et al. 2011; Giraud and Naismith 2000; Schmid et al. 2015), the hypothetical pathway of polysaccharide biosynthesis in the polysaccharide-based microbial flocculant production in strain F2 was finally proposed (Fig. 5). Microbial flocculants often have the complex structure, while polysaccharides are the main components in the microbial flocculants produced by strain F2 in this study. The proposed pathway highlights the biosynthesis of the main components (i.e., polysaccharides) in the microbial flocculants produced by strain F2 that uses glucose as the substrate. The production process mainly included the formation of nucleoside sugars, assembly of sugar repeating units and polymerization, and export of the polysaccharides. UDP-Glc and UDP-Gal were formed by the enzymes encoded by exoC, exoN, and exoB, while dTDP-Rha formation mainly required rfbA and rfbB. GDP-Man was formed by the enzymes encoded by manA, glmM, and manC. After the first nucleoside sugar was added to the lipid linker, other sugar residues were added to the extending sugar chain by glycosyltransferases (GTs), such as exoA, exoL, and exoM. Finally, the sugar chains were polymerized and secreted out of the bacterial cell by polysaccharide polymerization and export protein, such as enzyme coded by exoP and exoT.
Hypothetical biosynthesis pathway of polysaccharide in polysaccharide-based microbial flocculant produced by A. tumefaciens F2. Glc-6-P, glucose-6-phosphate; Glc-1-P, glucose-1-phosphate; UDP-Glc, uridine-5′-diphosphate glucose; UDP-Gal, uridine-5′-diphosphate galactose; dTDP-Glc, deoxythymidine diphosphate glucose; dTDP-Rha, deoxythymidine diphosphate rhamnose; Fru-6-P, fructose-6-phosphate; Man-6-P, mannose-6-phosphate; Man-1-P, mannose-1- phosphate; GDP-Man, guanosine-5′-diphosphate mannose
In summary, the present study provided new information regarding microbial flocculant biosynthesis in strain F2. The glucose-based biosynthetic pathway of polysaccharides in microbial flocculants in strain F2 was proposed, including three steps, namely, formation of nucleotide sugars, assembly of repeating units and polymerization, and export of the polysaccharide. The findings were expected to provide guidance for improving the yield of microbial flocculants. In future, research is still required to further confirm the biosynthesis pathway (e.g., by using metabolic flux analysis) and yield improvement of microbial flocculants that can be realized by enhancing the expression level of key genes in the biosynthetic pathway.
Change history
04 September 2020
In the original publication of the article, it was published under the title ���Applied microbiology and biotechnology uncovering the biosynthetic pathway of polysaccharide-based microbial flocculant in Agrobacterium tumefaciens F2���.
References
Becker A (2015) Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front Microbiol 6:687. https://doi.org/10.3389/fmicb.2015.00687
Cangelosi GA, Hung L, Puvanesarajah V, Stacey G, Ozga DA, Leigh JA, Nester EW (1987) Common loci for Agrobacterium tumefaciens and Rhizobium meliloti exopolysaccharide synthesis and their roles in plant interactions. J Bacteriol 169(5):2086–2091. https://doi.org/10.1128/jb.169.5.2086-2091.1987
Chouly C, Colquhoun IJ, Jodelet A (1995) NMR studies of succinoglycan repeating-unit octasaccharides from Rhizobium meliloti and Agrobacterium radiobacter. Int J Biol Macromol 17(6):357–363. https://doi.org/10.1016/0141-8130(96)81846-0
Evans LR, Linker A, Impallomeni G (2000) Structure of succinoglycan from an infectious strain of Agrobacterium radiobacter. Int J Biol Macromol 27:319–326. https://doi.org/10.1016/s0141-8130(00)00131-8
Ferreira AS, Silva IN, Oliveira VH, Cunha R, Moreira LM (2011) Insights into the role of extracellular polysaccharides in Burkholderia adaptation to different environments. Front Cell Infect Microbiol 1:16. https://doi.org/10.3389/fcimb.2011.00016
Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29(8):388–398. https://doi.org/10.1016/j.tibtech.2011.03.008
Giraud MF, Naismith JH (2000) The rhamnose pathway. Curr Opin Struct Biol 10(6):687–696. https://doi.org/10.1016/S0959-440X(00)00145-7
Glucksmann MA, Reuber TL, Walker GC (1993) Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol 175(21):7045–7055. https://doi.org/10.1111/j.1365-2672.1993.tb02806.x
Guan NZ, Du B, Li JH, Shin HD, Chen RR, Du GC, Chen J, Liu L (2018) Comparative genomics and transcriptomics analysis-guided metabolic engineering of Propionibacterium acidipropionici for improved propionic acid production. Biotechnol Bioeng 115(2):483–494. https://doi.org/10.1002/bit.26478
Janczarek M (2011) Environmental signals and regulatory pathways that influence exopolysaccharide production in Rhizobia. Int J Mol Sci 12:7898–7933. https://doi.org/10.3390/ijms12117898
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RI, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. https://doi.org/10.1016/0378-1119(95)00584-1
Li A, Geng JN, Cui D, Shu C, Zhang S, Yang JX, Xing J, Wang JN, Ma F, Hu SN (2011) Genome sequence of Agrobacterium tumefaciens strain F2, a bioflocculant-producing bacterium. J Bacteriol 193(19):5531. https://doi.org/10.1128/JB.05690-11
Quandt J, Hynes MF (1993) Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15–21. https://doi.org/10.1016/0378-1119(93)90611-6
Rehm BH (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8(8):578–592. https://doi.org/10.1038/nrmicro2354
Reinhold BB, Chan SY, Reuber TL, Marra A, Walker GC, Reinhold VN (1994) Detailed structural characterization of succinoglycan, the major exopolysaccharide of Rhizobium meliloti Rm1021. J Bacteriol 176(7):1997–2002. https://doi.org/10.1128/jb.176.7.1997-2002.1994
Reuber TL, Walker GC (1993) Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269–280. https://doi.org/10.1016/0092-8674(93)90418-P
Ruffing A, Chen RR (2006) Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microb Cell Factories 5:25. https://doi.org/10.1186/1475-2859-5-25
Salehizadeh H, Yan N, Farnood R (2018) Recent advances in polysaccharide bio-based flocculants. Biotechnol Adv 36:92–119. https://doi.org/10.1016/j.biotechadv.2017.10.002
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00496
Skorupska A, Janczarek M, Marczak M, Mazur A, Król J (2006) Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb Cell Factories 5:7. https://doi.org/10.1186/1475-2859-5-7
Stredansky M, Conti E (1999) Succinoglycan production by solid-state fermentation with Agrobacterium tumefaciens. Appl Microbiol Biotechnol 52:332–337. https://doi.org/10.1007/s002530051528
Stredansky M, Conti E, Bertocchi C, Matulova M, Zanetti F (1998) Succinoglycan production by Agrobacterium tumefaciens. J Ferment Bioeng 85(4):398–403. https://doi.org/10.1016/s0922-338x(98)80083-4
Wang LX, Wang Y, Pellock B, Walker GC (1999) Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J Bacteriol 181(21):6788–6796. https://doi.org/10.1038/228178a0
Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, Zhou Y, Chen L, Wood GE, Almeida NF Jr, Woo L, Chen Y, Paulsen IT, Eisen JA, Karp PD, Bovee D Sr, Chapman P, Clendenning J, Deatherage G, Gillet W, Grant C, Kutyavin T, Levy R, Li MJ, McClelland E, Palmieri A, Raymond C, Rouse G, Saenphimmachak C, Wu Z, Romero P, Gordon D, Zhang S, Yoo H, Tao Y, Biddle P, Jung M, Krespan W, Perry M, Gordon-Kamm B, Liao L, Kim S, Hendrick C, Zhao ZY, Dolan M, Chumley F, Tingey SV, Tomb JF, Gordon MP, Olson MV, Nester EW (2001) The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294(5550):2317–2323. https://doi.org/10.1126/science.1066804
Wu D, Li A, Yang JX, Ma F, Chen H, Pi SS, Wei W (2015) N-3-Oxo-octanoyl-homoserine lactone as a promotor to improve the microbial flocculant production by an exopolysaccharide bioflocculant-producing bacterium Agrobacterium tumefaciens F2. RSC Adv 5:89531–89538. https://doi.org/10.1039/c5ra15657b
Wu D, Li A, Ma F, Yang JX, Xie YT (2016) Genetic control and regulatory mechanisms of succinoglycan and curdlan biosynthesis in genus Agrobacterium. Appl Microbiol Biotechnol 100:6183–6192. https://doi.org/10.1007/s00253-016-7650-1
Yang R, Li HJ, Huang M, Yang H, Li AM (2016) A review on chitosan-based flocculants and their applications in water treatment. Water Res 95:59–89. https://doi.org/10.1016/j.watres.2016.02.068
Yu WC, Chen Z, Shen L, Wang YP, Li QB, Yan S, Zhong CJ, He N (2015) Proteomic profiling of Bacillus licheniformis reveals a stress response mechanism in the synthesis of extracellular polymeric flocculants. Biotechnol Bioeng 113(4):797–806. https://doi.org/10.1002/bit.25838
Yu WC, Chen Z, Ye H, Liu PZ, Li ZP, Wang YP, Li QB, Yan S, Zhong CJ, He N (2017) Effect of glucose on poly-γ-glutamic acid metabolism in Bacillus licheniformis. Microb Cell Factories 16:22. https://doi.org/10.1186/s12934-017-0642-8
Zevenhuizen LPTM (1997) Succinoglycan and galactoglucan. Carbohydr Polym 33(2–3):139–144. https://doi.org/10.1016/s0144-8617(97)00054-4
Zlosnik JEA, Hird TJ, Fraenkel MC, Moreira LM, Henry DA, Speert DP (2008) Differential mucoid exopolysaccharide production by members of the Burkholderia cepacia complex. J Clin Microbiol 46(4):1470–1473. https://doi.org/10.1128/JCM.02273-07
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 51578179).
Author contribution statement
A. Li and F. Ma conceived and designed the research. S.S. Pi and L. Feng conducted the experiments. J.G. Qiu and H.P. Zhao contributed new reagents or analytical tools. S.S. Pi and D. Wu analyzed the data. S.S. Pi wrote the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 698 kb)
Rights and permissions
About this article
Cite this article
Pi, S., Qiu, J., Li, A. et al. Applied microbiology and biotechnology uncovering the biosynthetic pathway of polysaccharide-based microbial flocculant in Agrobacterium tumefaciens F2. Appl Microbiol Biotechnol 104, 8479–8488 (2020). https://doi.org/10.1007/s00253-020-10850-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10850-2