Abstract
Gut microbiota dysbiosis, associated with insulin resistance, weak intestinal barrier integrity, and inflammation, may also play a role in the development of dietary-induced nonalcoholic fatty liver disease (NAFLD). This study investigates the effects of dietary Lactobacillus plantarum NA136 administration on gut microbiota composition in an insulin-resistant C57BL/6J mouse NAFLD model. Comparison of mice with and without L. plantarum NA136 treatment revealed that L. plantarum NA136 treatment not only relieved insulin resistance but also significantly increased relative proportions of Desulfovibrio, Alistipes, Prevotella, and Enterorhabdus in gut microbiota of NAFLD mice. Meanwhile, L. plantarum NA136 administration also inhibited pathogenic bacterial growth, while promoting growth of probiotics such as Allobaculum, Lactobacillus, and, most markedly, Bifidobacterium. Moreover, L. plantarum NA136 treatment of NAFLD mice improved intestinal barrier integrity and attenuated high-fat and fructose diet (HFD/F)-induced inflammation. These results implicate gut-liver-axis-dependent microbiota modulation as the underlying mechanism for L. plantarum NA136-induced amelioration of NAFLD.
Key points
• L. plantarum NA136 corrects gut microbiota disorders caused by a high-fat and fructose diet.
• L. plantarum NA136 strengthens the intestinal barrier and reduces inflammation in the liver.
• L. plantarum NA136 relieves NAFLD by improving the gut-liver axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the developed world, nonalcoholic fatty liver disease (NAFLD) has emerged as the most prevalent form of adult chronic liver disease, paralleling the epidemic rise in obesity there (Byrne and Targher 2015). Nevertheless, NAFLD cases are not restricted to developed countries, as evidenced by an increasingly global distribution of this disorder. Indeed, the rising incidence of liver disease in the world, especially in China, has been recently cited as a major contributor to worldwide disease burden (Fan and Farrell 2009; Wang et al. 2014).
In spite of the growing importance of NAFLD as a global disease, its pathogenesis has not yet been fully elucidated. In recent years, evidence for the “two hits hypothesis” of NAFLD development has accumulated and supported its widespread acceptance. With regard to this hypothesis, insulin resistance (IR) develops as a first hit that leads to excessive triglyceride accumulation in hepatic cells. Concomitantly, a second hit involving oxidative stress and increased generation of inflammatory cytokines leads to liver injury, inflammation, and fibrosis (Byrne 2010). Meanwhile, increasing evidence supports a role for gut microbiota dysbiosis in NAFLD development as yet another endogenous contributing factor to development of this disease (Abu-Shanab and Quigley 2010; Gkolfakis et al. 2015).
The gut-liver axis, a bidirectional interactive relationship between the gut and the liver, is currently under intense scrutiny as a key factor in NAFLD development (Caussy and Loomba 2018; Marra and Svegliati-Baroni 2018). This concept is supported by reports linking NAFLD to a disorder of the gut microbiota that causes the production of a gut microbiota-derived endotoxin, leading to impaired liver function (Safari and Gérard 2019). As another possible contributing factor, long-term consumption of a diet high in sugar and fat coupled with other factors, such as lack of physical exercise and lack of restful sleep, can cause gut Gram-negative bacterial overgrowth that is associated with increased lipopolysaccharide (LPS) production. Notably, it is known that LPS impairs intestinal barrier integrity, activates inflammation, and promotes insulin resistance (Compare et al. 2012). Meanwhile, studies have demonstrated that considerably fewer organisms belonging to Bacteroidetes and more members of Firmicutes exist in the gut microbiomes of individuals with NAFLD than in gut microbiomes of healthy individuals (Ley et al. 2006). Furthermore, numerous animal studies have demonstrated that probiotics or prebiotics can alleviate NAFLD by correcting gut microbiota dysbiosis (Cesaro et al. 2011), with recent evidence suggesting that probiotic administration of Lactobacilli and Bifidobacteria could inhibit proliferation of pathogenic bacteria and strengthen the intestinal barrier (Plaza-Diaz et al. 2014). Indeed, results of a similar study have even demonstrated that probiotic supplementation could ameliorate the liver inflammatory response and, thus, improve NAFLD patient health (Xue et al. 2017). Therefore, emerging evidence supports that the therapeutic targeting of gut microbiota composition restores the gut-liver axis function, which is a strategy to prevent and ameliorate NAFLD.
Lactobacillus plantarum NA136 is a newly acquired probiotic strain that is isolated from traditional Chinese pickles produced in Yanbian Korean Autonomous Prefecture, China. This strain has been reported to potentially alleviate NAFLD by regulating fatty acid metabolism and combating oxidative stress effects (Zhao et al. 2019). However, L. plantarum NA136 effects on the gut microbiome are still unknown. Here, we hypothesize that the main target acted upon by L. plantarum NA136 to achieve NAFLD symptom improvement lies within the gut-liver axis. Therefore, the goal of this study is to identify underlying mechanisms whereby L. plantarum NA136 administration alleviates insulin resistance, reduces liver inflammation, strengthens intestinal barrier integrity, and corrects gut microbiome dysbiosis.
Materials and methods
L. plantarum NA136 isolation and culture methods
L. plantarum NA136, isolated from Chinese traditional-style pickles and deposited in the China Center for Type Culture Collection (accession M2018112; CCTCC, Wuhan, China), was cultured at 37 °C for 18 h in de Man, Rogosa, and Sharpe (MRS) broth. Bacteria were harvested from MRS broth cultures by centrifugation (6000g for 10 min at 4 °C); then, pellets were washed twice with phosphate-buffered saline (PBS). Live bacteria intended for use as an oral probiotic supplement were suspended in sterile physiological saline. Bacterial suspensions were each adjusted to deliver an orally administered dose of 1.0 × 109 colony-forming units (CFU)/day/mouse.
Animals and their assignments to control and experimental groups
Mice (C57BL/6J male 8-week-old animals) were obtained from the Yisi Laboratory Animal Technology Company (Yisi, Changchun, China). After 1-week acclimatization with the administration of a normal diet and water, the mice were divided at random into three groups (10 mice per group): control (ND) group mice were fed a normal diet (with fat providing 10% of total energy); high-fat and fructose diet (HFD/F) group mice were fed a high-fat and fructose diet (with fat providing 65% of total energy and 30% dietary volume provided as fructose solution); and L. plantarum NA136 group mice (HFD/F + NA136) were fed the same diet as the HFD/F group, but concomitantly received a daily oral dose of L. plantarum NA136 (1.0 × 109 CFU/day/mouse). All animal experiments were conducted according to the Animal Care Committee of the Jilin Academy of Agricultural Sciences guidelines. The mice were sacrificed after 16 weeks administration of the dietary regimens, at which time blood and fecal samples were collected and intestinal and liver tissues were harvested and stored until needed for analyses.
Oral glucose tolerance tests
Oral glucose tolerance tests (OGTTs) were performed on the last day of breeding. All of the mice were fasted for 15 h, and fasting blood glucose was tested prior to glucose administration (time = 0). Then, the mice were administrated with glucose at a dose of 2 g/kg body weight (bw), and blood glucose was measured at 30, 60, 90, and 120 min.
Biochemical assays
Plasma glucose (10 μL of each blood sample) was measured using an Accu-Check Go glucose analyzer (Roche Diagnostics GmbH, Penzberg, Germany). Serum was extracted from blood samples; then, interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), insulin (INS), and LPS levels were measured via enzyme-linked immunosorbent assay (ELISA) kits (Jianglai, Shanghai, China). Insulin resistance was calculated using a homeostasis model-based insulin resistance index (HOMA-IR) formula, as follows:
Histological analysis
Small liver tissue pieces were fixed in a solution of 10% neutral formaldehyde. After 48 h, liver tissues were frozen and sliced to 5-μm-thick cryosections using a microtome. Cryosections were stained with Oil Red O and visualized using an optical microscope (Olympus, Tokyo, Japan).
Western blot analysis
Liver and intestinal tissue slices were lysed then homogenized in cold RIPA cell lysis buffer (Solarbio, Beijing, China). Insoluble tissue materials were removed by centrifugation at 12,000g (10 min at 4 °C). Nuclear extracts from the liver were prepared by using a Nuclear Protein Extraction Kit (Solarbio, Beijing, China). A bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Waltham, USA) was used to determine tissue lysate total protein concentration values. Next, samples containing 40 μg of protein were subjected to 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis followed by transfer of separated proteins to polyvinylidene fluoride (PVDF) membranes to create protein blots. After the blots were soaked in 5% bovine serum albumin (BSA) to block nonspecific antibody binding to membrane surfaces, they were incubated with antibodies: anti-nuclear factor kappa-B (NF-κB) P65, anti-P38, anti-pP38 (Bioss, Beijing, China), anti-inhibitor of nuclear factor kappa-B kinase (anti-IκB), anti-hypoxia-inducible factor 1α (anti-HIF-1α), anti-zonula occludens-1 (anti-ZO-1), anti-occludin, anti-claudin-1 (Abcam, Cambridge, USA), β-actin (Sangon Biotech, Shanghai, China), and lamin B (Solarbio, Beijing, China). After three washes with tris-buffered saline tween (TBST), the membranes were incubated with horseradish peroxidase-conjugated antibody and detection of antibody-bound proteins was performed via enhanced chemiluminescence. Relative protein expression levels were normalized to β-actin or lamin B.
Extraction of fecal genomic DNA for high-throughput sequencing and sequence analysis
Fecal sample DNA was extracted and purified using an E.Z.N.A™ Mag-Bind Soil DNA Kit (OMEGA, Norcross, USA) as per kit instructions. Polymerase chain reaction (PCR) amplification of bacterial 16S ribosomal RNA gene (V3–V4 region) DNA was performed using 341F primer 5′-CCTACGGGNGGCWGCAG-3′ and 805R primer 5′-GACTACHVGGGTATCTAATCC-3′. Successful amplification of 16S ribosomal DNA amplicons was assessed using the Illumina MiSeq platform. The gene sequencing data have been deposited in the National Center for Biotechnology Information (NCBI) and can be accessed in the Short Read Archive (SRA) under the accession number PRJNA604078. Microbiota composition analysis was performed using Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) (Caporaso et al. 2010). Operational taxonomic unit (OTU) assignments of high-quality sequences (sharing ≥ 97% similarity) to clusters were performed using UCLUST (Edgar 2010), with OTUs rarefaction metrics Shannon index and Chao index employed to assess alpha diversity. Concurrently, we conducted non-metric multidimensional scaling (NMDS) analysis using QIIIME to compare cluster groupings containing different samples.
Statistical analysis
Data presented in graphs and tables were expressed as means ± S.D. All data calculations were performed using SPSS software version 15.0 (Norusis 2007), with statistical analysis conducted using one-way analysis of variance (ANOVA). P values < 0.05 were deemed statistically significant. The area under the curve (AUC) of OGTTs was determined using the trapezoidal rule.
Results
L. plantarum NA136 effects on liver histopathology
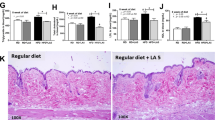
Long-term consumption of a high-fat and high-fructose diet can induce liver injury and increase hepatic lipid accumulation. In our previous study, the increasing activities of ALT and AST in the HFD/F group, compared with those of ALT and AST in the ND group, became normalized after treatment with L. plantarum NA136. We measured hepatic lipid droplet accumulation within hepatic tissues via Oil Red O staining (Fig. 1). Compared to control mice (the ND group) (Fig. 1a, b), mice in the HFD/F group exhibited significant liver lipid accumulation (Fig. 1c, d). By contrast, after 16 weeks of L. plantarum NA136 administration, the numbers and sizes of hepatic lipid droplets were reduced in the HFD/F + NA136 group mice (Fig. 1e, f), as determined using Oil Red O staining in combination with our previous hematoxylin-eosin (H&E) staining results. Collectively, these results demonstrate that L. plantarum NA136 administration effectively attenuates liver lipid accumulation.
Improved HFD/F-induced insulin resistance after L. plantarum NA136 treatment
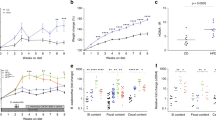
Because NAFLD is accompanied by IR, we measured OGTTs and serum insulin levels and then calculated HOMA-IR, with results shown in Fig. 2. After administration with glucose, the blood glucose values of the three groups reached the peak value at 30 min, and fell back rapidly at 30–60 min. After 90 min, the blood glucose values of each group tended to be stable (Fig. 2a). From Fig. 2b, it can be seen that the HFD/F + NA136 group had a significant lowering of the AUC value compared to the HFD/F group (P < 0.01). This suggested that L. plantarum NA136 could ameliorate impaired glucose tolerance.
Effect of L. plantarum NA136 on oral glucose tolerance tests (OGTTs) insulin resistance (IR). a OGTTs; b area under the glucose response curve (AUC); c serum insulin level; d HOMA-IR index. Data represent the mean ± SD of each group. ##P < 0.01 versus ND group; *P < 0.05 and **P < 0.01 versus HFD/F group
In Fig. 2c, a high-fat and high-fructose diet led to increased insulin levels relative to levels in the ND group mice, while relatively lower insulin levels were more evident in L. plantarum NA136-treated mice than in untreated HFD/F mice. In addition, the HOMA-IR index was apparently decreased in the HFD/F + NA136 group relative to its level in HFD/F group mice (Fig. 2d). These results collectively suggest that L. plantarum NA136 administration can effectively reverse insulin resistance.
L. plantarum NA136 effects on gut microbiota composition
Investigation of L. plantarum NA136 effects on gut microbiota composition that is obtained by Illumina MiSeq sequencing generated a total number of 1,035,886 clean sequence reads. Based on 97% sequence similarity, validated reads were selected and then grouped into OTUs. As shown in Table 1, the OTU number of the HFD/F group was the lowest in the three groups, and L. plantarum NA136 intervention had increased the similarity of OTUs of HFD/F-fed mice. After Chao index and Shannon metric calculations were conducted to determine gut microbiota species richness and diversity, the Chao index results indicated that gut microbiota richness of HFD/F mice was reduced relative to that of the ND group, while L. plantarum NA136 treatment significantly increased NAFLD mice gut microbiota richness (P < 0.05). Meanwhile, HFD/F + NA136 group Shannon index values were increased relative to those of the HFD/F group, suggesting that L. plantarum NA136 increased gut microbiota species diversity in NAFLD mice. However, there is no significant difference in Shannon index values of the ND group and the HFD/F group, which suggests that HFD/F could not change the diversity of gut microbiota, and such result was also observed by Lu’s study (Lu et al. 2016). Notably, assessments of structural alterations of gut microbiota using non-metric multidimensional scale analysis (NMDS) (Fig. 3a) generated a distinct gut microbiota composition clustering pattern for each group, with the most similar clustering patterns observed for the HFD/F and HFD/F + NA136 groups.
At the genus level, the number of Allobaculum, Lactobacillus, Bifidobacterium, Helicobacter, and Parasutterella was reduced significantly in the HFD/F group versus their corresponding levels in the ND group (Fig. 3b and Supplemental Fig. S1 and Supplemental Table S1). Meanwhile, the HFD/F diet may have boosted relative proportions of Desulfovibrio, Alistipes, Acetatifactor, Oscillibacter, Prevotella, and Enterorhabdus. Notably, Fig. 3b and Supplemental Table S1 show that L. plantarum NA136 treatment effectively regulated gut microbiota dysbiosis induced by long-term consumption of a high-fat and high-fructose diet. Interestingly, relative proportions of Barnesiella, Parabacteroides, and Bifidobacterium, key genus cited for anti-obesity effects (Fang et al. 2019; Leung et al. 2016; Wu et al. 2018), increased markedly after L. plantarum NA136 administration.
L. plantarum NA136 attenuated expression of HFD/F-induced tight junction proteins
It has been shown that high-fat and high-fructose dietary induction of Gram-negative intestinal bacterial growth can subsequently undermine intestinal barrier integrity (Hung et al. 2016). Thus, we examined intestinal HIF-1α, claudin-1, occludin, and ZO-1 expression levels (Fig. 4a) and observed that HIF-1α expression was obviously decreased in L. plantarum NA136-treated NAFLD mice. Because tight junction (TJ) proteins are thought to play key regulatory roles in intestinal permeability, it is noteworthy that consumption of a diet high in fat and fructose led to reduced ZO-1, claudin-1, and occludin expression levels (Fig. 4b, c) that were restored to normal levels after L. plantarum NA136 treatment.
Effects of L. plantarum NA136 treatment on intestinal barrier. a Protein expression of HIF-1α, claudin-1, occludin, ZO-1, and β-actin; b relative protein levels of HIF-1α and claudin-1; c relative protein levels of occludin and ZO-1. β-Actin was used as a loading control. Data represent the mean ± SD of each group. ##P < 0.01 versus ND group; **P < 0.01 versus HFD/F group
Effect of L. plantarum NA136 treatment on HFD/F diet-induced inflammatory responses
The concept that LPS, a component of Gram-negative bacterial outer membranes, may weaken the intestinal barrier prior to traversing it and inducing inflammation was supported here. Specifically, with increased serum LPS levels observed in HFD/F group mice, levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 were markedly higher in HFD/F group sera than in the sera of ND-fed mice (Table 2). Notably, oral administration of L. plantarum NA136 inhibited increases in serum LPS, TNF-α, IL-6, and IL-1β levels.
Activation of NF-κB, the key transcriptional regulator of immune and proinflammatory responses, is known to be influenced by a wide variety of factors, such as LPS, IL-1, TNF-α, and bacteria (Jobin and Sartor 2000). Here, western blotting revealed that nuclear NF-κB P65 expression increased in the HFD/F group mice relative to levels in ND group mice, while L. plantarum NA136 treatment of HFD/F mice led to markedly decreased NF-κB P65 protein expression with increased IκB protein expression (Fig. 5a, b). Furthermore, elevated P38 phosphorylation was observed in HFD/F group samples, while decreased P38 phosphorylation was observed in HFD/F + NA136 group samples (Fig. 5c).
L. plantarum NA136 attenuated the NF-κB P65-mediated inflammatory response in the liver. a Protein expression of IκB, cytoplasm NF-κB P65, nuclear NF-κB P65, P38, pP38, β-actin, and lamin B; b relative protein level of nuclear NF-κB P65; c quantification of P38 phosphorylation. β-Actin and lamin B were used as a loading control. Data represent the mean ± SD of each group. ##P < 0.01 versus ND group; **P < 0.01 versus HFD/F group
Discussion
Long-term high-fat and high-fructose consumption causes insulin resistance, inflammation, gut microbiota dysbiosis, and even NAFLD (Liu et al. 2017). Due to accumulating evidence for a pivotal role of gut microbiota in NAFLD development (Leung et al. 2016), the gut microbiome is a prime candidate for therapeutic targeting to ameliorate NAFLD (Caussy et al. 2019). Meanwhile, our previous study showed that administration of L. plantarum NA136, a strain isolated from Chinese traditional-style pickles produced in Yanbian Korean Autonomous Prefecture, could effectively decrease body weight, counteract hepatic lipid metabolic disorders, and activate antioxidant responses in NAFLD mice (Zhao et al. 2019), prompting this investigation of L. plantarum NA136 effects on insulin resistance, gut microbiota composition, gut barrier integrity, and inflammation in NAFLD mice.
In recent years, gut microbiota has been shown not only to influence energy acquisition and fat storage but also to play key roles in the development of insulin resistance and in the growing incidence of other related metabolic diseases (Vanni and Bugianesi 2010). Meanwhile, accumulating scientific evidence attributes NAFLD and associated gut microbiota dysbiosis to observed intestinal Gram-negative bacterial overgrowth that boosts both endotoxin (LPS) levels and production of toxic gases such as hydrogen sulfide (H2S) (Mani et al. 2014; Vanni and Bugianesi 2010). In this study, a high-fat and high-fructose diet decreased both the richness and the diversity of the gut microbiome community, as observed in previous studies that also linked low microbiota richness to inflammation, type 2 diabetes, inflammatory bowel disorder (IBD), and obesity (Chatelier et al. 2013). Here, L. plantarum NA136 treatment led to increased gut microbiome community diversity and noticeable enhancement of gut microbiota richness; conversely, untreated HFD/F mice exhibited relatively increased proportions of Alistipes, Prevotella, and Enterorhabdus, each of which produces LPS and which therefore may induce insulin resistance, obesity, and proinflammatory cytokine production (Chen et al. 2018; Li et al. 2018; Vanni and Bugianesi 2010). Furthermore, HFD/F mice also possessed a greater relative abundance of Desulfovibrio than ND group mice did. This result is noteworthy, since Desulfovibrio has been shown to interact with intestinal epithelial cells to form a surface biofilm and also produce H2S that triggers epithelial cell apoptosis and intestinal barrier weakening (Figliuolo et al. 2018). By contrast, L. plantarum NA136-treated HFD/F mice harbored greater proportions of beneficial intestinal bacteria, such as Allobaculum, Lactobacillus, Bifidobacterium, Helicobacter, Barnesiella, Parabacteroides, and Parasutterella, that produce short-chain fatty acids (SCFA) known to strengthen the intestinal barrier, reduce intestinal permeability and LPS levels, and competitively inhibit pathogenic bacterial growth (Eslamparast et al. 2013).
NAFLD is a disorder linked to an increased abundance of harmful bacteria that produce proinflammatory substances, such as endotoxin, ammonia, phenols, and H2S; such substances subsequently promote mucolysis and mucus layer degradation, epithelial cell apoptosis, and inflammatory activation (Figliuolo et al. 2018). Meanwhile, NAFDL-associated gut microbiota dysbiosis induced by long-term consumption of a high-fat and high-fructose diet may disrupt intestinal barrier function by reducing expression of intestinal tight junction proteins (occludin, claudin-1, and ZO-1) (Gangarapu et al. 2014). Here, we observed a reduction in expression levels of ZO-1, claudin-1, and occludin in the HFD/F group, which, when considered together with the results of our gut microbiota analysis, suggests that HFD/F consumption could cause gut microbiota dysbiosis that ultimately weakens the intestinal barrier. By contrast, after L. plantarum NA136 treatment, expression levels of tight junction proteins were normalized, in agreement with previous studies (Lim et al. 2016; Mujagic et al. 2017). This effect possibly stemmed from L. plantarum NA136 promotion of SCFA-producing bacterial growth. SCFA are known to exert multiple beneficial effects on the host, including promotion of lactic acid bacterial growth, reduction of insulin resistance and inflammation, improvement of intestinal barrier function, and stimulation of lipid oxidation (Hamer et al. 2008; Hosseini et al. 2011; van der Beek et al. 2016). Moreover, the function of HIF-1, a transcription factor composed of HIF-1α and HIF-1β subunits that may play a crucial role in hypoxia, intestinal injury, and intestinal mucosal integrity (Yang et al. 2014), was also evaluated in this work, since HIF-1 inhibition by L. plantarum C88 supplementation, as shown in our previous studies, prevented epithelial barrier dysfunction (Duan et al. 2018; Zhao et al. 2017). Here, gut bacterial translocation was inhibited by L. plantarum NA136 treatment, confirming that L. plantarum NA136 can also prevent intestinal barrier dysfunction.
Although the intestinal barrier normally prevents passage of harmful factors into blood, once this barrier is disrupted, Gram-negative bacteria-derived LPS has been shown to enter the blood and induce inflammation (Figliuolo et al. 2018). Here, increased blood inflammatory cytokine levels of TNF-α, IL-6, and IL-1β in HFD/F group mice were observed, reflecting increased serum LPS levels. However, administration of L. plantarum NA136 reversed this trend, suggesting that treatment reduced HFD/F diet-induced inflammatory cytokine expression, as observed for other probiotics (Kim et al. 2018; Xue et al. 2017). Meanwhile, proinflammatory cytokines, inflammatory cytokines, and HIF-1 are known to activate NF-κB expression and trigger inflammation (Yang et al. 2014). Importantly, in a noninflammatory state, NF-κB is retained within the cytoplasm in a form that is bound to inhibitory protein IκB; upon external cell stimulation, NF-κB is released from IκB, and the free NF-κB translocates from the cytoplasm to the nucleus, where it activates downstream inflammatory pathway target genes (Chen et al. 2015). Our results indicated that HFD/F could result in a decrease in IκB, and an increase in nuclear NF-κB translocation with elevated levels of phosphorylated P38, which subsided after L. plantarum NA136 treatment. When taken together, all of these findings demonstrate that L. plantarum NA136 administration improved intestinal barrier function and attenuated HFD/F-induced inflammation.
The gut-liver axis encompasses bidirectional interactions occurring between the gut and liver (Vajro et al. 2013), with studies supporting a key role for gut-liver axis functional imbalances in NAFLD development (Caussy and Loomba 2018; Marra and Svegliati-Baroni 2018). Such imbalances can be triggered through long-term consumption of a high-fat diet, whereby endotoxin and inflammatory factors produced in the gut then enter the liver through the gut-liver axis, triggering liver inflammation, oxidative stress, insulin resistance, and dysfunctional lipid metabolism (Frasinariu et al. 2012; Vajro et al. 2013). Here, we found that L. plantarum NA136 induced growth of probiotic organisms and inhibited growth of harmful bacteria to subsequently improve intestinal barrier integrity and reduce inflammatory responses. Evidence obtained here collectively suggests that L. plantarum NA136 can effectively improve gut microbiome composition.
This study demonstrated that L. plantarum NA136 treatment of mice with HFD/F-induced NAFLD led to significant increase of gut microbiota species richness and diversity, while correcting gut microbiota dysbiosis. Furthermore, L. plantarum NA136 treatment improved intestinal barrier strength, reduced liver inflammation, and decreased insulin resistance via gut-liver axis-dependent mechanisms. These results suggest that L. plantarum NA136 has therapeutic potential for ameliorating HFD/F-induced NAFLD.
References
Abu-Shanab A, Quigley EMM (2010) The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 7(12):691–701. https://doi.org/10.1038/nrgastro.2010.172
Byrne CD (2010) Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins, leukotrienes and essential fatty acids (PLEFA) 82(4–6):265–271. https://doi.org/10.1016/j.plefa.2010.02.012
Byrne CD, Targher G (2015) NAFLD: a multisystem disease. J Hepatol 62(1):S47–S64. https://doi.org/10.1016/j.jhep.2014.12.012
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Caussy C, Loomba R (2018) Gut microbiome, microbial metabolites and the development of NAFLD. Nat Rev Gastroenterol Hepatol 15:719–720. https://doi.org/10.1038/s41575-018-0058-x
Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, Downes MR, Evans RM, Brenner DA, Sirlin CB, Knight R, Loomba R (2019) A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun 10(1):1406. https://doi.org/10.1038/s41467-019-09455-9
Cesaro C, Tiso A, Prete AD, Cariello R, Tuccillo C, Cotticelli G, Blanco CV, Loguercio C (2011) Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 43(6):431–438. https://doi.org/10.1016/j.dld.2010.10.015
Chatelier EL, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Consortium M, Bork P, Wang J, Ehrlich SD, Pedersen O (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500(7464):541–546. https://doi.org/10.1038/nature12506
Chen YH, Yu Z, Fu L, Wang H, Wang H, Chen X, Zhang C, Lv ZM, Xu DX (2015) Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci Rep 5:10871. https://doi.org/10.1038/srep10871
Chen Y, Jin L, Li Y, Xia G, Chen C, Zhang Y (2018) Bamboo-shaving polysaccharide protects against high-diet induced obesity and modulates the gut microbiota of mice. J Funct Foods 49:20–31. https://doi.org/10.1016/j.jff.2018.08.015
Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G (2012) Gut–liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 22(6):471–476. https://doi.org/10.1016/j.numecd.2012.02.007
Duan C, Zhao Y, Huang C, Zhao Z, Gao L, Niu C, Wang C, Liu X, Zhang C, Li S (2018) Hepatoprotective effects of Lactobacillus plantarum C88 on LPS/D-GalN–induced acute liver injury in mice. J Funct Foods 43:146–153. https://doi.org/10.1016/j.jff.2018.02.005
Eslamparast T, Eghtesad S, Hekmatdoost A, Poustchi H (2013) Probiotics and nonalcoholic fatty liver disease. Middle East J Dig Dis 5(3):129–136. https://doi.org/10.15171/middle%20east%20j%20di.v5i3.1199
Fan JG, Farrell GC (2009) Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol 50(1):204–210. https://doi.org/10.1016/j.jhep.2008.10.010
Fang S, Wu D, Yang Z, Jiao N, Lin Y, Li X, Xiao J, Yuan M, Zhu R, Zhu L (2019) Keystone species in the pathogenic process of NAFLD. FASEB J 33(1_supplement):496.40. https://doi.org/10.1096/fasebj.2019.33.1_supplement.496.40
Figliuolo VR, Coutinho-Silva R, Coutinho CMLM (2018) Contribution of sulfate-reducing bacteria to homeostasis disruption during intestinal inflammation. Life Sci 215(15):145–151. https://doi.org/10.1016/j.lfs.2018.11.009
Frasinariu OE, Ceccarelli S, Alisi A, Moraru E, Nobili V (2012) Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis 45(7):543–551. https://doi.org/10.1016/j.dld.2012.11.010
Gangarapu V, Yıldız K, Ince AT, Baysal B (2014) Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol 25(2):133–140. https://doi.org/10.5152/tjg.2014.7886
Gkolfakis P, Dimitriadis G, Triantafyllou K (2015) Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 14(6):572–581. https://doi.org/10.1016/S1499-3872(15)60026-1
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Brummer RJ (2008) Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther 27(2):104–119. https://doi.org/10.1111/j.1365-2036.2007.03562.x
Hosseini E, Grootaert C, Verstraete W, Wiele TVd (2011) Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 69(5):245–258. https://doi.org/10.1111/j.1753-4887.2011.00388.x
Hung SC, Tseng WT, Pan TM (2016) Lactobacillus paracasei subsp. paracasei NTU 101 ameliorates impaired glucose tolerance induced by a high-fat, high-fructose diet in Sprague-Dawley rats. J Funct Foods 24:472–481. https://doi.org/10.1016/j.jff.2016.04.033
Jobin C, Sartor RB (2000) The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol 278(3):C451–C462. https://doi.org/10.1152/ajpcell.2000.278.3.C451
Kim WG, Kim HI, Kwon EK, Han MJ, Kim DH (2018) Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct 9:4255–4265. https://doi.org/10.1039/C8FO00252E
Leung C, Rivera L, Furness JB, Angus PW (2016) The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 13(7):412–425. https://doi.org/10.1038/nrgastro.2016.85
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023. https://doi.org/10.1038/nature4441022a
Li S, Li J, Mao G, Wu T, Hu Y, Ye X, Tian D, Linhardt RJ, Chen S (2018) A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct 9(10):5371–5380. https://doi.org/10.1039/C8FO01174E
Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH (2016) Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res 36(4):337–348. https://doi.org/10.1016/j.nutres.2015.12.001
Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X, Xia H, Liu Z, Cui B, Liang P, Xi L, Jin J, Ying X, Wang X, Zhao X, Li W, Jia H, Lan Z, Li F, Wang R, Sun Y, Yang M, Shen Y, Jie Z, Li J, Chen X, Zhong H, Xie H, Zhang Y, Gu W, Deng X, Shen B, Xu X, Yang H, Xu G, Bi Y, Lai S, Wang J, Qi L, Madsen L, Wang J, Ning G, Kristiansen K, Wang W (2017) Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 23(7):859–868. https://doi.org/10.1038/nm.4358
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K (2016) Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep 6:37589. https://doi.org/10.1038/srep37589
Mani S, Cao W, Wu L, Wang R (2014) Hydrogen sulfide and the liver. Nitric Oxide 41(18):62–71. https://doi.org/10.1016/j.niox.2014.02.006
Marra F, Svegliati-Baroni G (2018) Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 68(2):280–295. https://doi.org/10.1016/j.jhep.2017.11.014
Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HHM, de Wit NJW, Bron PA, Masclee AAM, Troost FJ (2017) The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep 7:40128. https://doi.org/10.1038/srep40128
Norusis M (2007) SPSS 15.0 advanced statistical procedures companion. Prentice Hall, Upper Saddle River
Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, Saez-Lara MJ, Campaña-Martin L, Muñoz-Quezada S, Romero F, Gil A, Fontana L (2014) Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One 9:e98401. https://doi.org/10.1371/journal.pone.0098401
Safari Z, Gérard P (2019) The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci 76(8):1541–1558. https://doi.org/10.1007/s00018-019-03011-w
Vajro P, Paolella G, Fasano A (2013) Microbiota and gut-liver axis: a mini-review on their influences on obesity and obesity related liver disease. J Pediatr Gastroenterol Nutr 56(5):461–468. https://doi.org/10.1097/MPG.0b013e318284abb5
van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE (2016) Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci 130(22):2073–2082. https://doi.org/10.1042/cs20160263
Vanni E, Bugianesi E (2010) The gut-liver axis in nonalcoholic fatty liver disease: another pathway to insulin resistance? Hepatology 49(6):1790–1792. https://doi.org/10.1002/hep.23036
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY (2014) The global burden of liver disease: the major impact of China. Hepatology 60(6):2099–2108. https://doi.org/10.1002/hep.27406
Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC (2018) Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 68(2):248–262. https://doi.org/10.1136/gutjnl-2017-315458
Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J, Geng Y (2017) Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep 7:45176. https://doi.org/10.1038/srep45176
Yang S, Yu M, Sun L, Xiao W, Yang X, Sun L, Zhang C, Ma Y, Yang H, Liu Y, Lu D, Teitelbaum DH, Yang H (2014) Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J Interf Cytokine Res 34(3):195–203. https://doi.org/10.1089/jir.2013.0044
Zhao L, Jiang Y, Ni Y, Zhang T, Duan C, Huang C, Zhao Y, Gao L, Li S (2017) Protective effects of Lactobacillus plantarum C88 on chronic ethanol-induced liver injury in mice. J Funct Foods 35:97–104. https://doi.org/10.1016/j.jff.2017.05.017
Zhao Z, Wang C, Zhang L, Zhao Y, Duan C, Zhang X, Gao L, Li S (2019) Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl Microbiol Biotechnol 103(14):5843–5850. https://doi.org/10.1007/s00253-019-09703-4
Acknowledgments
This work was sponsored by the Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2017ZD011 and C82230309), Modern Agroindustrial Technology Research Systems in China (CARS-36), and 2018 Funding Plan for Introducing High-Level Scientific and Technological Innovation Talents to Jilin scientific research institutes (2060399).
Author information
Authors and Affiliations
Contributions
Z.Z. designed the experiments; Z.Z., C.W., L.C., G.Y., and C.D. contributed to the experimental work; Z.Z. performed the data analysis and wrote the manuscript; L.C., C.N., and S.L. revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures involving animals were approved by the Animal Care and Ethic Committee of Jilin Academy of Agricultural Sciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 144 kb)
Rights and permissions
About this article
Cite this article
Zhao, Z., Chen, L., Zhao, Y. et al. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol 104, 5273–5282 (2020). https://doi.org/10.1007/s00253-020-10633-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10633-9