Abstract
The advantage of using acetogens such as Acetobacterium woodii as biocatalysts converting the cheap substrate and greenhouse gas carbon dioxide (CO2) into value-added chemicals comes together with the disadvantage of a low overall ATP gain due to the bioenergetics associated with the Wood-Ljungdahl pathway. Expanding the product spectrum of recombinant A. woodii strains to compounds with high ATP-demanding biosynthesis is therefore challenging. As a least invasive strategy for improved ATP generation, the exploitation of the arginine deiminase pathway (ADI) was examined under native conditions and via using heterologously expressed genes in A. woodii. Several promoters were analyzed for application of different gene expression levels in A. woodii using β-glucuronidase assays. Heterologous expression of the ADI pathway genes from Clostridium autoethanogenum was controlled using either the constitutive pta-ack promoter from Clostridium ljungdahlii or a tightly regulated tetracycline-inducible promoter Ptet. Unlike constitutive expression, only induced expression of the ADI pathway genes led to a 36% higher maximal OD600 when using arginine (OD600 3.4) as nitrogen source and a 52% lower acetate yield per biomass compared to cells growing with yeast extract as nitrogen source (OD600 2.5). In direct comparison, a 69% higher maximal OD600 and about 60% lower acetate yield per biomass in induced to non-induced recombinant A. woodii cells was noticed when using arginine. Our data suggests the application of the ADI pathway in A. woodii for expanding the product spectrum to compounds with high ATP-demanding biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the last decade, the use of and research on gas-fermenting acetogens (anaerobic bacteria employing the Wood-Ljungdahl pathway) for the production of bulk chemicals has enormously increased (Bengelsdorf et al. 2018). The key advantage of this gas fermentation technology is based on the utilization of waste gases as substrate (CO and CO2). In contrast to other biotechnological processes, these substrates are not competing for feedstock that can also serve human nutrition (Schiel-Bengelsdorf et al. 2013). Thus, gas fermentation technology has enormous potential to reduce the greenhouse gas output. In the last decade, the company LanzaTech has developed a steel mill waste gas fermentation process, which eventually reached first commercial scale production of ethanol in 2018 (LanzaTech 2018). Acetogens can be genetically engineered to produce a variety of products such as for instance acetone, butanol, isopropanol, 3-hydroxybutyrate (3-HB), and poly(3-hydroxybutyrate) (PHB) (Bengelsdorf et al. 2018; de Souza Pinto Lemgruber et al. 2019, Flüchter et al. 2019, Woolston et al. 2018) including only a few of already possible target compounds. However, the application of acetogens is limited to products that do not require a heavy ATP biosynthesis demand as they are on the thermodynamic edge of life (Schuchmann and Müller 2014).
In Clostridium ljungdahlii and Clostridium autoethanogenum, strategies such as supplementation of arginine or nitrate have been successfully exploited to address this issue and to enhance ATP yield within the cells (Valgepea et al. 2017; Emerson et al. 2018). While the addition of nitrate showed promising effects such as enhancing the H2-dependent growth and ATP yield of C. ljungdahlii, nitrate could not be utilized by Acetobacterium woodii (Emerson et al. 2018). Nevertheless, as one of the fastest CO2-consuming acetogens, A. woodii is a promising candidate for biotechnological application in gas fermentation (Groher and Weuster-Botz 2016). In C. autoethanogenum, supplementation of arginine caused a growth boost and higher ATP yields due to the expression of the arginine deiminase (ADI) pathway (Valgepea et al. 2017). A detailed analysis of A. woodii’s genome sequence revealed homologous genes to all genes encoding enzymes of the ADI pathway (CAETHG_3021 to CAETHG_3025) except for the gene encoding the arginine-ornithine antiporter (CAETHG_3023) from C. autoethanogenum (Poehlein et al. 2012; Valgepea et al. 2017). In C. autoethanogenum, the ADI pathway genes form an operon. However, in A. woodii, the respective homolog genes are scattered across the genome (Poehlein et al. 2012). This study aimed at elucidation whether A. woodii is capable of taking advantage of the ADI pathway either under native conditions or when the ADI operon is heterologously expressed using different promoters. Therefore, the tightly regulated promoter Ptet (Ransom et al. 2015) and the strong constitutive promoter Ppta-ack (Hoffmeister et al. 2016) were used for heterologous expression of the ADI pathway genes originating from C. autoethanogenum. In order to investigate which promoters are suitable for different levels of expression in A. woodii, several promoters were analyzed using β-glucuronidase assays.
Material and methods
Bacterial strains and cultivation
Strains and plasmids used in this study are listed in Table 1. For the construction of plasmids, Escherichia coli DH5α was used (Thermo Fisher Scientific Inc., USA). Cultivation of E. coli was conducted in LB (Luria-Bertani) medium (tryptone 10 g/l, NaCl 10 g/l, yeast extract 5 g/l) at 37 °C supplemented with 15 μg/ml chloramphenicol, if necessary (Green and Sambrook 2012). Acetobacterium woodii DSM 1030 was cultivated under strictly anaerobic conditions at 30 °C in modified DSMZ medium 135 as described by Hoffmeister et al. (2016). The modified DSMZ medium 135 was prepared without any NH4Cl and after autoclaving supplemented with either arginine (18 to 25 mM) or yeast extract (2 g/l) added from sterile and anaerobic stock solutions (720 mM, pH 6.0 for arginine and 100 g/l for yeast extract). The N2 + CO2 atmosphere was replaced by an H2 + CO2 atmosphere (1.1 bar overpressure) to exclude N2 as nitrogen source. In growth experiments under autotrophic conditions, bottles were pressurized again when overpressure dropped below 0.5 bar. Subsequently, if not indicated otherwise, growth experiments with A. woodii strains were performed in triplicates, in 125-mL Müller-Krempel culture flasks (Müller & Krempel AG, Bülach, Switzerland) with 50 mL of the respective medium. Therefore, cells of A. woodii strains were obtained from DMSO stock cultures and inoculated in 5 mL modified DSMZ medium 135. These 5-mL cultures were used to inoculate pre-cultures for subsequent growth experiments. Pre-cultures were cultivated in 50 mL modified DSMZ medium 135 either with yeast extract or with arginine as nitrogen source. If applicable, fructose was added from a sterile and anaerobic stock solution (1.11 M) to a final concentration of 28 mM. Thus, respective experiments were performed under mixotrophic growth conditions. For preparation of genomic DNA, Clostridium autoethanogenum DSM 10061 was also taken from the laboratory culture collection and grown in modified Tanner medium (Erz 2017).
DNA manipulation and construction of plasmids for promoter assays and ADI pathway genes
Plasmid preparations from E. coli were performed using the ZyppyTM plasmid miniprep kit (Zymo Research, USA) according to the manufacturer’s manual. For preparation of genomic DNA from C. autoethanogenum, the Master PureTM Gram-positive DNA purification kit (Epicentre, USA) was used following the instructions of the manufacturer. Purification of digested plasmids from agarose gels or of PCR amplicons was performed with the NucleoSpin® gel and PCR clean-up kit (Macherey-Nagel GmbH Co. & KG, Germany). All restriction enzymes used in this study were fast digest enzymes purchased from Thermo Fisher Scientific Inc. (Waltham, USA). For all DNA amplifications, KAPA Hifi DNA polymerase (Kapa Biosystem, Sigma-Aldrich Chemie GmbH, Germany) was used except for colony PCR amplifications, which were performed using KAPA2G Robust polymerase (Kapa Biosystem, Sigma-Aldrich Chemie GmbH, Germany). All cloning work, if not indicated otherwise, was performed using standard molecular cloning techniques (Green and Sambrook 2012). All primers are listed in Table 1, all constructed plasmids are listed in Table 2, and all recombinant A. woodii strains constructed via electroporation are listed in Table 3. Recombinant A. woodii strains were obtained by preparing electrocompetent cells and following the electroporation procedure as described by Hoffmeister et al. (2016). Recombinant A. woodii strains were further supplemented with 25 μg/ml thiamphenicol for plasmid maintenance.
The promoters PbgaL, Ptet, Pfac, PackA-theo, and Ppta-ack were fused with the gusA reporter gene of E. coli to obtain a pMTL83151 gusA plasmid series that can be used for a promoter survey by determining the respective β-glucuronidase activity in A. woodii. Initially, the intermediate plasmid pMTL83151_gusA was constructed by amplifying the gusA gene from the plasmid pMDY23-Pgap (Grimm et al. 2014) using the primers j5_gusA_fw and j5_gusA_rev (Table 1). The DNA fragment was purified and ligated into the pJET 1.2/blunt cloning vector (Thermo Fisher Scientific Inc., USA) according to the manufacturer’s protocol. The resulting plasmid was digested with XhoI and NheI, the gusA fragment purified, and then inserted into the XhoI- and NheI-digested pMTL83151. The resulting plasmid pMTL83151_gusA contained the gusA reporter gene without a promoter, and a ribosome-binding site upstream from gusA and was used for the subcloning of the aforementioned promoters.
The subcloned bgaR-PbgaL fragment contains its native ribosomal binding site from C. perfringens. In contrast to Banerjee et al. (2014), the first 13 codons of the cpe gene were omitted. The respective DNA fragment was amplified from the plasmid pKOD_mazF using the primers FW_PbgaL_BamHI and RV_PbgaL_XhoI (Table 1), purified, and ligated into the pJET 1.2/blunt cloning vector. The resulting plasmid was digested with XhoI and BamHI, the obtained bgaR-PbgaL fragment purified and then inserted into the XhoI- and BamHI-digested pMTL83151_gusA resulting in the plasmid pMTL83151_gusA_PbgaL.
The tetR-Ptet fragment was amplified from the plasmid pDSW1728 using the primers FW_PtetR_BamHI and RV_PtetR_XhoI (Table 1), purified, and ligated into the pJET 1.2/blunt cloning vector. The resulting plasmid was digested with XhoI and BamHI, the obtained tetR-Ptet fragment purified, and inserted into the XhoI- and BamHI-digested pMTL83151_gusA plasmid, resulting in the final plasmid pMTL83151_gusA_Ptet. The lacI-Pfac fragment was amplified from the plasmid pMTL82251-YZ2 using the primers FW_Pfac_IPTG_XbaI and RV_Pfac_IPTG_XhoI (Table 1), purified, and then directly digested with XhoI and XbaI and inserted into the XhoI- and XbaI-digested pMTL83151_gusA, resulting in the plasmid pMTL83151_gusA_Pfac. The synthetic PackA-theophylline riboswitch construct contains the ackA promoter from A. woodii (Hoffmeister et al. 2016) fused with a synthetic theophylline riboswitch (Seibold and Rückert, unpublished). The respective DNA fragment was obtained by amplifying the promoter region of the ackA gene using genomic DNA from A. woodii. Therefore, the primers FW_PackA and RV_PackA_theo were used. The primer RV_PackA_theo contains the synthesized theophylline riboswitch DNA element (Table 1). The PCR amplicon was purified and inserted into an amplified pMTL83151_gusA using the primers FW_p83_gusA_theo and RV_p83_gusA_PackA (Table 1) via the In-Fusion® HD cloning kit (Takara Bio USA Inc., USA) following the manufacturer’s protocol and resulting in the plasmid pMTL83151_gusA_PackA-theo. The pta-ack promoter fragment from C. ljungdahlii was amplified from pJIR750_actpta-ack using the primers FW_Ppta-ack1L_BamHI and RV_Ppta-ack1L_XhoI (Table 1), purified, then directly digested with XhoI and BamHI, and inserted into the XhoI- and BamHI-digested pMTL83151_gusA, resulting in the plasmid pMTL83151_gusA_Ppta-ack.
The ADI pathway genes (CAETHG_3021 to CAETHG_3025) were amplified using genomic DNA from C. autoethanogenum and the primers FW_CAETHG_Ptet and RV_CAETHG_Ptet (Table 1). The PCR amplicon was purified and inserted into an XhoI- and NheI-digested (excision of gusA) pMTL83151_gusA_Ptet via the In-Fusion® HD cloning kit (Takara Bio USA Inc., USA), following the manufacturer’s protocol and resulting in the plasmid pMTL83151_Ptet_Boost. The pta-ack promoter sequence from C. ljungdahlii was excised from pMTL83151_gusA_Ppta-ack via digestion with XhoI and BamHI, purified, and then inserted into the XhoI- and BamHI-digested (excision of Ptet) pMTL83151_Ptet_Boost, resulting in the plasmid pMTL83151_Ppta-ack_Boost.
Successful construction of plasmids was verified by Sanger sequencing (Eurofins Genomics GmbH, Luxemburg). Analysis of the obtained data regarding the nucleic acid sequences of the arcD gene from C. autoethanogenum revealed an additional guanine nucleotide following a poly-G (4) sequence in the 3′-region of CAETHG_3023. The annotated DNA sequence would result in a stop codon. The correct sequence, however, resulted in a read-through without a translational frameshift in CAETHG_3024. Further analysis of the respective coding region by comparison with the homologous gene from the closely related C. ljungdahlii (CLJU_c09290) revealed an identical nucleotide sequence (aside from other C. autoethanogenum–specific SNPs, data not shown) (Fig. S1). Taking this into account, we concluded that the cloned genes were intact suggesting that the arginine:ornithine antiporter ArcD is encoded by one single coding sequence instead of two (CAETHG_3023 and CAETHG_3024) as in C. ljungdahlii (CLJU_c09290).
β-Glucuronidase assays
GusA activity was measured in cell extracts of recombinant A. woodii strains each harboring a plasmid of the pMTL83151_gusA series possessing promoters PbgaL, Ptet, Pfac, PackA-theo, and Ppta-ack (see construction of plasmids). Recombinant A. woodii strains were cultivated in triplicates under heterotrophic conditions (42 mM fructose), until the mid or late log phase was reached (optical density at λ = 600 nm (OD600) ~ 0.40–1.3). The strain carrying the constitutive promoter Ppta-ack from C. ljungdahlii[pMTL83151_gusA_Ppta-ack] was harvested and processed as described below when the late log phase was reached. Depending on the inducible promoter system encoded on the different plasmids of the pMTL83151_gusA series, cells of recombinant A. woodii strains were induced via the supplementation of either 200 and 400 ng/ml anhydrotetracycline (atc), 1 mM lactose, 1 mM IPTG, or 1 mM theophylline. Furthermore, respective strains were also cultivated without supplementation of an inducer. After induction, cells were further cultivated either for additional four hours or overnight. Twenty-five to 50 ml of the cell suspension was harvested (3,773 g, 4 °C, 10 min) and washed twice with cold GusA buffer (50 mM sodium phosphate, 1 mM EDTA, pH 7.0 (Zhang et al. 2015a)), and suspended in 1 ml GusA buffer. Cells were disrupted in cryotubes with glass beads (0.1-mm diameter; Carl Roth GmbH & Co. KG, Germany) using the Ribolyser Precellys24 (Bertin Instruments, France; 3 × 30 s, 6500 rpm, 4 °C) with intermediate cooling steps on ice of 1 min. To remove cell debris and glass beads, cell lysates were centrifuged for 15 min at 17,968g and 4 °C, transferred to fresh reaction tubes, and kept on ice until further usage. Protein concentrations were determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific Inc., USA).
GusA activity of crude protein extract was measured in 96-well microplates (655900; Greiner Bio-One International GmbH, Austria) while the general procedures of the assay follow the technique reported by Zhang et al. (2015a). One hundred eighty microliters of pre-warmed (at 37 °C) MUG assay buffer (GusA buffer containing 4 mM MUG (4-methylumbelliferyl-β-d-glucuronide; Sigma-Aldrich Chemie GmbH, Germany) was added to suitable dilutions of crude protein extract (20 μl) to start the reaction. The respective fluorescence kinetic curves were recorded every 30 s for 10 min using either Infinite M200 multimode reader (Tecan Group AG, Switzerland) with a time-scan mode (λex = 365 nm, λem = 455 nm, excitation bandwidth = 9 nm, emission bandwidth = 20 nM, gain = 100) or Synergy™ H1 microplate reader (BioTek Instruments, Inc., Winooski, USA) using the identical wavelength configurations and measuring intervals. The amount of MUG converted into 4-MU (4-methylumbelliferone, Sigma-Aldrich Chemie GmbH, Germany) was calculated using a calibration curve determined via recording the fluorescence of 180 μl of MUG assay buffer supplemented with 20 μl of respective 4-MU stock solutions (0.5 μM–100 μM) in the same way as described above. The curve slope value of produced 4-MU and the protein concentration of the respective protein extract was used to calculate the specific GusA activity.
High-performance liquid chromatography
Acetate, fumarate, and fructose from the undiluted culture supernatants were analyzed by high-performance liquid chromatography (HPLC) using the method previously described (Hoffmeister et al. 2016) with the modification of a constant column temperature of 40 °C, using the organic acid resin 150 × 8 mm column or organic acid resin 300 × 8 mm column (CS-Chromatographie-Service GmbH, Germany).
According to the methods described by Schrumpf et al. (1991), quantification of the amino acids arginine, ornithine, and citrulline was performed via derivatization followed by separation using either the Agilent LC 1100 (Agilent Technologies, USA) HPLC equipped with a fluorescence detector (FLD) or the Agilent 1260 Infinity Series HPLC system (Agilent Technologies, USA) equipped with a diode array detector (DAD). Accordingly, detection was performed either fluorometrically or by UV absorbance. After automatic pre-column derivatization with ortho-phthaldialdehyde (OPA), culture supernatants were separated using the Multohyp ODS-5μ 125 × 4 mm (CS-chromatographie-Service GmbH, Germany) or the ISAspher 100-5 C18 125 × 4 mm (ISERA GmbH, Germany) column at 40 °C. Separation was mediated with an appropriate gradient of the mobile phase using varying proportions of sodium acetate (0.1 M, pH 7.2, 15 mM sodium azide) and methanol (100%). The gradient was applied using a flow rate of 0.7–0.8 ml/min starting with 20% methanol and increasing up to 100% methanol within 19 min of separation time. Supernatants were prepared by centrifugation (17,968g, 4 °C, 20 min) and aliquots of the supernatants were diluted with ddH2O to achieve an estimated amino acid concentration between 20 and 100 μM. As an internal standard, lysine (100 μM) was added to all analyzed supernatants. Moreover, an external standard series of arginine, ornithine, and citrulline ranging between 20 and 100 μM was recorded as well for calibration purposes.
Results
Establishment of an inducible promoter system in A. woodii
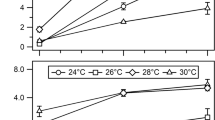
The characteristics of the promoters PbgaL, Ptet, Pfac, PackA-theo, and Ppta-ack were determined to cover a broad range of specific β-glucuronidase activities expressed in microunits per milligram of protein (Fig. 1). Highest specific GusA activities of 7.8·105 ± 1.6·105 μU/mg were measured using the constitutive promoter Ppta-ack from C. ljungdahlii in strain A. woodii [pMTL83151_gusA_Ppta-ack]. The second highest specific GusA activities were detected, when using the lactose/IPTG inducible promoter Pfac in induced (5.9·105 ± 1.7·105 μU/mg) and non-induced (2.6·105 ± 3.7·103 μU/mg) cells of the strain A. woodii [pMTL83151_gusA_Pfac]. The recombinant strain A. woodii [pMTL83151_gusA_PbgaL] harboring the lactose-inducible promoter PbgaL from C. perfringens exhibited slightly lower specific GusA activities of 2.2·104 ± 8.3·103 and 3.1·103 ± 2.4·102 μU/mg in induced and non-induced cells, respectively. However, tightly controlled regulation could only be observed, when gusA was expressed using the tetracycline-inducible promoter Ptet or the PackA promoter from A. woodii linked to the theophylline riboswitch PackA-theo in the respective strains A. woodii [pMTL83151_gusA_Ptet] and A. woodii [pMTL83151_gusA_PackA-theo]. Consequently, without the supplementation of the respective inducers, only very low specific GusA activities were measured when gusA was controlled by Ptet (17 ± 35 μU/mg) or PackA-theo (12 ± 18 μU/mg). Upon supplementation of the respective inducer, the specific GusA activities increased to 2.7·102 ± 48 μU/mg using PackA-theo and 1.2·103 ± 1.4·102 μU/mg with Ptet.
Specific GusA activities in relation to the respective promoters in recombinant A. woodii [pMTL83151_gusA] cells. gusA, gusA without promoter (negative control); gusA_Ppta-ack, gusA under control of the constitutive Ppta-ack promoter from Clostridium ljungdahlii; gusA_Pfac, gusA under control of the synthetic IPTG/lactose-inducible promoter Pfac without (-) and with (+) supplementation of 1 mM IPTG; gusA_PbgaL, gusA under control of the lactose-inducible promoter PbgaL from C. perfingens without (-) and with (+) supplementation of 1 mM lactose; gusA_PackA-theo, gusA under control of the ackA promoter from A. woodii linked downstream to a theophylline riboswitch without (-) and with (+) supplementation of 1 mM theophylline; gusA_Ptet, gusA under control of the tetracycline-inducible promoter Ptet without (-) and with supplementation of 200 (+) or 400 ng/ml (++) anhydrotetracycline; n.a., no activity; error bars indicate the standard deviations within biological triplicates with plotted mean values
The Ptet promoter was chosen for the controlled expression of the ADI pathway genes from C. autoethanogenum. In contrast to that, the high GusA activities, which were recorded when gusA was expressed using Ppta-ack without the necessity of an inducing reagent, led to the selection of Ppta-ack as the promoter of choice for strong and constitutive expression of the ADI pathway genes.
Expression of the arginine deiminase pathway in mixotrophically growing A. woodii: constitutive versus tetracycline-inducible control
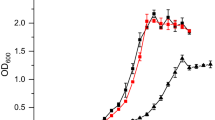
A fine-tuned inducible expression (Ptet promoter) compared to strong constitutive expression (Ppta-ack promoter) of heterologous ADI pathway genes showed considerable differences in growth behavior and product formation of respective A. woodii strains. The two recombinant strains A. woodii [pMTL83151_Ptet_Boost] and A. woodii [pMTL83151_Ppta-ack_Boost] expressed the ADI pathway from genes originating from C. autoethanogenum either in a tetracycline-inducible (Ptet) or in a constitutive (Ppta-ack) manner. Both strains were characterized via growth experiments using either arginine or yeast extract as nitrogen source under mixotrophic conditions with fructose and CO2 + H2 as carbon as well as energy source. Both recombinant strains consumed arginine and produced ornithine (Figs. 2 and 3), which is a clear proof for a functional ADI pathway, since the control strains (A. woodii wild type and A. woodii [pMTL83151]) did not show such a characteristic phenotype (Fig. S2). A. woodii wild type and A. woodii [pMTL83151] grew with arginine as nitrogen source (μmax 0.07 1/h and 0.14 1/h) and reached maximal optical densities (OD600) of 0.8 after 129 h and of 1.6 after 94 h, respectively. In a further experiment (Table 4), both strains were cultivated using yeast extract as a nitrogen source which promoted faster growth (μmax of 0.14 1/h and μmax of 0.2 1/h) and considerably higher maximal OD600 values (3.0 after 58 h and of 2.5 after 46 h). However, neither A. woodii wild type nor A. woodii [pMTL83151] produced ornithine under any condition tested (Fig. S2). Noteworthy is also the fact that only two thirds of fructose was converted into acetate in the recombinant ADI pathway-expressing strains when provided with arginine (see the Discussion section). In contrast, the control strains formed 3 moles of acetate from 1 mole of fructose (typical feature of acetogens). The data for the growth experiments are summarized in Table 4.
Mixotrophic growth experiment with A. woodii [pMTL83151_Ptet_Boost]. a Induced cells (300 ng/ml atc) growing with either yeast extract (empty symbols) or arginine (filled symbols) as nitrogen source. b Arginine as nitrogen source, cells were either induced (filled symbols) by 300 ng/ml anhydrotetracycline or non-induced (empty symbols). Fructose and CO2 were used as carbon, H2 and fructose as energy sources. Monitored OD600, squares (◻◼); fructose consumption, upward triangles (△▲); acetate production, circles (○●); arginine consumption, downward triangles (▽▼); ornithine production, pentagons (⬟); time of induction with anhydrotetracycline (300 ng/ml) is indicated by a vertical line. Error bars indicate the standard deviations within biological replicates (n = 3) with plotted mean values
Mixotrophic growth experiment with A. woodii [pMTL83151_Ppta-ack_Boost] growing with either yeast extract (empty symbols) or arginine (filled symbols) as nitrogen source. Fructose and CO2 were used as carbon, H2 and fructose as energy sources. Monitored OD600, squares (◻◼); fructose consumption, upward triangles (△▲); acetate production, circles (○●); arginine consumption, downward triangles (▽▼); ornithine production, pentagons (⬟); citrulline production, stars (★); error bars indicate the standard deviations within biological replicates (n = 3) with plotted mean values
The A. woodii [pMTL83151_Ptet_Boost] culture, in which cells expressed the ADI pathway in the induced state, reached a maximal OD600 of 3.4 after 71 h with arginine as nitrogen source (Fig. 2b). The substitution of arginine with yeast extract as nitrogen source resulted in a lower maximal OD600 of 2.5 after 71 h (Fig. 2a). Under both conditions tested, the addition of the inducer caused a drop of the growth rate by 50% using arginine and 77 % using yeast extract, respectively (Table 4). Besides the increase of 36% maximal OD600 in induced A. woodii [pMTL83151_Ptet_Boost] cells growing on arginine compared to yeast extract, another prominent difference was noticed in acetate production. While fructose consumption stayed in a similar range under tested conditions, A. woodii [pMTL83151_Ptet_Boost] produced 38.8 mM acetate per OD600 (97 mM total) using yeast extract and exhibited a 52% lower acetate yield (18.5 mM/OD600; 63 mM total) when using arginine, respectively (Table 4). Thus, the acetate production rates were also impacted considerably since the rates dropped from 1.34 to 0.45 mM/h after induction of the ADI pathway when using arginine. The decrease of acetate production in A. woodii [pMTL83151_Ptet_Boost] was concomitant with arginine consumption and ornithine production. About 6 mM of the provided 18 mM arginine was consumed and converted into approximately 6 mM ornithine (1.8 mM/OD600) at the end of the growth experiment.
To shed light on the direct impact of the Ptet-dependent induced expression of the heterologous ADI pathway genes in A. woodii [pMTL83151_Ptet_Boost], growth characteristics were analyzed simultaneously under induced and non-induced conditions. Figure 2b shows that the Ptet promoter is tightly regulated, while only induced cells notably consumed arginine and produced ornithine. The non-induced cultures reached the stationary growth phase within 30 h and a maximal OD600 of 1.3. The induced culture kept growing until a maximal OD600 of 2.2 was reached after 56 h (Fig. 2b). Besides this 69% higher final OD600 of the induced cultures, a further difference was noticed. The induced cultures showed about 60% lower acetate yield (17.3 mM/OD600 compared to 43.1 mM/OD600; 38 mM compared to 56 mM total), which was also mirrored in the acetate production rates during the growth experiments (Table 4). Fructose was consumed within 120 h in the induced and non-induced cultures with similar rates (Table 4). The non-induced A. woodii [pMTL83151_Ptet_Boost] cultures showed only low arginine consumption (approximately 2 mM arginine, sufficient for growth), but did not exhibit any ornithine production. The induced cells used approximately 10 mM of arginine and produced 7 mM of ornithine (3.2 mM/OD600) within the same time frame (Fig. 2b).
To address the question, if constitutive expression of the heterologous ADI pathway genes by the Ppta-ack promoter affects the growth behavior of A. woodii [pMTL83151_Ppta-ack_Boost], growth characteristics were analyzed in parallel using either arginine or yeast extract as nitrogen source. When cultures were grown with yeast extract, a maximal OD600 of 1.2 was reached after 55 h, whereas when cultures used arginine a maximal OD600 of 0.8 was reached after 73 h (Fig. 3). Cultures completely metabolized fructose when growing with yeast extract, but did only consume 74% of the provided fructose when using arginine as nitrogen source. Fructose consumption rates and acetate production rates are shown in Table 4. A. woodii [pMTL83151_Ppta-ack_Boost] consumed all of the provided arginine (18 mM) and produced 11 mM ornithine in total with the highest detected yield of 13.8 mM/OD600. Additional to ornithine, this strain produced a total of 5 mM citrulline (6.3 mM/OD600), which is an intermediate of the ADI pathway.
Furthermore, fumarate was also produced by A. woodii [pMTL83151_Ppta-ack_Boost] to concentrations of 1.4 mM when growing using arginine, in contrast to 0.3 mM when using yeast extract as nitrogen source (Fig. S3a). Among all of the strains analyzed besides A. woodii [pMTL83151_Ppta-ack_Boost], only A. woodii [pMTL83151] produced very low amounts of fumarate (0.1 mM) when using arginine as nitrogen source (Fig. S3b).
Induced expression of the arginine deiminase pathway in autotrophically growing A. woodii
Analogous to the mixotrophic growth experiments, heterologous expression of the ADI pathway has been proved using A. woodii [pMTL83151_Ptet_Boost] growing autotrophically with H2 + CO2 as carbon and energy source. The strain (induced cells) reached similar OD600 values with arginine (OD600 1.0) as with yeast extract (OD600 0.92) as nitrogen source. Induced A. woodii [pMTL83151_Ptet_Boost] cells consumed provided arginine (16 mM) and produced a corresponding amount of ornithine. A. woodii [pMTL83151_Ptet_Boost] showed prior induction (300 ng/ml anhydrotetracycline) of the ADI pathway growth rates of 0.02 1/h (arginine) up to 0.04 1/h (yeast extract). After induction, induced cells of A. woodii [pMTL83151_Ptet_Boost] continued growing with a rate of 0.02 1/h (arginine) while growth of non-induced cells stagnated. Non-induced cells consumed about 3.5 mM arginine, however, without production of detectable amounts of ornithine. Furthermore, non-induced A. woodii [pMTL83151_Ptet_Boost] cells showed very inconsistent growth while reaching a final OD600 of 0.52 (Fig. 4). It has to be noted that A. woodii [pMTL83151_Ppta-ack_Boost] did not show detectable autotrophic growth using H2 + CO2 with neither arginine nor with yeast extract as nitrogen source.
A. woodii [pMTL83151_Ptet_Boost] growing autotrophically with either yeast extract (half-filled symbols; n = 3) or arginine (filled and empty symbols; n = 2) as nitrogen source. Cells growing with arginine were either induced (filled symbols) by 300 ng/ml anhydrotetracycline or non-induced (empty symbols). CO2 was used as carbon and H2 as energy source. Monitored OD600, squares (◻◼); gas consumption (absolute value of accumulated pressure loss (bar)), upward triangles (△▲◮); acetate production, circles (○●); arginine consumption, downward triangles (▽▼); ornithine production, pentagons (⬟); time of induction with anhydrotetracycline (300 ng/ml) is indicated by a vertical line. Error bars indicate the standard deviations within biological replicates with plotted mean values
Discussion
The intention of lowering energetic barriers in acetogenic metabolism becomes a challenging endeavor, especially because it is well known that these bacteria live on the thermodynamic edge of life (Schuchmann and Müller 2014). The necessity of this task becomes more and more clear since the relevance of acetogens in biotechnology is steadily increasing (Schiel-Bengelsdorf and Dürre 2012; Bengelsdorf et al. 2018; LanzaTech 2018). Valgepea et al. (2017) could demonstrate that the ADI pathway can be exploited in Clostridium autoethanogenum to beneficially affect the metabolism via producing additional ATP and improving the growth of cells. So far, A. woodii is one of the fastest gas-consuming acetogens (Groher and Weuster-Botz 2016). The data presented in this study show that only recombinant A. woodii strains can make use of a heterologously expressed ADI pathway, controlled either by the Ptet or by the Ppta-ack promoter, when arginine is supplemented to the medium. A. woodii cells are at least to some extent able to use arginine as nitrogen source, since a native arginine deiminase could cleave off ammonium and produce citrulline. Small amounts of fumarate detected in the supernatants of A. woodii [pMTL83151] hint to a native utilization of an urea cycle (Jackson et al. 1986), executed by the action of arginine deiminase, argininosuccinate synthetase (AS), and argininosuccinate lyase (AL), whose genes are all present in the genome of A. woodii and are encoded by argA (Awo_c08610), argG (Awo_c13540), and argH (Awo_c13530), respectively (Poehlein et al. 2012). The activity of AS and AL are responsible for the conversion of citrulline into argininosuccinate (Ratner 1954) and the subsequent cleavage to arginine and fumarate (Davison and Elliott 1952). This, however, would not result in a net gain of ammonium since the gained ammonium in the ADI reaction is eventually reinvested by the activity of argininosuccinate synthase reaction into aspartate (Ratner 1954). Puzzling, however, remains the question from where remaining nitrogen was acquired supporting the poor growth of A. woodii and A. woodii [pMTL83151] without significantly consuming provided arginine. One possibility could be the use of the amino group of cysteine hydrochloride which serves as reducing agent (1.7 mM) in the medium.
Our data showed great differences in the level of expression of the ADI pathway as well as in the determined specific GusA activities depending on whether the ADI operon or gusA was controlled by a constitutive or an inducible promoter. This strongly indicates that such differences in the level of (heterologous) expression very likely occur when any other gene is controlled by one of the latter promoters.
The expression on ADI genes controlled by the tetracycline-inducible promoter system Ptet caused beneficial effects on growth of recombinant cells consuming arginine compared to strong constitutive expression of the ADI genes using the Ppta-ack promoter. In contrast, the strong, constitutive expression of the ADI genes in A. woodii [pMTL83151_Ppta-ack_Boost] led to poor or no growth in mixotrophically and autotrophically growing cells. This is surprising considering the highest theoretical ATP production from an active ADI pathway being equivalent to the ornithine excretion (Table 4). Most likely, the poor growth of A. woodii [pMTL83151_Ppta-ack_Boost] could be due to an excessive expression of arcD encoding the arginine:ornithine antiporter ArcD which is considered to be a homolog of transport membrane protein ArcD in Lactococcus lactis (Trip et al. 2013; Noens et al. 2015). It was shown from other membrane proteins that overexpression of the respective encoding genes can cause toxic effects to the cells (Wagner et al. 2008; O’Brien and Wright 2011). The fact that strong continuous expression in general may cause harmful effects also in other cells was shown for instance in Saccharomyces cerevisiae (Mumberg et al. 1994) and E. coli (Carrier et al. 1983). In addition, detection of citrulline in the supernatant of A. woodii [pMTL83151_Ppta-ack_Boost] indicates that the strong expression of the ADI genes caused an imbalance of metabolic flux of the ADI pathway. An elevated citrulline formation most likely dragged A. woodii [pMTL83151_Ppta-ack_Boost] cells to a further metabolic shift towards fumarate via the action of AS and AL. Citrulline excretion in relation to the ADI pathway was reported before in lactic acid bacteria with so far unknown physiological relevance (Liu and Pilone 1998; Noens and Lolkema 2017). However, it may be associated with a fast rate of citrulline formation (hydrolysis of arginine mediated by ADI) in contrast to slow removal of citrulline in the thermodynamically unfavorable ornithine carbamoyltransferase reaction (phosphorylation of citrulline resulting in the formation of carbamyl-phosphate and ornithine) (Liu and Pilone 1998; Zúñiga et al. 2002).
On the other hand, the more moderate expression of the ADI pathway genes controlled by the Ptet promoter in A. woodii [pMTL83151_Ptet_Boost] cells resulted in a balanced arginine consumption and ornithine production promoting a significant growth advantage in terms of higher optical densities compared to non-induced cells as well as to cells using yeast extract as nitrogen source. This finding suggests that A. woodii [pMTL83151_Ptet_Boost] gains 1 extra mole of ATP per mole of arginine (Poolman et al. 1987; Liu and Pilone 1998) from the arginine deiminase pathway. This extra mole of ATP could be added to the theoretical amount of 4.3 moles of ATP per mole of fructose from glycolysis in combination with the Wood-Ljungdahl pathway (Schuchmann and Müller 2014), if all acetyl-CoA would be converted to acetate by the phosphotransacetylase reaction followed by substrate level phosphorylation (SLP) in the acetate kinase reaction. Remarkably, this was obviously not the case, since all recombinant strains expressing the ADI pathway showed considerably reduced acetate production yields (Table 4). The estimated fructose conversion into acetate in cells expressing the ADI pathway heterologously and consuming arginine was only two thirds compared to the respective control experiments. This is equivalent to a potential loss of 2 mol of CO2 per fructose (or one mole of acetate) in the pyruvate:ferredoxin oxidoreductase reaction without further reduction to acetate. Thus, under the applied mixotrophic growth condition, there was presumably a downregulation of the Wood-Ljungdahl pathway. The surplus of electrons obtained from glycolysis and the pyruvate:ferredoxin oxidoreductase reaction were presumably converted into H2 via the electron-bifurcating hydrogenase. Even if the production of hydrogen was not determined in the presented experiments, it is known that A. woodii is capable of H2 production when growing chemoorganotrophically (Braun and Gottschalk 1981). The incomplete fructose conversion into acetate is an indication that less ATP was generated via SLP during the acetate kinase reaction. The loss of ATP from this reaction was more than compensated by the additional ATP gain obtained from arginine via the carbamate kinase (CK) reaction. Subsequently, the accessory acetyl-CoA has then been used for the production of biomass underlining the significant differences in final OD600 values. Under autotrophic growth conditions, A. woodii [pMTL83151_Ptet_Boost] cells had no detectable growth advantage when using arginine compared to yeast extract as nitrogen source (Fig. 4). However, this lack of evidence is most likely due to the very simple mode of performed batch experiments in bottles. It can be assumed that more advanced cultivation methods using gas fermentation reactor configurations will lead to a data set that allows a detailed evaluation (Grohner and Weuster-Botz 2016). Bioreactor experiments with continuous gas supply, pH control, and stirring of the culture broth usually have beneficial effects on growth and metabolism of cells compared to batch experiments in bottles. Moreover, detailed expression studies using RNA sequencing technologies can provide more insight in this matter and shed light on regulatory mechanisms as shown already for a variety of prokaryotes (Sorek and Cossart 2010). In Lactococcus lactis, it has been postulated that the ADI pathway (and the arginine biosynthetic operon) are both regulated by two transcriptional ArgR-type regulators (Larsen et al. 2004; Larsen et al. 2005). One of the two ArgR-type homologs (ArgR (llmg_2315)) binds to the arcA promoter region controlling the ADI pathway genes in an arginine-independent manner (Larsen et al. 2005). On the other hand, the second ArgR-type homolog (AhrC (llmg_1686)) represses the arginine biosynthesis and activates the expression of the ADI pathway genes only in combination of ArgR with arginine via reducing the affinity of ArgR to the operator (Larsen et al. 2005). Blastp analyses revealed that C. autoethanogenum as well possesses homologous proteins with 38% amino acid identity to ArgR (llmg_2315) and 58% amino acid identity to AhrC (llmg_1686) encoded in CAETHG_3019 and CAETHG_3208, respectively. A smart use of the regulators ArgR and AhrC could further improve the growth and lower energetic barriers in A. woodii metabolism.
The presented data show that A. woodii benefits from the supply of additional ATP using the recombinant ADI pathway; this might lead to an extended product range. This is in general desirable, since the current biotechnological application of acetogens is limited to a reduced amount of metabolic end products (Valgepea et al. 2017). Furthermore, the utilization of a defined medium such as the arginine-containing medium presented here, promoting optimal growth to A. woodii strains moderately expressing the ADI pathway, should be preferred over media containing complex ingredients such as yeast extract for the setup of precise carbon flux balances and for identification of limiting factors in growth experiments (Monod 1949). Additionally, pH regulation could be managed via sophisticated arginine supplementation in biotechnological processes minimizing the need of further addition of bases to the process, and thus lowering the production costs. Furthermore, the herein-described tetracycline-inducible promoter can be exploited as a valuable tool for general research in A. woodii regarding the cloning and expression of genes which cannot be accomplished using commonly used strong and constitutively active promoters (Hoffmeister et al. 2016; Bengelsdorf et al. 2018).
References
Al-Hinai MA, Fast AG, Papoutsakis ET (2012) Novel system for efficient isolation of Clostridium double-crossover allelic exchange mutants enabling markerless chromosomal gene deletions and DNA integration. Appl Environ Microbiol 78:8112–8121. https://doi.org/10.1128/AEM.02214-12
Banerjee A, Leang C, Ueki T, Nevin KP, Lovley DR (2014) Lactose-inducible system for metabolic engineering of Clostridium ljungdahlii. Appl Environ Microbiol 80:2410–2416. https://doi.org/10.1128/AEM.03666-13
Bengelsdorf FR, Beck MH, Erz C, Hoffmeister S, Karl MM, Riegler P, Wirth S, Poehlein A, Weuster-Botz D, Dürre P (2018) Bacterial anaerobic synthesis gas (syngas) and CO2 + H2 fermentation. In: Advances in Applied Microbiology, vol 103. Elsevier, Amsterdam, pp 143–221
Braun K, Gottschalk G (1981) Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch Microbiol 128:294–298
Carrier MJ, Nugent ME, Tacon WCA, Primrose SB (1983) High expression of cloned genes in E. coli and its consequences. Trends Biotechnol 1:109–113. https://doi.org/10.1016/0167-7799(83)90033-1
Davison DC, Elliott WH (1952) Enzymic reaction between arginine and fumarate in plant and animal tissues. Nature 169:313–314. https://doi.org/10.1038/169313a0
de Souza Pinto Lemgruber R, Valgepea K, Tappel R, Behrendorff JB, Palfreyman RW, Plan M, Hodson MP, Simpson SD, Nielsen LK, Köpke M, Marcellin E (2019) Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab Eng 53:14–23. https://doi.org/10.1016/j.ymben.2019.01.003
Emerson DF, Woolston BM, Liu N, Donnelly M, Currie DH, Stephanopoulos G (2018) Enhancing hydrogen-dependent growth of and carbon dioxide fixation by Clostridium ljungdahlii through nitrate supplementation. Biotechnol Bioeng 116:294–306. https://doi.org/10.1002/bit.26847
Erz C (2017) 2,3-Butanediol production using acetogenic bacteria. PhD thesis, University of Ulm, Germany
Flitsch SK (2016) Identifikation und Charakterisierung von Regulatoren der Lösungsmittelbildung in Clostridium acetobutylicum. PhD thesis, University of Ulm, Germany
Flüchter S, Follonier S, Schiel-Bengelsdorf B, Bengelsdorf FR, Zinn M, Dürre P (2019) Anaerobic production of poly(3-hydroxybutyrate) and its precursor 3-hydroxybutyrate from synthesis gas by autotrophic clostridia. Biomacromolecules. https://doi.org/10.1021/acs.biomac.9b00342
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Grimm V, Gleinser M, Neu C, Zhurina D, Riedel CU (2014) Expression of fluorescent proteins in bifidobacteria for analysis of host-microbe interactions. Appl Environ Microbiol 80:2842–2850. https://doi.org/10.1128/AEM.04261-13
Groher A, Weuster-Botz D (2016) Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J Biotechnol 228:82–94. https://doi.org/10.1016/j.jbiotec.2016.04.032
Heap JT, Pennington OJ, Cartman ST, Minton NP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78:79–85. https://doi.org/10.1016/j.mimet.2009.05.004
Hoffmeister S, Gerdom M, Bengelsdorf FR, Linder S, Flüchter S, Öztürk H, Blümke W, May A, Fischer R-J, Bahl H, Dürre P (2016) Acetone production with metabolically engineered strains of Acetobacterium woodii. Metab Eng 36:37–47. https://doi.org/10.1016/j.ymben.2016.03.001
Jackson MJ, Beaudet AL, O’Brien WE (1986) Mammalian urea cycle enzymes. Annu Rev Genet 20:431–464. https://doi.org/10.1146/annurev.ge.20.120186.002243
LanzaTech (2018) World’s first commercial waste gas to ethanol plant starts up. https://www.lanzatech.com/2018/06/08/worlds-first-commercial-waste-gas-ethanol-plant-starts/2018/. Accessed 11 July 2019
Larsen R, Buist G, Kuipers OP, Kok J (2004) ArgR and AhrC are both required for regulation of arginine metabolism in Lactococcus lactis. J Bacteriol 186:1147–1157. https://doi.org/10.1128/JB.186.4.1147-1157.2004
Larsen R, Kok J, Kuipers OP (2005) Interaction between ArgR and AhrC controls regulation of arginine metabolism in Lactococcus lactis. J Biol Chem 280:19319–19330. https://doi.org/10.1074/jbc.M413983200
Liu S-Q, Pilone GJ (1998) A review: arginine metabolism in wine lactic acid bacteria and its practical significance. J Appl Microbiol 84:315–327. https://doi.org/10.1046/j.1365-2672.1998.00350.x
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394. https://doi.org/10.1146/annurev.mi.03.100149.002103
Mumberg D, Müller R, Funk M (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res 22:5767–5768
Noens EEE, Lolkema JS (2017) Convergent evolution of the arginine deiminase pathway: the ArcD and ArcE arginine/ornithine exchangers. Microbiol Open 6:e00412. https://doi.org/10.1002/mbo3.412
Noens EEE, Kaczmarek MB, Żygo M, Lolkema JS (2015) ArcD1 and ArcD2 arginine/ornithine exchangers encoded in the arginine deiminase pathway gene cluster of Lactococcus lactis. J Bacteriol 197:3545–3553. https://doi.org/10.1128/JB.00526-15
O’Brien J, Wright GD (2011) An ecological perspective of microbial secondary metabolism. Curr Opin Biotechnol 22:552–558. https://doi.org/10.1016/j.copbio.2011.03.010
Poehlein A, Schmidt S, Kaster A-K, Goenrich M, Vollmers J, Thürmer A, Bertsch J, Schuchmann K, Voigt B, Hecker M, Daniel R, Thauer RK, Gottschalk G, Müller V (2012) An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7:e33439. https://doi.org/10.1371/journal.pone.0033439
Poolman B, Driessen AJ, Konings WN (1987) Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol 169:5597–5604
Ransom EM, Ellermeier CD, Weiss DS (2015) Use of mCherry red fluorescent protein for studies of protein localization and gene expression in Clostridium difficile. Appl Environ Microbiol 81:1652–1660. https://doi.org/10.1128/AEM.03446-14
Ratner S (1954) Urea synthesis and metabolism of arginine and citrulline. Adv Enzymol Relat Subj Biochem 15:319–387
Schiel-Bengelsdorf B, Dürre P (2012) Pathway engineering and synthetic biology using acetogens. FEBS Lett 586:2191–2198. https://doi.org/10.1016/j.febslet.2012.04.043
Schiel-Bengelsdorf B, Montoya J, Linder S, Dürre P (2013) Butanol fermentation. Environ Technol 34:1691–1710. https://doi.org/10.1080/09593330.2013.827746
Schrumpf B, Schwarzer A, Kalinowski J, Pühler A, Eggeling L, Sahm H (1991) A functionally split pathway for lysine synthesis in Corynebacterium glutamicum. J Bacteriol 173:4510–4516
Schuchmann K, Müller V (2014) Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol 12:809–821. https://doi.org/10.1038/nrmicro3365
Sorek R, Cossart P (2010) Prokaryotic transcriptomics: a new view on regulation, physiology and pathogenicity. Nat Rev Genet 11:9–16. https://doi.org/10.1038/nrg2695
Trip H, Mulder NL, Lolkema JS (2013) Cloning, expression, and functional characterization of secondary amino acid transporters of Lactococcus lactis. J Bacteriol 195:340–350. https://doi.org/10.1128/JB.01948-12
Valgepea K, Loi KQ, Behrendorff JB, de Lemgruber RSP, Plan M, Hodson MP, Köpke M, Nielsen LK, Marcellin E (2017) Arginine deiminase pathway provides ATP and boosts growth of the gas-fermenting acetogen Clostridium autoethanogenum. Metab Eng 41:202–211. https://doi.org/10.1016/j.ymben.2017.04.007
Wagner S, Klepsch MM, Schlegel S, Appel A, Draheim R, Tarry M, Hogbom M, van Wijk KJ, Slotboom DJ, Persson JO, de Gier J-W (2008) Tuning Escherichia coli for membrane protein overexpression. Proc Natl Acad Sci U S A 105:14371–14376. https://doi.org/10.1073/pnas.0804090105
Woolston BM, Emerson DF, Currie DH, Stephanopoulos G (2018) Rediverting carbon flux in Clostridium ljungdahlii using CRISPR interference (CRISPRi). Metab Eng 48:243–253. https://doi.org/10.1016/j.ymben.2018.06.006
Zhang J, Liu Y-J, Cui G-Z, Cui Q (2015a) A novel arabinose-inducible genetic operation system developed for Clostridium cellulolyticum. Biotechnol Biofuels 8:36. https://doi.org/10.1186/s13068-015-0214-2
Zhang Y, Grosse-Honebrink A, Minton NP (2015b) A universal mariner transposon system for forward genetic studies in the genus Clostridium. PLoS One 10:e0122411. https://doi.org/10.1371/journal.pone.0122411
Zúñiga M, Pérez G, González-Candelas F (2002) Evolution of arginine deiminase (ADI) pathway genes. Mol Phylogenet Evol 25:429–444
Acknowledgments
We thank Eric Ransom and David Weiss for kindly providing plasmid pDSW1728. We thank Sophie Moormann, Julia Nothelfer, Judith Cammerer, and Lucille Exler for conducting enzymatic assays. The provision of the theophylline riboswitch sequence by Gerd Seibold and Christian Rückert is gratefully acknowledged. We thank the reviewers of this paper for constructive criticism and numerous helpful comments.
Funding
This study was funded by the ERA-IB5 program (project CO2CHEM, grant 031A566A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matthias H. Beck declares that he has no conflict of interest. Maximilian Flaiz declares that he has no conflict of interest. Frank R. Bengelsdorf declares that he has no conflict of interest. Peter Dürre declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 519 kb)
Rights and permissions
About this article
Cite this article
Beck, M.H., Flaiz, M., Bengelsdorf, F.R. et al. Induced heterologous expression of the arginine deiminase pathway promotes growth advantages in the strict anaerobe Acetobacterium woodii. Appl Microbiol Biotechnol 104, 687–699 (2020). https://doi.org/10.1007/s00253-019-10248-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10248-9