Abstract
Sophorolipids (SLs), mainly synthesized by yeasts, were a sort of biosurfactant with the highest fermentation level at present. In recent years, SLs have drawn extensive attention for their excellent physiochemical properties and physiological activities. Besides, issues such as economics, sustainability, and use of renewable resources also stimulate the shift from chemical surfactants towards green or microbial-derived biosurfactants. SLs’ large-scale production and application were restricted by the relatively high production costs. Currently, waste streams from agriculture, food and oil refining industries, etc., have been exploited as low-cost renewable substrates for SL production. Advanced cultivation method, uncommonly used substrates, and new genetically modified SL-producing mutants were also designed and applied to improve the productivity or the special properties of SLs. In this review, a systematic and detailed description of primary and secondary metabolism pathways involved in SL biosynthesis was summarized firstly. Furthermore, based on the pathways of SL biosynthesis from different carbon substrates, we reviewed the current knowledge and advances in the exploration of cost-effective and infrequently used hydrophilic and hydrophobic substrates for large or specialized SL production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the beginning of the new millennium, the development of economical and sustainable bioprocesses replacing petrochemical-based synthesis of established products has significantly increased. Surfactants based on renewable primary products, generally called biosurfactants, are one promising substance class currently under investigation (Maneerat 2005). Sophorolipids and rhamnolipids are biosurfactants of microbial origin, which show biodegradability, low toxicity, excellent surface-active properties, and biological activities (Makkar et al. 2011; Saharan et al. 2011; Van Bogaert et al. 2007; Vatsa et al. 2010).
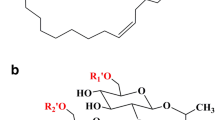
Sophorolipids (SLs), secondary metabolites mainly secreted by non-pathogenic yeasts (in contrast to rhamnolipids), are one of the most promising biosurfactants. Structurally, they are composed of a disaccharide sophorose linked by a β-glycosidic bond to a long fatty acid chain (Fig. 1). In fermentation broth, SLs are synthesized as a mixture of slightly different molecules with three major points of variation of the lactonization, acetylation pattern, and the fatty acid part (chain length, saturation, and position of hydroxylation). The different structural classes cause wide variation in physicochemical and biological properties. Nowadays, SLs have been reported to apply in fields such as agriculture, food, biomedicine, bioremediation, cosmetics, nanotechnology, and oil (Darne et al. 2016; Oliveira et al. 2015; Oliveira et al. 2014; Shah et al. 2005; Van Bogaert et al. 2007; Van Bogaert et al. 2011b; Vaughn et al. 2014).
Despite the numerous applications which SLs possess, the high costs of large-scale production of SLs are still obstacles for its economic competitiveness. Among them, the synthetic culture medium and the downstream process may attribute to 60% of the total cost of the fermentative process (Saharan et al. 2011). There are two basic strategic options available for overcoming the obstacles: (a) using low-cost substrates for culture media preparation and (b) development of efficient and optimized bioprocesses for SL production and recovery (high production with maximum recovery) (Oliveira et al. 2014). Carbon source substrates account for 10–30% of the total cost of SL production medium. SL production will be significantly high when both the hydrophilic carbon source and the hydrophobic carbon source are presented in the fermentation medium (Van Bogaert et al. 2007). Choosing cheap agricultural and industrial wastes instead of commonly used glucose and rapeseed oil/oleic acid is one of the most effective ways to reduce the cost. Additionally, the conversion of waste and renewable resources into biosurfactants and other related metabolic products through microorganisms will partly solve a wide range of liquid and solid waste disposal problems (Huaimin et al. 2018).
At present, some studies on SL fermentation have been carried out to explore SL production from different cheap or unusual substrates. Biosynthesis pathway, accumulation rate, and composition of SLs are obviously different when different carbon sources are provided. Besides, the yields of SLs are substantially diverse not only depending on the kinds of carbon substrates but also related to the methods of cultivation (batch, fed-batch, continuous culture, or solid-state fermentation). Furthermore, not all cheap substrates lead to production cost reduction effects. Hence, it is critical to investigate the relationships between SL production and composition from various substrates and SL bioconversion efficiency through different pathways.
There have been some reviews on biosynthesis, production, and application of SLs (Van Bogaert et al. 2007; Van Bogaert et al. 2011a, b). Generally, based on primary metabolism, SL-producing yeast undergoes secondary metabolism to synthesize SLs. The biosynthesis pathway of SLs had been preliminarily described in the reviews. With further discovery and identification of key enzymes in the SL biosynthesis pathway in recent years, the primary and secondary metabolism pathways involved in SL biosynthesis need to be combined. In this review, a systematic and detailed description of the SL metabolism network in yeast was provided. SL biosynthesis and factors affecting SL conversion efficiency from complex carbon substrates or sole carbon substrate were also discussed, respectively.
Furthermore, all relevant studies on the effects of carbon sources utilization, nitrogen sources, and cultivation methods on the conversion and composition of SLs were organized and summarized. Based on the pathway of SL biosynthesis from different substrates, cost-effective alternative substrates were first divided into two major categories of hydrophilic and hydrophobic, then subdivided into four categories of sugars, biodiesel co-product of glycerol, food industry wastes, and agricultural biomass wastes in hydrophilic substrates and three categories of hydrocarbons & alkanols, fatty acids, and oil & food processing industry wastes in hydrophobic substrates. Through classification discussion, the effects and mechanisms of various carbon sources on SL production and composition were compared and analyzed, which could provide favorable support for the cost-effective production of SLs by selecting suitable carbon substrates according to the production area, source of raw materials, application fields, etc.
Biosynthesis of sophorolipids
SLs are secondary metabolites secreted in the stationary phase under nitrogen limiting conditions (Davila et al. 1994; Kim et al. 2009). SL production could be strongly stimulated when both lipophilic and hydrophilic carbon sources, such as glucose and fatty acid, were present in the medium (Asmer et al. 1988). SL yield is relatively low when only one substrate is supplied in the medium (Cooper and Paddock 1984). Figure 2 shows the schematic overview of primary and secondary metabolism pathways involved in SL biosynthesis from glucose and fatty acid.
Proposed primary and secondary metabolism pathways involved in SL biosynthesis from glucose and fatty acids (Van Bogaert et al. 2013; Saerens et al.; Ciesielska et al. 2016). Abbreviations: in the fatty acid synthesis pathway: ACL: ATP-citrate lyase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthase. In the fatty acid oxidation pathway: ACS: acetyl-CoA synthetase; CPT I and CPTII: carnitine palmitoyltransferase I and II. In the TCA cycle: PDC: pyruvate dehydrogenase complex, ME: malic enzyme. In the glycolysis pathway: HK: hexokinase; PK: pyruvate kinase. In the SL synthesis pathway: PGM: phosphoglucomutase; UGPASE: UDP-glucose pyrophosphorylase; CYP52M1: cytochrome P450 monooxygenase, UGTA1 and UGTB1: UDP-glucose-dependent glycosyltransferase 1 and 2; AT: acetyltransferase; SBLE: lactone esterase
When reducing sugars, glycerol, molasses, whey, or lignocellulose are used as hydrophilic substrates, they will be converted to corresponding reducing sugar firstly and broken down into pyruvate by the glycolysis pathway. Then, pyruvate dehydrogenase catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA, which enters the Krebs cycle and provides energy and intermediate metabolites for microbial growth and metabolism. Meanwhile, part of glucose converts to activated UDP-glucose for the synthesis of glycogen and other complex carbohydrates by phosphoglucomutase (PGM) and UDP-glucose pyrophosphorylase (UGPASE), serving as the primary composition part of SLs (Oliveira et al. 2014; Saerens et al. 2015; Van Bogaert et al. 2007; Van Bogaert et al. 2011b).
For hydrophobic substrates, such as hydrocarbons, long-chain alcohols, aldehydes, oils, esterified oils, fatty acids, and fatty acid esters, they will be transformed into fatty acids by fatty aldehyde dehydrogenase (FAD) or long-chain alcohol oxidase (FAO) firstly and then enter the SL biosynthesis pathway. SLs usually have fatty acid residues with 16 or 18 carbon atoms. The modifications, as the appearance of double bond or carbon chain length, are performed by the common enzymes of fatty acid biosynthesis and do not need the specific enzymes involved in SL biosynthesis. If the medium contains no fatty acids, acetyl-CoA derived from glycolysis will convert into fatty acids by de novo synthesis. When only hydrophobic substrates are existing in the medium, part of the fatty acids will be converted to acetyl-CoA by β-oxidation for cell maintenance (Saerens et al. 2015).
When the two essential components (UDP-glucose and fatty acids) simultaneously are existing, fatty acids are converted into ω-/ω-1 hydroxylated fatty acid under the catalytic reaction of cytochrome P450 monooxygenase (CYP52M1). Then, UDP-glucose is coupled (position C1′) to the hydroxyl group of the fatty acid and generates glycolipids by glucosyltransferase I (UGTA1). In a subsequent step, a second UDP-glucose is coupled to the C2′ position of the first glucose moiety by glycosyltransferase II (UGTB1) and non-acetylated acidic SLs are formed. Further modifications are performed by acetyltransferase (AT) to obtain acetylated acidic SLs at the 6′- and/or 6″-position (Esders and Light 1972; Saerens et al. 2015). The genes involved in the SL biosynthesis pathway are found in the gene cluster (Van Bogaert et al. 2013). In addition to the genes encoding the enzymes of SL biosynthesis, the cluster contains a gene encoding SL transport protein. Finally, acidic acetylated and non-acetylated SLs are transported to the outside of cells and further catalyzed to the lactonized acetylated and non-acetylated SLs by lactone esterase (SBLE). SBLE is responsible for SL lactonization and not located in the cluster (Fig. 2) (Ciesielska et al. 2016; Waele et al. 2018; Saerens et al. 2011a, b; Saerens et al. 2015; Saerens et al. 2011c).
In summary, although carbon substrates provided in the medium are different, eventually they can be almost converted into activated UDP-glucose and long-chain fatty acids, thereby starting the biosynthesis of SLs. Therefore, it is critical to explore the pathway and conversion efficiency of different complex substrates to glucose and fatty acids. SL yield, conversion efficiency, and production cost are bound to be influenced by the supplied substrates. SL production from different renewable hydrophilic and hydrophobic substrates or raw materials reported in the literature is reviewed. The influence of nitrogen sources and cultivation methods on SLs production is also compared.
Sophorolipid production from renewable hydrophilic substrates
Sophorolipid production from various sugars without or with vegetable oils as co-substrates
Available sugars that have been reported to be used for SL production mainly include two broad categories: monosaccharides (glucose, fructose, mannose, etc.) and di- and oligosaccharides (sucrose, lactose, maltose, raffinose, etc.) (Table 1). Related research was mainly carried out to investigate and compare the biosynthesis pathway of SLs. In most cases, glucose and one of a variety of hydrophobic carbon sources are used as combined carbon sources for SL production. Moreover, glucose is usually regarded as the reference for comparing the substitution effects of different hydrophilic substrates.
When glucose is supplied as a sole precursor, microbes absorb glucose from the culture medium firstly and then break them into pyruvate by Embden-Meyerhof pathway (EMP); some part of pyruvate is converted into new glucose molecules through gluconeogenesis. The other part of pyruvate produces acetyl CoA under the action of pyruvate dehydrogenase. Part of acetyl CoA enters the tricarboxylic acid (TCA) cycle, providing energy for the growth and primary metabolism of microbes, while others are converted into fatty acids by de novo synthesis. When other monosaccharides (non-glucose) are used as the carbon source, the biosynthesis pathway of SLs is the same as glucose. However, due to the way and rate of substrate entering the glycolysis pathway, the accumulation rate of SLs is relatively slow. The EMP is usually as glucose → glucose-6-phosphate → fructose-6-phosphate → fructose 1,6-bisphosphate → 3-phosphoglyceraldehyde → dihydroxyacetone phosphate → 1,3-diphosphoglyceric acid → D-3-phosphoglyceric acid + ATP → D-2-phosphoglyceric acid → phosphoenolpyruvic acid → pyruvate + ATP. Taking fructose, galactose, and mannose for example, their EMP is (1) D-fructose→Fructose-1-phosphate → glyceraldehyde → glyceraldehyde-3-phosphate + dihydroxyacetone phosphate; (2) D-galactose → galactose-1-phosphate → glucose-1-phosphate → glucose-6-phosphate; and (3) D-mannose → mannose-6-phosphate → fructose-6-phosphate, respectively. Thus, monosaccharides are firstly converted to glycolysis intermediates to enter the glycolysis pathway. Catalytic rates and efficiency of intermediate production affect the accumulation rate of SLs by affecting the efficiency of entering glycolysis. When di- and trisaccharides are used as carbon sources, it is necessary that, by enzymatic hydrolysis, they are converted into the corresponding monosaccharides which enables them to participate in SL synthesis. Due to different types of reducing sugars, the pathways and steps of converting them into new glucose molecules are different accordingly; the accumulation rates of SLs are not the same according to the pyruvate production efficiency.
Types of reducing sugars basically do not affect the structure of SLs. On the one hand, different types of sugars enter the glycolysis pathway by converting into an intermediate of pyruvate through the EMP pathway. Pyruvate can be considered as the starting point of converting to activated glucose and/or fatty acids. On the other hand, most of the hydrophilic carbon sources added to the medium are used for microbial growth and primary metabolism; only part of glucose is transformed into UDP-glucose and incorporated with fatty acid moieties (Hommel et al. 1994; Saerens et al. 2015; Van Bogaert et al. 2008).
As shown in Table 1, Göbbert et al. (1984) first described that glucose was the most suitable sugar for SL synthesis and monosaccharide was more favorable than trisaccharide and disaccharide for SL accumulation. Growing cell culture and resting cell culture basically did not affect the yield and chemical structure of SLs. The energy gained from glucose metabolism of resting cells was high enough to synthesis SLs over a long period. SL biotransformation studies using 13C-labeled D-glucose as the sole carbon source by Hommel et al. (1994) revealed that the glucose moiety of sophorose was synthesized de novo, and this explained why the replacement of sophorose moiety by different sugars failed. However, when mixed substrates like glucose and hexadecane were used, part of the added glucose would be directly incorporated into the sophorose moiety of SLs. Klekner et al. (1991) reported that high yeast extract (YE) concentration can significantly damage the production SLs and change the composition of crude SLs, whether in glucose-based medium or sucrose-based medium. They also demonstrated that cultivation carried out in a fermentor or supplied with more air had a greater demand for nitrogen source and a higher carbon substrate(s) conversion capacity than in a flask. Lactose without or with vegetable oils also was explored for SL production. Zhou and Kosaric (1993) revealed that T. bombicola did not grow when only lactose was provided, suggesting T. bombicola lacking the lactose transport systems or lactase. SLs could be synthesized in the presence of both lactose and olive oil suggesting that oil had an effect in enhancing either the lactose transport systems or inducing lactase or both. They also found that lactose in low concentrations (less than 4.0 %) could promote the biosynthesis of SLs. Glucose with canola oil was the optimum carbon composition, and the maximum SL production of 160 g/L was obtained in a 1-L fermentor. Compared with 80% of SL conversion from glucose with canola oil, only 45% of SL conversion from canola oil with lactose was achieved (Zhou 1995). In this period, reducing production costs is not the primary research goal. However, through testing SL production from different sugars without or with vegetable oils by Torulopsis bombicola, researchers proved that glucose could be converted into SLs with the highest conversion efficiency.
Sophorolipid production from co-products of glycerol without or with hydrophobic substrates as co-substrates
Biodiesel is generally produced from soybean, sunflower, coconut, palm, and rapeseed oil by transesterification with methanol or ethanol. Glycerol is the major inevitable by-product of biodiesel and does not find many applications compared with pure glycerol (Koganti 2012). Such low-priced glycerol was explored as an alternative carbon source to reduce SL production costs (Table 2).
Due to the higher osmotic stress created by pure glycerol and the lack of fatty acid source, only a few SLs could be produced when pure glycerol was used as the sole carbon source (Solaiman 2005; Konishi et al. 2018). Just like glucose, the addition of fatty acid esters, vegetable oils, or fatty acids could significantly enhance SL production from pure glycerol (Ashby et al. 2006; Bajaj and Annapure 2015). By comparing cell growth and SL production by Candida bombicola from glucose or biodiesel glycerol (88% pure) with soybean oil, Koganti (2012) firstly confirmed that biodiesel glycerol almost showed no inhibitory effect on the cell growth and SL production. Bajaj and Annapure (2015) investigated SL production from glycerol and ricinoleic acid (RA)–rich castor oil. Glycerol with castor oil resulted in lower SL yield than glycerol with oleic acid due to the bactericidal and fungicidal properties of RA. Besides, they found that castor oil gave SLs with novel structures by hydroxylating RA at the ω-1 position but incorporating into SLs through the already available hydroxyl group at the 12th position. Recently, Konishi et al. (2018) also utilized waste glycerol to selectively produce acid-form SLs. By combined with alkyl C18 esters, which obtained on-site from oleo-chemical industries, a final acid-form SL production of 169.0 g/L was acquired with high-concentration cultivation in a 2-L jar fermentor using the fed-batch cultivation technique. Under this condition, the highest SL Yp/s of 56.3% and SL productivity Pv of 0.939 g/L/h from glycerol were obtained by Candida floricola ZM1502. Redox balance in the cell and glucose-caused catabolite repression of fatty acid assimilation were provided as reasons for the hydrophobic substrates and glycerol which are preferable over glucose for efficient acid-form SL production. Besides biodiesel, glycerol is readily available at a lower cost from commercial fat-splitting plants as sweetwater (14.4% of glycerol). Without any preconcentration or purification treatment, Starmerella bombicola could grow on sweetwater and give a comparable SL yield with pure glycerol. These works indicated that glucose can be replaced by a biodiesel co-product of glycerol and further be replaced by the more cost-effective sweetwater.
Sophorolipid production from hydrophilic food industry wastes without or with hydrophobic substrates as co-substrates
Large amounts of wastes, both liquid and solid, are generated during the process of food production, preparation, and consumption. Food waste management in an environmentally sustainable manner has become an urgent problem for all the food industries. Reusing and recycling food industry wastes and treating wastes for value-added product production can decrease the cost of food consumption and minimize pollution hazards. The by-products from food industries are not in a real sense of wastes, but are sources of sugar, minerals, dietary fiber, and bioactive compounds and could be used for SL production. Among them, sugars are of the utmost importance.
Sophorolipid production from whey with hydrophobic substrates as co-substrates
Cheese whey is a by-product of the cheese industry. After the production of most cheeses, about 50% of milk solid remains in the whey, including most of the lactose and lactalbumin. The rising cost of lactose disposal and cost reduction of SL production encourage studies on cost-effective SL production from whey (Table 3).
The study of Zhou and Kosaric (1993) first demonstrated the possibility of using cheese whey for SL production. However, T. bombicola could hardly survive when cheese whey was the sole carbon source. Only a few SLs could be obtained by the addition of olive oil to cheese whey; even lactose was consumed quickly. However, a high production of 280.0 g/L SLs was obtained from deproteinized whey concentrate (DWC-20) with repeated feeding of rapeseed oil without lactose consuming in Daniel et al.’s (1998a) work. They assumed that cell growth and SL production only relied on rapeseed oil. High lipase activity and no β-galactosidase activity detected in the crude cell extract supported the assumption that the gluconeogenesis pathway would be employed when glycerol and fatty acids from rapeseed oil were used. The high SL yield obtained was because lactose was not consumed and only lipidic substrate in the medium was available. It was considered that these results accord with the work of Asmer et al. (1988) who showed that the combination of glucidic and lipidic substrates led to lower SL production compared with the lipidic/lipidic combination. Then, they developed a two-step batch cultivation process for SL production to lower the lactose content and biological oxygen demand simultaneously by cultivating Cryptococcus curvatus ATCC 20509 and C. bombicola ATCC 22214. However, due to the unfavorable C/N ratio, only 12.0 g/L of SLs was obtained (Daniel et al. 1999). Subsequently, they described a two-stage fed-batch process to overcome low SL output by feeding cheap rapeseed oil during the production phase. With the great advantages of total lactose consumption and distinct reduction of the COD value, the highest SL production of 422.0 g/L with a Pv of 1.029 g/L/h was obtained in a 3-L fermentor (Daniel et al. 1998b). Achlesh and Kannan (2010b) also investigated SL production from deproteinized whey, glucose, and oleic acid by C. bombicola in a 3-L bioreactor with or without pH control. However, the maximum SL production and Pv values were only 33.3 g/L and 0.172 g/L/h, respectively, far away from the data reported above. Although whey has been successfully utilized as the hydrophilic substrate for SL production, more studies are still needed to overcome the batch variability problems.
Sophorolipid production from molasses without or with hydrophobic substrates as co-substrates
Soy molasses, containing about 30% of fermentable carbohydrate, is a by-product of soybean oil processing. The major soluble carbohydrate components of molasses are glucose, arabinose, sucrose, raffinose, stachyose, and other oligosaccharides (Makkar et al. 2011). Molasses from refining sugarcane or sugar beets into sugar are composed of water, carbohydrates, vitamin B6, and several dietary minerals but do not contain protein or fat. The main components of molasses make it suitable for being used as ingredients for the economical production of SLs (Table 4).
Solaiman et al. (2004) was the first to employ soy molasses for SL production in a fed-batch fermentor. Then, they demonstrated the applicability of the low-cost soy molasses as combined nitrogen and carbon sources with oleic acid for SL production (Solaiman et al. 2007). Besides, 97% and 87% of the obtained SLs from soy molasses and oleic acid were in lactone form, which suggested that soy molasses was beneficial to produce lactonic SLs. Achlesh and Kannan (2009) reported the production of SLs from a cheap fermentative medium containing sugarcane molasses, yeast extract, urea, and soybean oil in both flask and bioreactor by C. bombicola. SL production showed a trend of initial increase and then decrease in the bioreactor because of the substrate limitation. The maximum SLs of 63.7 g/L and Pv of 0.531 g/L/h could be achieved after 120 h of cultivation. Daverey and Pakshirajan (2009) optimized the sugarcane molasses and soybean oil concentrations along with physical parameters of temperature, agitation, inoculum size, inoculum age, and pH control to enhance SL production. They also found that costly glucose and nitrogen sources of yeast extract and urea could be replaced by sugarcane molasses, almost without the decrease in SL production. Makoto et al. (2011) investigated the biosurfactant-producing capability of 15 yeast strains by cultivating them in the medium consisting of only sugarcane molasses and water. The results showed that only S. bombicola NBRC 10243 could excrete biosurfactant of SLs from the sole sugarcane molasses medium. Moreover, the feeding of the molasses in the fermentor could significantly increase the production of SLs. Minucelli et al. (2016) reported that only relatively low SL yields could be obtained from sugarcane molasses or sugarcane juice as the hydrophilic source and chicken fat or sunflower oil as the hydrophobic source. They considered that low concentrations of glucose (4%) in sugarcane molasses and sugarcane juice resulted in the less efficient production of SLs. At low glucose concentration, part of the fatty acids available is targeted for cell maintenance but not for biosurfactant synthesis (Van Bogaert et al. 2007). For economic reasons and the verification of the capabilities of the organism of C. bombicola, low market honey was selected for SLs production by Pekin et al. (2005). They designed a special two-stage fed-batch cultivation, and eventually an SL concentration of above 400.0 g/L with the highest Pv of 0.917 g/L/h from molasses was obtained in a 3-L bioreactor. SLs are conventionally largely produced from glucose and oleic acid by submerged fermentation (SmF). Recently, Jiménez-Peñalver et al. (2018) demonstrated an alternative fermentation approach of solid-state fermentation (SSF) to produce SLs with stearic acid (C18:0) and sugar beet molasses. During this SSF process, the media cost was reduced by replacing glucose and nitrogen source with sugar beet molasses and the problems of foaming and high viscosity were avoided. Interestingly, the produced SLs by SSF from sugar beet molasses also were mainly composed of lactonic SLs. The studies mentioned above confirmed the potential ability of molasses and related substrates for SL production by different cultivation methods. However, it still requires the interdisciplinary effects and research to make mass production of SLs to be full realization.
Sophorolipid production from hydrophilic agricultural biomass wastes without or with hydrophobic substrates as co-substrates
Population growth and living standard improvement lead to intensive agriculture, which in turn leads to a rapid increase in the amount and types of agricultural biomass wastes. Management of agricultural biomass wastes from wheat, rice, corn, sorghum, etc., are contributing towards both environmental protection and economic benefits. Some studies on converting agricultural biomass wastes to the cost-effective product of SLs have been carried out (Table 5).
Ma et al. (2014) demonstrated a conversion process from lignocellulosic material of delignined corncob residue (DCCR) to SLs by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 (now known as S. bombicola CGMCC 1576) and C. curvatus ATCC 96219 for the first time. In the work, glucose, oleic acid, and yeast extract were replaced by delignined corncob residue hydrolysate (DCCRH), single cell oil (SCO) and single cell protein (SCP), respectively. The introduction of DCCR for SL production broadened the range of utilization of low-cost substrates and helped to promote SL fermentation on a large scale. Due to the fact that lignocellulosic material pretreatment will produce wastewater and accordingly increase the total production cost of SLs, Masaaki et al. (2015) developed a simple process for lignocellulosic biomass saccharification and an effective cultivation protocol to increase the cost efficiency of SL production. Under optimal conditions, a relatively high SL production of 49.2 g/L with the highest Pv of 0.513 g/L/h was obtained from the corncob hydrolysate (CCH) medium by batch cultivation in a 1-L fermentor. Subsequently, Samad et al. (2015) reported SL production on hydrolysates derived from sweet sorghum bagasse and corn fiber. The results demonstrated that sorghum bagasse gave a higher yield of SLs than corn fiber. The highest SL production of 84.6 g/L with a SL yield YP/S of 0.423 g/g was achieved from sorghum bagasse hydrolysates with the addition of soybean oil. In keeping with the results of Ma et al. (2014), they also found that the addition of yeast extract to hydrolysate medium only led to slightly better cell growth but no promotion to SL production (Samad 2015). By employing a novel pretreatment method of the SO3 microthermal explosion, Liu et al. (2016) further deceased the production of wastewater and increased the yield of SLs. The highest SL yield of 53.7 g/L with the highest YP/S of 0.448 g/g was acquired under the YE concentration of 0.15% in flask by W. domercqiae. The yeast could also survive and produce a considerable amount of SLs even when there is no extra nitrogen source added to the hydrolysate medium due to the existing residual cellulase used in the hydrolysis process. Recently, Samad et al. (2017) reported SL production on bagasse hydrolysate combined with yellow grease and corn stover hydrolysate combined with soybean oil. Among them, bagasse hydrolysate was derived from a simple acid pretreatment and corn stover hydrolysate was developed from an extensive alkaline-based pretreatment procedure.
As mentioned above, lignocellulosic-rich agricultural residues could be employed for the cost-effective production of SLs. SLs are mainly achieved by hydrolyzing lignocellulose with cellulase to obtain glucose-rich hydrolysate to replace glucose for yeast growth and SL production. However, several problems still exist in lignocellulose utilization: (1) the sources of different lignocellulosic materials are greatly affected by seasons and regions. (2) All of the lignocellulosic biomass is rich in lignin, which needs to be deprived before use to improve saccharification efficiency. Raw lignocellulosic biomass pretreatment increases the production cost of alternative carbon substrates. (3) To seeking for higher SL productivity, hydrolysates need to be detoxified to reduce the inhibitor and improve the utilization of fermentable sugars, which in turn increases the production cost of SLs to a certain extent. Hence, many aspects should be taken into consideration before SL industrial production using these biomass conversion processes.
Sophorolipid production from diverse hydrophobic substrates
Sophorolipid production from alkanes, alkanols, and alkanones without or with hydrophilic substrates as co-substrates
Alkane, alkanol, and alkyl ketone, especially with the carbon chain length from 12 to 20, had been reported as the hydrocarbon and alkanol stocks for SL production. The use of unconventional hydrophobic carbon sources could help to obtain some novel glycolipids. In the biosynthesis pathway of SLs, glucose connected to fatty acid at the ultimate or penultimate carbon through the action of cytochrome P450 and it is this step that determines the range of C16 and C18 aliphatic chains which are readily converted into SLs.
In the biosynthesis pathway of SLs from hydrocarbons, hydrocarbons in the range of C16 and C18 are firstly converted into fatty acids under the catalysis of the aldehyde dehydrogenase/fatty alcohol oxidase (ALDH/FAO). Then, the newly formed fatty acids are converted to hydroxy fatty acids by cytochrome P450 monooxygenase, and directly used for SL production. Fatty acid chains that fall short of this C16 and C18 range will be used as the energy source for cell growth and primary metabolism through β-oxidation and gluconeogenesis. Longer fatty acid chains will be shortened until they reached an adequate length. Hence, new-to-nature SLs could result if a lipophilic substrate has already been suitably oxidized. With a view from this aspect, different hydrocarbons and alkanols were mainly applied for novel SL production (Table 6).
To investigate whether sugar or hydrocarbons determine the hydrophilic moieties of SLs or not, Göbbert et al. (1984) incubated the resting cells with octadecane/paraffin S in a buffer medium. However, the results showed that both the sugar and hydrocarbon did not influence either the composition of the hydrophilic nor the lipophilic moiety of SLs. Davila et al. (1994) determined the influence of the carbon number of long-chain n-alkane on SL production. The results showed that SL production increased with the number of carbons of n-alkane tested. Fermentations on C16 and C18 alkanes were several-fold higher than those on C12 and C14 alkanes. Lower SL performances of C12 and C14 alkanes were accounted to the requirement of alteration into hydroxy acid moiety before their incorporation. Contrasting to SL production from hydrocarbon with resting cells (Göbbert et al. 1984), lipidic compositions of SLs obtained from alkanes were largely influenced by the nature of lipidic precursors with SmF (Tulloch et al. 1962). SL products were discriminated according to their carbon number, unsaturation degree, and hydroxy group location. Only hydrophobic substrates of C16 and C18 alkanes could be incorporated in hydroxy acid moieties without changing the length of the carbon chain. A large portion of hydrophobic substrates with shorter carbon chains would be extended by 2, 4, or 6 carbons before incorporated. The site specificity of hydroxylation (terminal or subterminal) was also influenced by the length of the fatty acid chain. The higher the number of the carbon chain, the lower the terminal-hydroxylation ratio.
Studies on the successful production of SLs with high structural diversity by wild strains are rare. Only Jones (1968) showed that Torulopsis gropengiesseri could synthesize glycolipids with mono- and dihydroxy alkane components under the consumption of glucose and 2-alkanols/2-acetoxy alkanes/methyl-branched alkanes. However, the chain length of the obtained SLs was not shorter than 16 C atoms. To produce acid-free and short-chain SLs, Brakemeier et al. (1995) investigated the production and types of SLs using glucose and 2-alkanols with 12, 14, and 16 carbon atoms by C. bombicola. Compared with glucose as the sole carbon source, 2-alkanols could significantly increase SL production and slightly inhibit yeast growth. Moreover, 2-alkanol was found as the major hydrophobic moiety (>75%) of the three newly formed acid-free SLs differing only in acetylation degree. Besides, additional monooxygenation of the alcohol led to the incorporation of 2,(ω-1)-alkandiol and glycolipids with up to four glucose units were obtained. Brakemeier et al. (1998a) reported the use of 2-dodecanol for novel glycolipid production. Due to the fact that one part of the racemic secondary alcohol was directly connected with glucose or sophorose unit, 22.0 g/L of novel alkyl glycolipids containing glycosidically/esterically bound ω- or (ω-1)-hydroxy C16 or 18 fatty acid was successfully obtained. Because of the high cost of secondary alcohols, Brakemeier et al. (1998b) continued their studies by employing primary alcohol and some alkanones for novel types of SL production. The results showed that the primary alcohol of 1-dodecanol was directly transformed into glycolipids and novel surface-active SLs with glycosidically linked primary or secondary fatty alcohols were secreted. Cavalero and Cooper (2003) investigated the effects of alkane substrates on the structure and physical state of SLs by C. bombicola. When alkanes and glucose were used as carbon sources, SL yields increased with chain lengths increased from 12 to 16 and then decreased with chain lengths increased from 17 to 20. They also found that the amount of direct incorporation increased with increasing chain length to a maximum for pentadecane, hexadecane, and heptadecane. As the length of the alkane substrates increased further, the amount of direct incorporation then decreased until there was no apparent incorporation for eicosane. SL production was also carried out from lauryl alcohol C12-14 and glucose by C. bombicola (Dengle-Pulate et al. 2014). The primary characterization of the obtained SLs depicted the presence of alkyl sophorosides/SLs. Moeover, the antimicrobial activity of these SLs is remarkably better compared with SLs produced from oleic acid or linolenic acid.
As mentioned above, the naturally occurred SLs synthesized by C. bombicola possess little variation in the length of the lipid tails. To obtain novel SLs with shorter chain lengths to improve their water solubility and increase the range of their applications, Van Bogaert et al. (2010b) blocked the β-oxidation pathway of SL synthesis on the genome level by knocking out the multifunctional enzyme type 2 (MFE-2) gene. Several knockout mutants with the correct genotype and phenotype were obtained and evaluated with fermentation on 1-dodecanol. They reported that clearly better SL yields of 2.2~3.1 times higher than wild type for all mutant strains were obtained. The best-performing MFE-2 negative C. bombicola, designated as C. bombicola M30, was selected to convert 1-dodecanol into medium-chained SLs, subsequently. However, only 15.0 g/L of medium-chained SLs was obtained from 1-dodecanol by this β-oxidation-deficient strain (Fleurackers et al. 2010). Van Bogaert et al. (2011a) described two strategies to break the limitation of C16–C18 fatty acids of SLs. One was avoiding the controlling effect of the P450 enzyme by adding already hydroxylated substrates. The other was employing a hydrophobic substrate with a stearic acid–like structure or chain length, which could be hydroxylated by cytochrome P450 and incorporated into SL molecules. The results demonstrated that 1,12-dodecanediol could be utilized for medium-chain SL production successfully. Due to the symmetric character of 1,12-dodecanediol, glycolipids with sophorose units introduced at both sites were also obtained. The use of unconventional stearic acid–like substrates opened perspectives of the production of new-to-nature glycolipids. Blocking the β-oxidation pathway could also achieve higher production of medium-chain length SLs (Van Bogaert et al. 2010b). In the S. bombicola genome, FAO1 plays a major role in the long-chain alcohol oxidation pathway. Takahashi et al. (2016) reported that the KSM-fao1Δ strain with disruption of the FAO gene could efficiently produce novel glycolipids from primary alcohols of 1-tetradecanol. The deletion of FAO1 could improve the production of tetradecanol-based SLs and tetradecanediol-based SLs from 0 to 16.9 and 46.2%, respectively.
The main purposes of these studies are altering the structures of SLs, rather than to increase SL production or reduce costs. Both strategies of enzyme-targeted substrate supplement and gene recombination have been successfully applied for the production of SLs in special structure. However, more works are required to overcome the problems associated with productivity increase and cost reduction.
Sophorolipid production from fatty acids without or with hydrophilic substrates as co-substrates
Fatty acids, including oils, esterified oils, fats, and fatty acid esters, are defined as feedstocks carrying or containing a structure of fatty acid. Depending on the length of the carbon chain, fatty acids are divided into short-chain, medium-chain, and long-chain fatty acids. As the secondary carbon source, fatty acid plays an important role in the pathway of SL biosynthesis. When the fatty acid is used as the sole carbon source, partial fatty acid enters the Krebs cycle to synthesize glucose, providing energy for biological metabolism and performing as the hydrophilic substrate for SL biosynthesis. Another part of fatty acid is converted to ω-/ω-1 hydroxy fatty acid under the catalytic of cytochrome P450 monooxygenase, providing a direct hydrophobic substrate for SL biosynthesis. Similarly, the lengths of fatty acids in natural formed SLs mainly are 16 and 18. Fatty acids with longer carbon chains or branched fatty acids are degraded by β-oxidation, while shorter fatty acids are extended to 16 or 18 carbon atoms for the effective synthesis of SLs (Felse et al. 2007).
The reported renewable fatty acids used for SL production mainly divided into plant oils, animal fats, and fatty acid esters. Safflower oil, corn oil, soybean oil, sunflower oil, rapeseed oil, palm oil, coconut oil, grape seed oil, olive oil, linseed oil, germ oil, jatropha oil, karanja oil, neem oil, meadowfoam oil, and other vegetable oils had been applied for SL fermentation. Fatty acid esters, especially esters of plant oils, like stearic acid methyl stearate, sunflower fatty acid methyl ester, palm oil fatty acid methyl ester, linseed fatty acid methyl ester, soybean oil fatty acid methyl ester, rapeseed fatty acid ethyl ester, soybean oil fatty acid ethyl ester, and soybean oil fatty acid propyl ester, were also developed for higher production of SLs. Besides, animal fats such as fish oil, beef tallow, chicken fat, and other animal oils were also employed for SL biosynthesis (Table 7).
Cooper and Paddock (1984) investigated the influence of carbohydrates and vegetable oils on the production of SLs by T. bombicola. They considered that both the yield and composition of SLs would not be essentially affected by various vegetable oils as the sole carbon source, just as the results reported by Göbbert et al. (1984). However, when two carbon sources are provided in the medium, the composition of the two crude SL products was different. Interestingly, they found that T. bombicola cultivated in oleic acid alone led to a significantly higher yield than that from glucose and oleic acid (Asmer et al. 1988). Davila et al. (1994) reported that oils or esters rich in C18:1 and C18:0 fatty acids contributed better SL production performances. In the cases of rapeseed, sunflower, and palm oils, enhanced productions were obtained from their esters because the esters were easily hydrolyzed. Ethyl/methyl esters of rapeseed oil were beneficial to the high yield of SLs than fatty acids or oils by C. bombicola. SL production values of 320, 340, and 317 g/L with high Pv values of 1.684, 2.031, and 1.921 g/L/h were obtained from ethyl/methyl esters of rapeseed oil with glucose by cultivation of fed-batch, continuous fed-batch, and two-step process fed-batch in a 4-L fermentor, respectively (Davila et al. 1992; Davila et al. 19924; Davila et al. 1997). Additionally, oil-based SLs always exhibited a higher level of diacetylated lactones than that from corresponding esters. Moreover, SLs obtained from polyunsaturated fatty acid–predominated sunflower oil and linseed oil contained increased levels of acidic classes of SLs while SLs from stearic acid or oleic acid were lactonic class–predominated (Davila et al. 1994). Furthermore, they found that the substrate feeding condition markedly affected the acetylation extent of sophorose and distribution of the acidic and lactonic forms (Davila et al. 1997). Besides esters of rapeseed oil, refined rapeseed oil was also conductive for SL production. SL production of more than 300.0 g/L and increased productivities of 2.375 g/L/h (feed-batch) from rapeseed oil and glucose were obtained with C. bombicola (Rau et al. 2001). They attributed the highly efficient conversion of rapeseed oil and glucose carbon into SL carbon to resting cell conditions, ATP, and/or de novo synthesis of SLs by glycerol uptake and the ability of the cells to convert non-oleic fatty acids into primary C18:0 and C18:1 fatty acid (Rau et al. 1996).
SL production could be substantially changed depending on not only the kinds of carbon substrates but also the culture methods. Attributing to fed-batch culture in the fermentor was more beneficial than batch culture for input carbon channeling to the product rather than CO2, fed-batch was frequently used to enhance SL production (Solaiman et al. 2007; Nuñez et al. 2001). Lee and Kim (1993) reported that SL production was remarkably increased from 80.0 g/L (0.37 g/g substrate) in batch culture to 120.0 g/L (0.60 g/g substrate) from soybean oil and glucose in fed-batch culture. Moreover, SL production of 180.0 g/L with the highest Yp/s of 0.922 g/g was achieved from oleic acid and glucose in an extended fed-batch cultivation (Rau et al. 1996). Kim et al. (1997) applied the continuous culture for SL fermentation from soybean oil and glucose. They found that the specific consumption rate of soybean oil was closely related to the specific production rate of SLs and a high concentration of soybean oil performed an inhibiting effect on SL production. This phenomenon was explained as a high concentration of soybean oil in the medium which repressed NADPH production, reduced the synthesis of hydroxy fatty acid, and consequently decreased SL production. Furthermore, when applying the results of feed-batch cultivation to the new two-stage N-limited continuous process in a 40-L bioreactor, SL productivity was further increased to 3.167 g/L/h from oleic acid combined with glucose (Rau et al. 2001). To simplify the fermentation control strategy, Kim et al. (2009) developed a novel feeding rate–controlled fed-batch culture for SL production. The feeding rate of rapeseed oil was dependent on pH and calculated by NaOH and rapeseed oil consumption rate. Finally, up to 365.0 g/L with Yp/s of 1.901 g/g of crude SLs was produced from a 2.5-L fermentor.
Due to a significantly higher oxygen transfer rate in a stirred tank bioreactor than in shaken flask, shake flask cultures were not as productive as fermentor cultures (Casas and Garcia-Ochoa 1999). However, they were essential for culture optimization and structure-property studies (Vedaraman and Venkatesh 2010; Minucelli et al. 2016). Based on the oxygen transfer rate, Guilmanov et al. (2002) developed a fed-batch shake flask method for the efficient production of SLs by C. bombicola. Maximum values for both volumetric product formation of 1–1.5 g/L/h and SL yield of 350.0 g/L were resulted in at optimal oxygen transfer rate between 50 and 80 mM O2/L/h. Moreover, the fatty acid unsaturation degree of SLs could be controlled by adjusting oxygenation conditions at the initial fermentation period.
We can clearly see that vegetable oils like rapeseed oil, soybean oil, and sunflower oil are commonly used second carbon sources for SL fermentation. In recent years, some non-traditional oils like coconut oil, meadowfoam oil, α-linolenic acid, fish oil, and ricinoleic acid (RA)–rich castor oil had also been employed as newer feedstocks not for SL production enhancement, but for novel SL fermentative production (Bhangale et al. 2014; Gupta and Prabhune 2012; Li et al. 2013; Van Bogaert et al. 2010a). Oils with medium-chain fatty acids containing coconut oil and very-long-chain fatty acids containing meadowfoam oil did not contribute to enhancing SL production and have a toxic effect on stationary C. bombicola cells. Besides, the fatty acid composition of the meadowfoam-based SLs was like the one observed for de novo SLs (Van Bogaert et al. 2010a). Gupta and Prabhune (2012) cultivated C. bombicola in MGYP media containing α-linolenic acid (ALA) and glucose for novel SL production. Although only 4.0 g/L of SLs was produced, three different forms of C18:3 SL molecules, free acid, lactone, and a diacetylated lactone, were reported firstly. Due to the fact that fish oil is rich in long-chain polyunsaturated fatty acid components of docosahexaenoic acid (DHA; 22:6) and eicosapentaenoic acid (EPA; 20:5), Li et al. (2013) used fish oil to produce SLs with long-chain hydroxy fatty acids. Structural analysis results displayed that several unconventional acidic and lactonic SL molecules with EPA, DHA, 22:3, or 20:0 and different acetylation degrees were obtained. Castor oil contains 80–90% of RA, which is a mono-unsaturated fatty acid with 18 carbon atoms and a hydroxyl functional group in the 12th position. The presence of the hydroxyl group in RA makes the production of certain novel SLs possible. Structure analysis of castor oil–produced SLs showed that RA was incorporated into SLs at the ω-6 position, without the oleic acid incorporation requiring the step of ω-1 oxygenation (Bhangale et al. 2014).
Uncommon regional vegetable oils like jatropha oil, karanja oil, and neem oil, and crude oils like tapis oil, melita oil, and ratawi oil were also employed to reduce SL production cost and improve certain specific properties of SLs. Although the yields of SLs were relatively low, the physicochemical properties such as surfactant property, emulsification activity, and emulsion stability and biological properties such as antibacterial action and stain removal capability of the newly formed SLs were improved to some extent (Wadekar et al. 2012b; Shah et al. 2017; Imura et al. 2013).
Studies on the effects of fatty acids or esters alone or mixed with other carbon sources on SL fermentation arouse wide concern. Although the influences of medium composition, cultivation method, and oxygen transfer rate on production and composition of SLs have been illustrated in detail, the researchers should keep looking at more economic substrates and processes for industrial applications.
Sophorolipid production from oil and food processing industry wastes without or with hydrophilic substrates as co-substrates
The cost of the raw material makes up 40–50% of the whole expense of SL production. The selection and development of cheaper raw materials is an effective way to reduce the entire production cost. Currently, waste frying oil, soybean dark oil, industrial fatty acid waste, motor oil waste, etc., had been involved as hydrophobic substrates to replace the conventionally used hydrophobic substrates for SLs production (Table 8).
Soybean dark oil, an inedible oil with black color, is a by-product of vegetable oil processing. Like soybean oil and corn oil, oleic acid is the main fatty acid contained in soybean dark oil. The price is less than half the price of other oils; hence, SL production from soybean dark oil by the yeast C. bombicola was examined (Kim et al. 2005; Kim et al. 2009). The results showed that a competitive production of 90.0 g/L with a Pv value of 0.536 g/L/h of SL was obtained from soybean dark oil and glucose. Besides, the derived SLs showed excellent antimicrobial activity and could be used in health care products as an antimicrobial agent. Thousands of gallons of cooking oil are used each week in restaurants around the world. With the availability of this huge amount of low-cost raw material of waste cooking oil, SL production from these wastes was designed (Fleurackers 2006; Felse et al. 2007; Shah et al. 2007). Although using waste cooking oil for SL production looks promising, there are still some difficulties existing such as indirect use of waste frying oil as the raw material. Degradation products contained in the used frying oil can interfere in the overall primary and secondary metabolism of SL production. Wadekar et al. (2012a) reported that SL production from waste frying oils was lower than the fresh oils and activated earth treatment of fried oils was found to improve SL yields significantly. Besides, attributing to crude SLs obtained from frying sunflower oil containing 70–80% of acidic SLs, their CMC value was lower than that from fresh sunflower oil. Combined with activated earth treatment, the presence of ultrasound and fed-batch was also studied to improve SL synthesis from waste cooking oil in a fermentor (Maddikeri et al. 2015). Attributing to the cavitation effects, 55.6 g/L of SLs (mainly in the lactonic form) was observed from waste cooking oil by fed-batch cultivation assisted with ultrasound. The compositions and proportions of industrial fatty acid residues are more complicated. In addition to waste fatty acids, they also contain trace contaminants like nickel, which can inhibit yeast cell growth and SL production. Felse et al. (2007) investigated the effects of industrial lipid feedstocks, nickel content and culture methods on SL production. The results showed that tallow fatty acid residue gave the highest SL production of 120.0 g/L with the highest YP/S of 0.6 g/g under fed-batch culture. SLs produced from nickel-contaminated stearic acid had sufficiently low nickel contents and could be used in the fields of industrial cleaning and oil recovery enhancing without further processing.
To further increase SL productivity and reduce cost, solid-state fermentation (SSF) is employed as an alternative technology. Using SSF for SL production presents the advantages of allowing the use of inexpensive solid substrates and avoiding potential problems associated with foaming. Mango kernel fat is obtained from mango seeds and found to have a high content of stearic and oleic acid. Oil cakes are by-products containing starch, proteins, and little amount of lipids obtained after oil extraction from oil industry processes. Both mango kernel olein and oil cakes were used as low-cost feedstocks for SL production by SSF (Parekh et al. 2012; Rashad et al. 2014a, b; Jiménez-Peñalver et al. 2016). Compared with mango kernel fat and stearin fraction of mango kernel fat, olein fractions of mango kernel fat were found to be beneficial for SL production under both SmF and SFF conditions. After extraction by ethyl acetate, 17.5 g of SLs with a conversion 17.5% was obtained from 100 g of the substrate by SSF, while SmF only resulted in a yield of 5.8 g SLs/100 g of the substrate with a conversion rate of 5.8%. Furthermore, SL production from the mango kernel olein medium by SSF was comparable to that from the oleic acid medium. It is the first report on the efficient production of SLs by SSF (Parekh et al. 2012). The highest SL yield of 0.495 g/g substrates was obtained from sunflower oil cake plus crude soybean oil by SSF when employing a new concept for extraction by methanol followed by ethyl acetate, then partially purified with hexane. Only SL yield of 0.206 g/g substrates was obtained by SmF with ethyl acetate extraction (Rashad et al. 2014a, b). Motor oil waste, a petroleum industrial waste, is considered as the worst environmental impact because it contained toxic chemicals, carcinogenic hydrocarbons, and heavy metals which harm the environment and public health. For the economic production process with a reduction in environmental pollutants, SL bioconversion from motor oil waste plus sunflower oil cake by SmF and SSF was also studied. SLs Pv of 0.132 g/g mixed substrates was obtained from SmF, while total SLs Pv of 0.458 g/g substrates was attained by adopting the abovementioned extraction technique with SSF (Rashad et al. 2014a, b). Jiménez-Peñalver et al. (2016) investigated SL production from winterization oil cake (WOC) and sugar beet molasses (MOL) by SSF. They suggested that intermittent mixing during the process can improve substrate bioavailability and reduce nutrients and biomass composition gradients in the solid mass. They also found that there were significant correlations between SL yield and oxygen & fats consumed, suggesting that the respirometer can be used to monitor the biological activity of the processes.
These studies signify that SL production from oil and food processing industry wastes with new cultivation methods could ease off the cost factor for the overall SL production with a reduction in environmental pollutants. The efficient conversion of nutrient-rich, low-value industrial and agricultural wastes or by-products to SLs is an important strategy to produce low-cost SLs which can be used in oil recovery and industrial cleaning fields.
Conclusion and recommendations
The characteristics of their being secreted by nonpathogenic yeasts, high yield, and excellent surface-lowering properties and biological activities of SLs make them an environment-friendly alternative biosurfactant for the petrochemical-based surfactants. Currently, SLs are not yet competitive with those chemical surfactants from an economic point of view. Employment of low-cost and renewable fermentation substrates, development of efficient fermentation culture methods, sustainable optimization of downstream separation, and purification and genetic engineering of SL-producing strains have been used as effective strategies to overcome the obstacle of the high production cost of SLs.
Although reducing sugar, by-products of glycerol, deproteinized whey, molasses, different straw wastes, waste fatty acids, and other inexpensive substrates have been explored for SL bioconversion, the use of cost-efficient substrates under most conditions still bring negative impacts on the yield of SLs. The optimization of the medium and culture conditions are essential and critical factors in SL fermentative processes with food and agricultural and industrial wastes as substrates. Besides, the preparation of renewable substrates and fermentation of SLs from these alternative substrates may require both chemical and biochemical processes, with the use of water, energy, and chemical or biological reagents. With respect to the above, whether low-cost substrates or waste streams indeed reduce the cost of SL production should be evaluated, and comprehensive consideration should also be given to raw material origin, cost and continuous supply, SL production efficiency, environmental effects, and other factors.
Additionally, employing different feedstocks for SL production depending on the fields of use might be another choice to make SLs more competitive with petrochemical origin surfactants. Well-defined, pure compounds can be used as a hydrophobic source without compromising the economic viability of the process to produce SLs designated towards specialty applications in fields of pharmaceutical. Inexpensive feedstocks can be employed to produce SLs used in application fields like oil recovery and industrial cleaning. However, the transition from a petroleum-based economy to a biobased economy requires the exploitation of synergies, scientific innovations, breakthroughs, continuous promotion of environmental awareness, and step changes in the infrastructure of the chemical industry.
References
Achlesh D, Kannan P (2009) Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 158:663–774
Achlesh D, Kannan P (2010a) Kinetics of growth and enhanced sophorolipids production by Candida bombicola using a low-cost fermentative medium. Appl Biochem Biotechnol 160:2090–2101
Achlesh D, Kannan P (2010b) Sophorolipids from Candida bombicola using mixed hydrophilic substrates: production, purification and characterization. Colloids Surf B Biointerfaces 79:246–253
Ashby RD, Dky S, Foglia TA (2006) The use of fatty acid esters to enhance free acid sophorolipid synthesis. Biotechnol Lett 28:253–260
Asmer HJ, Lang S, Wagner F, Wray V (1988) Microbial production, structure elucidation and bioconversion of sophorose lipids. J Am Oil Chem Soc 65:1460–1466
Bajaj VK, Annapure US (2015) Castor oil as secondary carbon source for production of sophorolipids using Starmerella bombicola NRRL Y-17069. J Oleo Sci 64:315–323
Bhangale A, Wadekar S, Kale S, Bhowmick D, Pratap A (2014) Production of sophorolipids synthesized on castor oil with glucose and glycerol by using Starmerella bombicola (ATCC 22214). Eur J Lipid Sci Technol 116:336–343
Brakemeier A, Lang S, Wullbrandt D, Merschel L, Benninghoven A, Buschmann N, Wagner F (1995) Novel sophorose lipids from microbial conversion of 2-alkanols. Biotechnol Lett 17:1183–1188
Brakemeier A, Wullbrandt D, Lang S (1998a) Candida bombicola: production of novel alkyl glycosides based on glucose/2-dodecanol. Appl Microbiol Biotechnol 50:161–166
Brakemeier A, Wullbrandt D, Lang S (1998b) Microbial alkyl-sophorosides based on 1-dodecanol or 2-, 3- or 4-dodecanones. Biotechnol Lett 20:215–218
Casas JA, Garcia-Ochoa F (1999) Sophorolipid production by Candida bombicola: medium composition and culture methods. J Biosci Bioeng 88:488–494
Cavalero DA, Cooper DG (2003) The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J Biotechnol 103:31–41
Ciesielska K, Roelants SL, Van Bogaert IN, De Waele S, Vandenberghe I, Groeneboer S, Soetaert W, Devreese B (2016) Characterization of a novel enzyme Starmerella bombicola lactone esterase (SBLE) responsible for sophorolipid lactonization. Appl Microbiol Biotechnol 100:9529–9541
Cooper DG, Paddock DA (1984) Production of a biosurfactant from Torulopsis bombicola. Appl Environ Microbiol 47:173–176
Daniel HJ, Otto RT, Reuss M, Syldatk C (1998a) Sophorolipid production with high yields on whey concentrate and rapeseed oil without consumption of lactose. Biotechnol Lett 20:805–807
Daniel HJ, Reuss M, Syldatk C (1998b) Production of sophorolipids in high concentration from deproteinized whey and rapeseed oil in two stage fed-batch process using Candida bombicola ATCC 22214 and Cryptococcus curvatus ATCC 20509. Biotechnol Lett 20:1153–1156
Daniel HJ, Otto RT, Binder M, Reuss M, Syldatk C (1999) Production of sophorolipids from whey: development of a two-stage process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 using deproteinized whey concentrates as substrates. Appl Microbiol Biotechnol 51:40–45
Darne PA, Mehta MR, Agawane SB, Prabhune AA (2016) Bioavailability studies of curcumin–sophorolipid nano-conjugates in the aqueous phase: role in the synthesis of uniform gold nanoparticles. RSC Adv 6:68504–68514
Daverey A, Pakshirajan K (2009) Production of sophorolipids by the yeast Candida bombicola using simple and low cost fermentative media. Food Res Int 42:499–504
Davila AM, Marchal R, Vandecasteele JP (1992) Kinetics and balance of a fermentation free from product inhibition: sophorose lipid production by Candida bombicola. Appl Microbiol Biotechnol 38:6–11
Davila AM, Marchal R, Vandecasteele JP (1994) Sophorose lipid production from lipidic precursors: Predictive evaluation of industrial substrates. J Ind Microbiol Biot 13:249–257
Davila AM, Marchal R, Vandecasteele JP (1997) Sophorose lipid fermentation with differentiated substrate supply for growth and production phases. Appl Microbiol Biotechnol 47:496–501
Dengle-Pulate V, Chandorkar P, Bhagwat S, Prabhune AA (2014) Antimicrobial and SEM studies of sophorolipids synthesized using lauryl alcohol. J Surfactants Deterg 17:543–552
Esders TW, Light RJ (1972) Glucosyl- and acetyltransferases involved in the biosynthesis of glycolipids from Candida bogoriensis. J Biol Chem 247:1375–1386
Felse PA, Shah V, Chan J, Rao KJ, Gross RA (2007) Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme Microb Technol 40:316–323
Fleurackers SJJ (2006) On the use of waste frying oil in the synthesis of sophorolipids. Eur J Lipid Sci Technol 108:5–12
Fleurackers SJJ, Bogaert INAV, Develter D (2010) On the production and identification of medium-chained sophorolipids. Eur J Lipid Sci Technol 112:655–662
Göbbert U, Lang S, Wagner F (1984) Sophorose lipid formation by resting cells of Torulopsis bombicola. Biotechnol Lett 6:225–230
Guilmanov V, Ballistreri A, Impallomeni G, Gross RA (2002) Oxygen transfer rate and sophorose lipid production by Candida bombicola. Biotechnol Bioeng 77:489–494
Gupta R, Prabhune AA (2012) Structural determination and chemical esterification of the sophorolipids produced by Candida bombicola grown on glucose and alpha-linolenic acid. Biotechnol Lett 34:701–707
Hommel RK, Weber L, Weiss A, Himmelreich U, Rilke O, Kleber HP (1994) Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J Biotechnol 33:147–155
Huaimin W, Roelants S, To M, Patria R, Kaur G, Sze Lau N, Yin Lau C, Van Bogaert I, Soetaert W, Lin C (2018) Starmerella bombicola: recent advances on sophorolipid production and prospects of waste stream utilization. J Chem Technol Biotechnol 94:999–1007
Imura T, Kawamura D, Morita T, Sato S, Fukuoka T, Yamagata Y, Takahashi M, Wada K, Kitamoto D (2013) Production of sophorolipids from non-edible jatropha oil by Stamerella bombicola NBRC 10243 and evaluation of their interfacial properties. J Oleo Sci 62:857–864
Jiménez-Peñalver P, Gea T, Sánchez A, Font X (2016) Production of sophorolipids from winterization oil cake by solid-state fermentation: Optimization, monitoring and effect of mixing. Biochem Eng J 115:93–100
Jiménez-Peñalver P, Castillejos M, Koh A, Gross R, Sánchez A, Font X, Gea T (2018) Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J Clean Prod 172:S0959652617328111
Jones DF (1968) Microbiological oxidation of long-chain aliphatic compounds. V. Mechanism of hydroxylation. J Chem Soc Perkin Trans 22:2827–2833
Joshi-Navare K, Khanvilkar P, Prabhune A (2013) Jatropha oil derived sophorolipids: production and characterization as laundry detergent additive. Biochem Res Int 2013:169797
Kim SY, Oh DK, Lee KH, Kim JH (1997) Effect of soybean oil and glucose on sophorose lipid fermentation by Torulopsis bombicola in continuous culture. Appl Microbiol Biotechnol 48:23–26
Kim HS, Kim YB, Lee BS, Kim EK (2005) Sophorolipid production by Candida bombicola ATCC 22214 from a corn-oil processing byproduct. J Microbiol Biotechnol 15:55–58
Kim YB, Yun HS, Kim EK (2009) Enhanced sophorolipid production by feeding-rate-controlled fed-batch culture. Bioresour Technol 100:6028–6032
Klekner V, Kosaric N, Zhou QH (1991) Sophorose lipids produced from sucrose. Biotechnol Lett 13:345–348
Koganti S (2012) Conversion of biodiesel byproduct glycerol to arabitol and sophorolipids through microbial fermentation. University of Akron, Dissertation
Konishi M, Morita T, Fukuoka T, Imura T, Uemura S, Iwabuchi H, Kitamoto D (2018) Efficient production of acid-form sophorolipids from waste glycerol and fatty acid methyl esters by Candida floricola. J Oleo Sci 67:489–496
Lee KH, Kim JH (1993) Distribution of substrates carbon in sophorose lipid production by Torulopsis bombicola. Biotechnol Lett 15:263–266
Li H, Ma XJ, Wang S, Song X (2013) Production of sophorolipids with eicosapentaenoic acid and docosahexaenoic acid from Wickerhamiella domercqiae var. sophorolipid using fish oil as a hydrophobic carbon source. Biotechnol Lett 35:901–908
Liu XG, Ma XJ, Yao RS, Pan CY, He HB (2016) Sophorolipids production from rice straw via SO3 micro-thermal explosion by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. AMB Express 6:1–11
Ma XJ, Li H, Wang DX, Song X (2014) Sophorolipid production from delignined corncob residue by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576 and Cryptococcus curvatus ATCC 96219. Appl Microbiol Biotechnol 98:475–483
Maddikeri GL, Gogate PR, Pandit AB (2015) Improved synthesis of sophorolipids from waste cooking oil using fed batch approach in the presence of ultrasound. Chem Eng Sci 263:479–487
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1:1–19
Makoto T, Tomotake M, Koji W, Naoto H, Tokuma F, Tomohiro I, Dai K (2011) Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. J Oleo Sci 60:267–273
Maneerat S (2005) Production of biosurfactants using substrates from renewable resources. J Sci and Tech 27:675–683
Masaaki K, Yuka Y, Jun-Ichi H (2015) Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. J Biosci Bioeng 119:317–322
Minucelli T, Ribeiro-Viana RM, Borsato D, Andrade G, Cely MVT, Oliveira MRD, Baldo C (2016) Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in soil bioremediation. Waste & Biomass Valorization 8:1–11
Nuñez A, Ashby RA, Foglia T, Solaiman D (2001) Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 53:673–677
Oliveira MRD, Camilios-Neto D, Baldo C, Magri A, Celligoi MAPC (2014) Biosynthesis and production of sophorolipids. Int J Sci Tech Res 3:133–146
Oliveira MRD, Magri A, Baldo C, Camilios-Neto D, Minucelli T, Celligoi MAPC (2015) Minireview: sophorolipids a promising biosurfactant and its applications. Int J Adv Biotechnol Res 6:161–174
Parekh VJ, Patravale VB, Pandit A (2012) Mango kernel fat: A novel lipid source for the fermentative production of sophorolipid biosurfactant using Starmerella bombicola NRRL-Y 17069. Ann Biol Res 3:1798–1803
Pekin G, Vardar-Sukan F, Kosaric N (2005) Production of sophorolipids from Candida bombicola ATCC 22214 using Turkish corn oil and honey. Eng Life Sci 5:357–362
Rashad MM, Al-kashef AS, Nooman MU, Mahmoud AEE (2014a) Engco-utilization of motor oil waste and sunflower oil cake on the production of new sophorolipids by Candida bombicola NRRL-Y 17069. Res J Pharm Biol Chem Sci 5:1515–1528
Rashad MM, Nooman MU, Ali MM, Al-kashef AS, Mahmoud AE (2014b) Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas Aceites 65:e017
Rau U, Manzke C, Wagner F (1996) Influence of substrate supply on the production of sophorose lipids by Candida bombicola ATCC 22214. Biotechnol Lett 18:149–154
Rau U, Hammen S, Heckmann R, Wray V, Lang S (2001) Sophorolipids: a source for novel compounds. Ind Crops Prod 13:85–92
Saerens KM, Roelants SL, Van Bogaert IN, Soetaert W (2011a) Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res 11:123–132
Saerens KM, Saey L, Soetaert W (2011b) One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnol Bioeng 108:2923–2931
Saerens KM, Zhang J, Saey L, Van Bogaert IN, Soetaert W (2011c) Cloning and functional characterization of the UDP-glucosyltransferase UgtB1 involved in sophorolipid production by Candida bombicola and creation of a glucolipid-producing yeast strain. Yeast 28:279–292
Saerens KM, Van Bogaert IN, Soetaert W (2015) Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola. FEMS Yeast Res 15:1–9
Saharan B, Sahu R, Sharma D (2011) A review on biosurfactants: fermentation, current developments and perspectives. Genet Eng Biotechnol J 29:1–39
Samad A (2015) Sophorolipid production from lignocellulosic biomass feedstocks. Southern Illinois University Carbondale, Dissertation
Samad A, Zhang J, Chen D, Liang Y (2015) Sophorolipid production from biomass hydrolysates. Appl Biochem Biotechnol 175:2246–2257
Samad A, Zhang J, Chen D, Chen X, Tucker M, Liang Y (2017) Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. J Ind Microbiol Biotechnol 44:353–362
Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R (2005) Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother 49:4093–4100
Shah V, Jurjevic M, Badia D (2007) Utilization of restaurant waste oil as a precursor for sophorolipid production. Biotechnol Prog 23:512–515
Shah MUH, Sivapragasam M, Moniruzzaman M, Talukder MMR, Yusup SB, Goto M (2017) Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem Eng J 127:60–67
Solaiman DKY (2005) Sophorolipid biosynthesis from biodiesel co-product stream. J Am Oil Chem Soc 82:625–630
Solaiman DKY, Ashby RD, Nuñez A, Foglia TA (2004) Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnol Lett 26:1241–1245
Solaiman DKY, Ashby RD, Zerkowski JA, Foglia TA (2007) Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnol Lett 29:1341–1347
Takahashi F, Igarashi K, Hagihara H (2016) Identification of the fatty alcohol oxidase FAO1 from Starmerella bombicola and improved novel glycolipids production in an FAO1 knockout mutant. Appl Microbiol Biotechnol 100:9519–9528
Tulloch AP, Spencer JFT, Gorin PAJ (1962) The fermentation of long-chain compounds by Torulopsis magnoliae. I. Structures of the hydroxy fatty acids obtained by the fermentation of fatty acids and hydrocarbons. Can J Chem 40:1326–1338
Van Bogaert IN, Saerens K, De Muynck C, Develter D, Soetaert W, Vandamme EJ (2007) Microbial production and application of sophorolipids. Appl Microbiol Biotechnol 76:23–34
Van Bogaert IN, Develter D, Soetaert W, Vandamme EJ (2008) Cerulenin inhibits de novo sophorolipid synthesis of Candida bombicola. Biotechnol Lett 30:1829–1832
Van Bogaert IN, Roelants S, Develter D, Soetaert W (2010a) Sophorolipid production by Candida bombicola on oils with a special fatty acid composition and their consequences on cell viability. Biotechnol Lett 32:1509–1514
Van Bogaert IN, Sabirova J, Develter D, Soetaert W, Vandamme EJ (2010b) Knocking out the MFE-2 gene of Candida bombicola leads to improved medium-chain sophorolipid production. FEMS Yeast Res 9:610–617
Van Bogaert IN, Fleurackers S, Van Kerrebroeck S, Develter D, Soetaert W (2011a) Production of new-to-nature sophorolipids by cultivating the yeast Candida bombicola on unconventional hydrophobic substrates. Biotechnol Bioeng 108:734–741
Van Bogaert IN, Zhang J, Soetaert W (2011b) Microbial synthesis of sophorolipids. Process Biochem 46:821–833
Van Bogaert IN, Holvoet K, Roelants S, Li B, Lin YC, Van de Peer Y, Soetaert W (2013) The biosynthetic gene cluster for sophorolipids: a biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol Microbiol 88:501–509
Vatsa P, Sanchez L, Clement C, Baillieul F, Dorey S (2010) Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int J Mol Sci 11:5095–5108
Vaughn SF, Behle RW, Skory CD, Kurtzman CP, Price NPJ (2014) Utilization of sophorolipids as biosurfactants for postemergence herbicides. Crop Prot 59:29–34
Vedaraman N, Venkatesh N (2010) The effect of medium composition on the production of sophorolipids and the tensiometric properties by Starmerella bombicola MTCC 1910. Pol J Chem Technol 12:9–13
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012a) Sophorolipid production by Starmerella bombicola (ATCC 22214) from virgin and waste frying oils, and the effects of activated earth treatment of the waste oils. J Am Oil Chem Soc 89:1029–1039
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012b) Jatropha oil and karanja oil as carbon sources for production of sophorolipids. Eur J Lipid Sci Technol 114:823–832
Wadekar SD, Kale SB, Lali AM, Bhowmick DN, Pratap AP (2012c) Utilization of sweetwater as a cost-effective carbon source for sophorolipids production by Starmerella bombicola (ATCC 22214). Prep Biochem Biotechnol 42:125–142
Waele S, Vandenberghe I, Laukens B, Planckaert S, Verweire S, Van Bogaert IN, Soetaert W, Devreese B, Ciesielska K (2018) Optimized expression of the Starmerella bombicola lactone esterase in Pichia pastoris through temperature adaptation, codon-optimization and co-expression with HAC1. Protein Expr Purif 143:62–70
Zhou QH (1995) Utilization of canola oil and lactose to produce biosurfactant with Candida bombicola. J Am Oil Chem Soc 72:67–71
Zhou QH, Kosaric N (1993) Effect of lactose and olive oil on intra- and extracellular lipids of Torulopsis bombicola. Biotechnol Lett 15:477–482
Funding
This work was supported by the Fundamental Research Funds for the Central Universities of China (No. JZ2019YYPY0029), the National Natural Science Foundation of China (No. 31400049), and the China Postdoctoral Science Foundation (No. 2015T80646).
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, X., Meng, L., Zhang, H. et al. Sophorolipid biosynthesis and production from diverse hydrophilic and hydrophobic carbon substrates. Appl Microbiol Biotechnol 104, 77–100 (2020). https://doi.org/10.1007/s00253-019-10247-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10247-w