Abstract
Positive modulation of gut microbiota in laying chickens may offer a strategy for reduction of Salmonella Typhimurium shedding and production of safer poultry products. In the current study, the caecal luminal microbiota of laying chicks was studied using 16S rRNA amplicon sequencing on DNA obtained from the chicks that were offered supplementation with commercial probiotics, synbiotics and/or Salmonella Typhimurium challenge. The load of Salmonella Typhimurium in various organs was quantified. Irrespective of the probiotics and synbiotics supplementation and Salmonella Typhimurium challenge, caecal microbiota was dominated by 22 distinct bacterial genera and 14 families that clustered into Actinobacteria, Proteobacteria and Firmicutes at phylum level. Taken together, probiotics and synbiotics supplementation increased (false discovery rate; FDR < 0.05) the abundance of Ruminococcus, Trabulsiella, Bifidobacterium, Holdemania and Oscillospira, indicating their role in maintaining gut health through lowering luminal pH and digestion of complex polysaccharides. Salmonella Typhimurium challenge decreased the abundance of Trabulsiella, Oscillospira, Holdemania, Coprococcus, Bifidobacterium and Lactobacillus and increased Klebsiella and Escherichia, indicating its role in caecal dysbiosis. Although probiotics and synbiotics supplementation positively modulated the caecal microbiota, they were not effective in significantly (P > 0.05) reducing Salmonella Typhimurium load in caecal tissue and invasion into vital organs such as liver and spleen. The early colonisation of laying chick caeca by probiotics and synbiotics had the potential to positively influence luminal microbiota; however, the microbial abundance and diversity were not sufficient to significantly reduce the shedding of Salmonella Typhimurium in faeces or invasion into internal organs during this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caecal microbiota in chickens is linked with host health and productive traits that reflect its importance in colonisation resistance to zoonotic pathogens (Shini et al. 2013). Positively influencing the host gut microbiota helps in digestion and metabolism (Stanley et al. 2012), regulation of intestinal angiogenesis (Stappenbeck et al. 2002), development and regulation of host immune system (Hooper et al. 2001) and even in brain function (Benakis et al. 2016). Microbial communities are influenced by tissue type, flock age, disease and rearing conditions (Cui et al. 2017; Luoma et al. 2017; Ngunjiri et al. 2019). Studies suggested that the host genotype can exert a strong influence on gut microbiota composition (Goodrich et al. 2016) and, therefore, the microbiome of egg-type birds is not the same as that of broilers (Ocejo et al. 2019). In layer chicks, the lowest complexity of caecal microbiota is around day 1 of hatch, where it usually consists of five different species (Crhanova et al. 2011). The microbial diversity slowly increases with bird age to 14 species on day 3 and approximately 42 species around day 19 of hatch (Crhanova et al. 2011). After 2 weeks post-hatch, the Ruminococcus and Firmicutes increase to a greater extent than the Enterobacteriaceae (Ballou et al. 2016). Newly hatched chicks are prone to colonisation by pathogenic microorganisms present in the rearing environment. Higher microbial diversity is commonly associated with healthy host conditions, while reduced microbial diversity affects the intestinal health negatively (Sommer et al. 2017).
To modulate the gut microbiota composition in chickens, diets are often supplemented with pre- and probiotics. Prebiotics are non-living fibrous feed additives (non-digestible oligosaccharides) that promote the growth and multiplication of the indigenous gut microbiota. Therefore, prebiotics serve as feed for beneficial indigenous gut bacteria. The proposed mechanisms of action of prebiotics include the production of antimicrobial substances (Chen et al. 2007), modulation of host immune system (Babu et al. 2012) and improving gut morphology (Pourabedin et al. 2014). In contrast to prebiotics, probiotics are live microbial feed supplements that beneficially affect the host by improving its intestinal microbial balance (Fuller 1989). The protective effects of probiotics in the gut could be due to the production of organic acids (lactic acid) and adhesion inhibitors, and the secretion of antimicrobial substances such as hydrogen peroxide and bacteriocins (Spinler et al. 2008). Probiotics can also secrete enzymes that hydrolyse bacterial toxins and modify toxin receptors (Buts et al. 1994).
In laying hens, diets supplemented with different strains of probiotics have significantly improved gut microbial balance, blood and yolk cholesterol levels, egg production and overall egg quality. For example, Lactobacillus improved the equilibrium of gut microbiota by increasing the population of Bifidobacteria and decreased potentially harmful bacteria (Forte et al. 2016). Lactobacillus and Bacillus improved overall birds’ performance, caecal microbiota and gut morphology (Forte et al. 2016). Lactobacillus salivarius and Bacillus subtilis improved egg production, serum low-density cholesterol and serum antibody level against avian influenza virus (Zhang et al. 2012). Bacillus licheniformis and B. subtilis improved egg production, damaged egg ratio, egg yolk cholesterol and serum cholesterol (Kurtoglu et al. 2004). Pediococcus acidilactici positively influenced overall egg quality, feed efficiency and yolk fatty acid composition and yolk content (Mikulski et al. 2012). Enterococcus faecium improved overall egg quality, gut microbiota, serum cholesterol level, nutrient digestibility and excreta ammonia emission (Park et al. 2016; Zhang and Kim 2013). Apart from competitive exclusion theory, probiotics may offer protection against pathogens by modulating host immune response. For example, chickens infected with Salmonella Typhimurium resulted in reduced production of IFN-γ when fed with Lactobacillus, Bifidobacterium and Streptococcus-based probiotic (Haghighi et al. 2008). Similarly, pre-treatment of human intestinal epithelial cells with Bifidobacterium or Lactobacillus resulted in the inhibition of Salmonella-induced IL-8 expression (O'Hara et al. 2006). In laying hens, prebiotics supplementation has been shown to influence gut health and birds performance positively. For example, xylo-oligosaccharides improved intestinal epithelial morphology, caecal Bifidobacterial population, caecal butyrate level, plasma immunoglobulins concentrations and plasma vitamin D3 level (Ding et al. 2017). Isomalto-oligosaccharide improved overall egg quality, egg production, feed intake and serum cholesterol level (Tang et al. 2017). Mannan-oligosaccharides positively influenced egg production, egg weight, liver antioxidant status and feed conversion ratio (Bozkurt et al. 2016). Fructooligosaccharides reduced Salmonella Enteritidis colonisation in the liver and ovary (Donalson et al. 2008). Inulin reduced yolk cholesterol concentration, caecal pH and coliform bacteria count (Shang et al. 2010).
In healthy adult chickens, Salmonella infection generally does not lead to the development of clinical signs (Barrow and Lovell 1991), while in young chicks, it can cause morbidity and mortality (Williams Smith and Tucker 1980). However, Salmonella infection potentially changes gut microbial communities dominated by Enterobacteriaceae (Liu et al. 2018) with more visible effects in younger chicks (Juricova et al. 2013). In 1-week-old layer chicks, Salmonella Enteritidis challenge altered the caecal microbial communities (Mon et al. 2015). The effects of Salmonella Enteritis on changes in gut microbiota were greater in day-old chicks compared with 4 and 16-day-old Isa-Brown chicks (Juricova et al. 2013). Pre- and pro-biotics are effective in clearing Salmonella from the chicken gut through modification of the gut microbiome (Azcarate-Peril et al. 2018; Bratburd et al. 2018) and host immune system modulation (Chang et al. 2019; Haghighi et al. 2008). Lactobacillus has been shown to reduce Salmonella Enteritidis load in chickens caeca significantly (Penha Filho et al. 2015). In a mouse colitis model, probiotic Escherichia coli Nissle 1917 was effective in reducing Salmonella Typhimurium colonisation (Deriu et al. 2013). Multiple strains based probiotic was effective in reducing the shedding level of Salmonella Typhimurium in pigs (Casey et al. 2007). A significant interaction of pre- and pro-biotics on host immune response against Salmonella Typhimurium has been observed in pigs (Naqid et al. 2015). In humans, gastroenteritis caused by Salmonella Typhimurium is often traced back to contaminated poultry produce (Fearnley et al. 2011); hence, the poultry industry is under constant pressure to contain this pathogen at farm level. Based on the intended use of probiotics and synbiotics for controlling Salmonella in chickens, we hypothesised that, if used in the first week of the chick’s hatch, commercial probiotics and synbiotics can provide colonisation resistance through competitive exclusion against Salmonella Typhimurium in the caeca at an early age. To test this hypothesis, we used next-generation sequencing targeting hypervariable regions within microbial 16S rRNA genes to compare the caecal luminal microbiota of layer chicks exposed to short-term probiotics and synbiotics supplementation and subsequently challenged with Salmonella Typhimurium or left as a control. To understand the effects of different commercial probiotics and synbiotics on Salmonella Typhimurium colonisation and invasion into internal organs, culture methods were used for organ load determination. The outcome of the study has broadened our understanding of the interaction of Salmonella Typhimurium with gut microbiota in the presence or absence of probiotic and synbiotic supplements.
Materials and methods
Rearing of birds
Fertile eggs from an Isa-Brown parent flock were obtained from a local breeder farm. Following fumigation (by formaldehyde and potassium permanganate (3:1)), the eggs were incubated in clean conditions for hatching in the School of Animal and Veterinary Sciences. From the incubator, the hatching tray papers with chicks’ meconium samples were processed by culture methods for Salmonella isolation. Before the chicks’ placement, the entire experimental facility was cleaned and then tested for Salmonella. The hatched chicks were reared in a house with strict biosecurity protocols as per the protocol of the ISA General Management Guide 2009-10. Next, the chicks (n = 90) were equally divided into ten treatment groups (Table 1) and reared in pens (different treatment groups in separate rooms) with water and feed provided ad libitum. The feed was fumigated as described previously and the drinking water was autoclaved. The fumigated feed was routinely tested by culture method for the presence of Salmonella spp. The commercially available probiotics and synbiotics used in this study were selected based on their claimed efficacy for control of Salmonella through gut microbiota modulation in poultry. These products are usually used in layer industry for improving birds’ performance.
Four commercial probiotic and synbiotic products were purchased from the market and used in this study. For all the probiotic and synbiotic treatment groups, 1 g of each of the product was mixed in either 1 kg of fumigated feed or 1 L of autoclaved water. Every day, a freshly prepared batch of the products was offered to the treatment groups from the day of hatch to day 7 of chicks’ age. At day 8 of chicks’ age, birds from the probiotics and synbiotics supplemented and Salmonella Typhimurium treatment and positive control groups (Table 1) were challenged via the oral route with Salmonella Typhimurium phage type 9. The probiotics, synbiotics and negative control groups received phosphate buffered saline (PBS) only.
Inoculum preparation and birds challenge
Salmonella Typhimurium previously isolated from a layer farm (Gole et al. 2014) was used in this study. Salmonella Typhimurium inoculum was prepared by following the method described previously (McWhorter and Chousalkar 2018). In the challenge groups, each bird received an oral dose of 103 colony forming unit (CFU; 0.1 mL) of Salmonella Typhimurium. The inoculation dose was kept low to understand its effects on gut microbiota modulation and colonisation of internal organs. Studies suggest that approximately 103 CFU of Salmonella per chicken is enough to activate the host immune system (Chart et al. 1992; Marcq et al. 2011). A 100 μL of the original inoculum with serial dilutions was plated onto Xylose Lysine Deoxycholate (XLD; Thermo Fisher Scientific, Victoria, Australia) media to confirm the CFU received by each bird. From each treatment group at each time-point (days 3, 5 and 7 post-infection), three birds were processed by cervical dislocation for collection of caecal contents and tissues. Previous study showed a minor variation in gut microbiota between individual birds (Videnska et al. 2014). Number of other studies also used three birds at each time-point for gut microbiota analysis (Juricova et al. 2013; Kubasova et al. 2019).
Salmonella Typhimurium enumeration in tissue
Pieces of liver, spleen and caecal tissues were aseptically collected and weighed into 1.5 mL Eppendorf tubes containing stainless steel beads 0.5–2.0 mm and PBS. The samples were maintained on ice until further use. Tissues were homogenised using a Bullet Blender Storm homogeniser (Next Advance, NY, USA) on full speed for 5–10 min. Serial dilutions were prepared from the original tissue homogenates, plated onto XLD agar and incubated overnight at 37 °C. The Salmonella Typhimurium colonies were counted to determine the bacterial load (log10 CFU) in 1 g of tissue. A 100-μL sample from the original homogenates was also enriched in 900 μL buffered peptone water (BPW; Thermo Fisher, Victoria, Australia) and incubated overnight at 37 °C. A 100-μL sample of the incubated BPW samples was added to 10 mL of Rappaport Vassiliadis soya peptone (RVS) broth (Thermo Fisher Scientific, Victoria, Australia) and incubated overnight at 42 °C for selective growth of Salmonella. Suspected Salmonella cultures from XLD agar were sub-cultured on Brilliance Salmonella agar (BSA; Thermo Fisher Scientific, Victoria, Australia) plates for confirmation. A 100 mg of each of the probiotics and synbiotics was cultured in De Man, Rogosa and Sharpe (MRS) media and characteristic colonies were gram stained. For approximate CFU count in 1 g of the probiotics and synbiotics, a 100 mg of individual products was suspended into PBS, serially diluted and plated (100 μL) on MRS media.
Caecal contents DNA extraction
To obtain a quality DNA from caecal contents, the manufacturer’s protocol (QIAamp DNA Stool Mini Kit; Qiagen, Victoria Australia) was modified slightly. Briefly, approximately 180 mg of caecal contents was weighed into a 1.5-mL tube (Eppendorf Safe-Lock; Eppendorf, Hamburg, Germany). Glass beads (acid-washed ≤ 106 μm and 425–600 μm; Sigma Aldrich, St. Louis, MO) were added to the samples and maintained on ice. Next, the samples were processed for DNA extraction as per the kit protocol except for the inclusion of the step of homogenisation in a bullet blender. DNA was eluted in 100 μL of buffer ATE (10 mM Tris-Cl pH 8.3, 0.1 mM EDTA and 0.04% NaN3) as per protocol of QIAamp DNA Stool Mini Kit. The purity (260/280 ratio 1.70–2.05; 230/260 ratio 1.80–2.30) and concentrations (20~300 ng/μL) were measured in a NanoDrop-1000 spectrophotometer (Thermo Fisher Scientific, Victoria, Australia). The DNA samples were stored at − 80 °C until used for downstream applications. Three samples at each sampling time-point per treatment group were submitted to the Australian Genome Research Facility (Melbourne, Australia) for diversity profiling analysis (16S: 341F–806R (V3–V4)) using the forward (CCTAYGGGRBGCASCAG) and reverse (GGACTACNNGGGTATCTAAT) primer pair.
16S rRNA analysis
PCR amplification and MiSeq sequencing
PCR amplicons were generated using the primers designed from V3–V4 region amplification of bacterial DNA using AmpliTaq Gold 360 master mix (Thermo Fisher Scientific, Victoria, Australia) for the primary PCR. The PCR conditions were initial heating at 95 °C for 7 min and 29 cycles of dissociation at 94 °C for 30 s, annealing at 50 °C for 60 s and extension at 72 °C for 60 s with a final finish of 72 °C for 7 min. A secondary PCR to index the amplicons was performed with TaKaRa Taq DNA Polymerase (Takara Bio, CA, USA). The resulting amplicons were measured by Qubit 4 fluorometer (Thermo Fisher Scientific, Victoria, Australia) and normalised. The equimolar pool was then measured by qPCR (KAPA) followed by sequencing on the Illumina MiSeq Platform (San Diego, CA, USA) with 2 × 300 base pairs paired-end chemistry.
Paired-end reads were assembled by aligning the forward and reverse reads using PEAR (version 0.9.5) (Zhang et al. 2013). From the sequences, primers were identified and trimmed. Trimmed sequences were processed using Quantitative Insights into Microbial Ecology (qiime 1.8) (Caporaso et al. 2010), USEARCH (version 7.1.1090) (Edgar 2010; Edgar et al. 2011) and UPARSE (Edgar 2013) software. Using USEARCH, sequences were quality filtered, and full length duplicate sequences were removed and sorted by abundance. Singletons or unique reads in the data set were discarded. Sequences were clustered followed by chimera filtered using “rdp_gold” database as a reference. To obtain the number of reads in each operational taxonomic unit (OTU), sequences were mapped back to OTUs with a minimum identity of 97%. In qiime, taxonomy was assigned using GreenGenes database (version 13_8, Aug 2013) (DeSantis et al. 2006). The OTU file was uploaded into Calypso software (version 8.72) (Zakrzewski et al. 2016) and the data were further analysed for group comparisons at false discovery rate (FDR) < 0.05. During data analysis in the Calypso, the OTU table was filtered to exclude taxa with low abundance (< 0.01%) and was total sum normalised (TSS) square root transformed. The processed data in the Calypso were subsequently used for univariate (one- and two-way ANOVA), multivariate and diversity analyses. The Shannon index was used to calculate the microbial alpha diversity affected by probiotics/synbiotics treatment and sampling time-point (or birds’ age) at OTU, genus and family levels. In the Calypso, the Shannon index at OTU level measures how the microbes are balanced and how species (evenness) are at a similar or dominant level to each other.
Statistical analysis
The Salmonella Typhimurium load (log10 CFU) per gram of tissue was analysed in Statview v.5.0.1.0 by taking sampling time-point and treatment group as the main effects. Repeated measure analysis was used to investigate the effects of time-point or treatment on Salmonella load in the organs. Level of significance was determined by PLSD at P < 0.05.
Availability of data and materials
The metagenome raw dataset supporting the results of this study have been deposited at the National Center for Biotechnology Information (NCBI), Sequence Read Archive (SRA) under the BioProject Accession Number PRJNA548995.
Results
Before the placement of chicks, the experimental facility tested was negative for Salmonella spp. Similarly, the fumigated feed tested was also negative for the presence of Salmonella. No Salmonella was recovered from the chicks’ meconium or from chicks sampled at regular intervals until the selected groups were challenged with Salmonella Typhimurium (ST). The faecal samples collected from the challenged groups on days 3, 5 and 7 post-infection (p.i.) were positive for Salmonella by culture method. All the control groups were negative for Salmonella throughout the experiment. No mortality or clinical signs of salmonellosis were observed after the chicks were challenged with ST. However, during sample collection, some of the challenged birds showed partially emptied caeca with mucous plugs. On day 3 p.i., one bird from the positive control group showed necrotic foci on the liver, while one bird from the probiotic A supplemented and ST challenge group showed haemorrhage in the spleen. The characteristic clinical signs observed in the caeca of the ST challenged groups have been summarised in Table 2.
Gut microbiota sequencing and quality of generated data
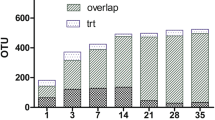
A total of 6.45 Gb sequences data for the ten different treatment groups were generated using Illumina sequencing. The average reads numbers per treatment group and quality have been tabulated in Table 3. To further check the depth of microbial communities’ coverage, a rarefaction analysis curve was calculated for all the treatment groups. In rarefaction analysis, the number of observed species was counted and plotted as a function of the number of sampled sequences. The slope of the curve indicates how well the sequenced data represent the underlying microbial community. The rarefaction analysis curve showed that the underlying microbial communities in all the treatment groups were well covered by the sequenced data (Supplementary file Fig. S1).
Caecal luminal microbial communities
A total of 22 known genera was identified with some sequences reads mapped to unclassified bacterial families (Fig. 1). At family level, the communities of caecal bacteria mainly comprised of Bacillaceae, Bifidobacteriaceae, Clostridiaceae, Enterobacteriaceae, Enterococcaceae, Erysipelotrichaceae, Lachnospiraceae, Lactobacillaceae, Paenibacillaceae, Peptostreptococcaceae, Pseudomonadaceae, Ruminococcaceae, Streptococcaceae and Veillonellaceae in addition to unclassified bacteria. The three most abundant phyla were Actinobacteria, Firmicutes and Proteobacteria.
Caecal luminal bacterial abundance and diversity are affected by probiotic and synbiotic supplementation and Salmonella Typhimurium challenge
Overall, the probiotic and synbiotic supplementation and ST challenge significantly (FDR < 0.05) affected microbial abundance in the caecal lumen of layer chicks. Within each treatment group, sampling time-point (or flock age) significantly affected (P < 0.05) the microbial diversity. To gain insight into microbial abundance and diversity, individual probiotic or synbiotic supplemented group samples (with or without ST challenge) were analysed against the positive control and negative control groups. Data from the different probiotic or synbiotic treatment groups were not compared with one another because the contents of each product were different. Only the genera and families significantly affected (FDR < 0.05) by probiotic, synbiotic treatments or ST challenge have been presented here.
Effect of probiotic A supplementation
At the genus level, in the probiotic A control group, the abundance of Trabulsiella and Oscillospira was higher compared to the probiotic A supplemented and ST challenged, ST negative control and ST positive control groups (Fig. 2a). Unclassified Ruminococcaceae, Klebsiella and Anaerotruncus abundance was higher in the ST positive control compared with the other treatment groups. ST challenge decreased the abundance of Paenibacillus and increased Anaerotruncus in the ST positive control and probiotic A supplemented and ST challenged groups compared with the probiotic A control and ST negative control groups. Compared with the ST positive control group, in the presence of probiotic A supplementation, ST challenge affected the abundance of Coprococcus, Butyricicoccus, Eubacterium and Blautia differently as seen in the probiotic A supplemented and ST challenged group. A complete list of the significant genera affected by the probiotic A supplementation and/or ST challenge is depicted in Fig. 2a. The core caecal microbiome was affected by the probiotic A supplementation and ST challenge (Supplementary file Fig. S2). At OTU level, there were 32 common taxa in the probiotic A supplemented group.
Caecal luminal microbial abundance and diversity affected by probiotic A supplementation and Salmonella Typhimurium (ST) challenge. a Microbial abundance at genera level. b Stripchart showing the Shannon index of the OTU (diversity) affected by bird age (or sampling time-point). c RDA+ of each of the treatment group. P < 0.024 was equivalent of FDR < 0.05 in Calypso software. Probiotic A was comprised of Lactobacillus acidophilus, L. delbrueckii subspecies bulgaricus, L. plantarum, L. rhamnosus, Bifidobacterium bifidum, Enterococcus faecium and Streptococcus salivarius subspecies thermophilus
In the probiotic A supplemented group, at OTU level, the Shannon index showed that the bacterial communities per group were at the same level (P = 0.6600) across all the treatment groups (Supplementary file Fig. S3). Within each treatment group, bird age (or sampling time-points) increased (P = 0.0230) the diversity of microbial communities in the negative control (P = 0.0091) and the probiotic A control (P = 0.0046) groups, while ST challenge with (P = 0.2723) or without (P = 0.3128) probiotic A supplementation had no significant effect on the microbial diversity (Fig. 2b). The Shannon index at the genus and family level produced very similar results to OTU level diversity measurements. The redundancy analysis (RDA+) is a multivariate method that is used to explore complex associations between community composition and multiple explanatory variables. The RDA+ showed that overall the treatment groups clustered separately showing the significant effects (P = 0.001) of probiotic A supplementation and ST challenge on the composition of bacterial communities (Fig. 2c).
Effect of probiotic B supplementation
Probiotic B supplementation and ST challenge had a significant effect (FDR < 0.05) on caecal luminal microbial abundance at the genus and family levels. At the genus level, ST challenge increased the abundance of unclassified bacteria and decreased Ruminococcaceae both in the probiotic B supplemented and ST challenged and ST positive control groups (Fig. 3a). The abundance of Ruminococcus and Bifidobacterium was higher in the probiotic B supplemented control group compared with the probiotic B supplemented and ST challenged, ST positive control and negative control groups. Paenibacillus, Lactococcus, Eubacterium, Enterococcus and Coprobacillus were abundant in the probiotic B supplemented and ST challenged group compared with the probiotic B control, ST positive control and negative control groups. The core caecal microbiome was affected by the probiotic B supplementation and ST challenge (Supplementary file Fig. S4). At OTU level, there were 30 common taxa in the probiotic B supplemented group.
Caecal luminal microbial abundance and diversity affected by probiotic B supplementation and Salmonella Typhimurium (ST) challenge. a Microbial abundance at genera level. b Stripchart showing the Shannon index of the OTU (diversity) affected by bird age (or sampling time-point). c RDA+ of each of the treatment group. P < 0.019 was equivalent of FDR < 0.05 in Calypso software. Probiotic B contained Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325 and Bacillus amyloliquefaciens in its composition
Probiotic B supplementation and ST challenge did not significantly affect (P = 0.0860) microbial diversity among the treatment groups (Supplementary file Fig. S5). Alpha diversity measured by the Shannon index based on sampling time-point (or birds’ age) showed a significant (P = 6e−05) variation in microbial diversity (Fig. 3b). Probiotic B supplementation reduced (P = 0.0032) the microbial diversity on day 5 and day 7 compared with the day 3 in the probiotic B control group, although it was not altered (P = 0.1252) in the probiotic B supplemented and ST challenged group. The RDA+ showed that probiotic B supplementation and ST challenge changed (P = 0.001) the composition of bacterial communities (Fig. 3c).
Effect of synbiotic A supplementation
At the genus level, in the synbiotic A supplemented and ST challenge group, the abundance of Anaerotruncus, Lactococcus, Lactobacillus, Eubacterium and Bifidobacterium was significantly (FDR < 0.05) higher compared with the synbiotic A control, ST positive control and negative control groups (Fig. 4a). Synbiotic A supplementation without ST challenge significantly increased the abundance of Trabulsiella and Ruminococcus as seen in the synbiotic A control group. A list of all of the significant genera has been provided in Fig. 4a. The core caecal microbiome was mainly affected by the synbiotic A supplementation interaction with ST challenge (Supplementary file Fig. S6). At OTU level, there were 28 common taxa in the synbiotic A supplemented group. There were no specific taxa attributed to the synbiotic A control group.
Caecal luminal microbial abundance and diversity affected by the synbiotic A supplementation and Salmonella Typhimurium (ST) challenge. a Microbial abundance at genera level. b Stripchart showing the Shannon index of the OTU (diversity) affected by bird age (or sampling time-point). c RDA+ of each of the treatment group. P < 0.021 was equivalent of FDR < 0.05 in Calypso software. Synbiotic A was composed of Enterococcus sp., Pediococcus sp., Bifidobacterium sp., Lactobacillus sp. and fructooligosaccharides
A significant effect (P = 0.0072) of synbiotic A supplementation was observed on microbial diversity in different treatment groups (Supplementary file Fig. S7). The microbial diversity was significantly lower in the synbiotic A control group compared with the synbiotic A supplemented and ST challenged, ST positive control and negative control groups. Within each treatment group (P = 0.0012), microbial diversity significantly decreased (P = 0.0191) with bird age (or sampling time-point) in the synbiotic A control and negative control groups, while in the synbiotic A supplemented and ST challenged (P = 0.4262) and ST positive control (P = 0.3128) groups, there was no difference in the microbial diversity (Fig. 4b). The RDA+ showed that synbiotic A supplementation and ST challenge changed (P = 0.001) the composition of bacterial communities, although some overlap between the synbiotic A control and negative control groups was observed (Fig. 4c).
Effect of synbiotic B supplementation
At the genus level, in the synbiotic B control group, the abundance of Trabulsiella, Oscillospira and Holdemania significantly increased compared with the synbiotic B supplemented and ST challenged, ST positive control and negative control groups (Fig. 5a). Escherichia and Coprococcus genera were higher in abundance in the synbiotic B supplemented and ST challenged group compared with the other three treatment groups. Overall, the ST challenge increased the abundance of unclassified bacteria in the ST positive control group. The overall genera significantly affected by the synbiotic B supplementation and ST challenge have been depicted in Fig. 5a. The core caecal microbiome was affected by the synbiotic B supplementation and ST challenge (Supplementary file Fig. S8). At OTU level, there were 36 common taxa in the synbiotic B supplemented group.
Caecal luminal microbial abundance and diversity affected by synbiotic B supplementation and Salmonella Typhimurium (ST) challenge. a Microbial abundance at genera level. b Stripchart showing the Shannon index of the OTU (diversity) affected by bird age (or sampling time-point). c RDA+ of each of the treatment group. P < 0.019 was equivalent of FDR < 0.05 in Calypso software. Synbiotic B contained Lactobacillus acidophilus, L. casei, L. salivarius, L. plantarum, L. rhamnosus, L. brevis, Bifidobacterium bifidum, B. lactis, S. thermophiles, prebiotic inulin (chicory root extract), protease, amylase, cellulase, hemicellulase, lipase, papain and bromelain in its composition
In the synbiotic B supplemented group, at the OTU level, the diversity of the microbial communities was not affected (P = 0.4200) by the synbiotic supplementation or ST challenge (Supplementary file Fig. S9). Overall, sampling time-point (or birds age) had a significant effect (P = 0.004) on microbial diversity (Fig. 5b) only in the negative control group (P = 0.0091). The RDA+ showed that synbiotic B supplementation and ST challenge changed (P = 0.001) the composition of bacterial communities (Fig. 5c).
Salmonella Typhimurium load in different organs
Salmonella spp. were not recovered from either the negative control group or the probiotics and synbiotics control groups. Probiotics A and B supplementation in young layer chicks for a week did not significantly reduce (P > 0.05) the ST load (log10 CFU/g of tissue) in the caeca, liver and spleen (Fig. 6a–f). In the probiotics A and B supplemented and challenged with ST groups, the bacterial load in all three organs increased with day p.i. except for the caeca and liver of the probiotic A challenged group (Fig. 6a–f).
Effect of probiotics A and B on mean load (log10 CFU/g of tissue) of Salmonella Typhimurium (ST) in caeca, liver and spleen collected on days 3, 5 and 7 p.i. aST load in caecal tissue of probiotic A supplemented chicks. bST load in liver tissue of probiotic A supplemented chicks. cST load in spleen tissue of probiotic A supplemented chicks. dST load in caecal tissue of probiotic B supplemented chicks. eST load in liver tissue of probiotic B supplemented chicks. fST load in spleen tissue of probiotic B supplemented chicks. Superscripts (a,b) represent significant difference affected by day p.i. in positive control group. Superscripts (x,y) represent significant difference affected by day p.i. in probiotic supplemented group. In each graph, line across the bars represents significant differences (*P < 0.05; **P < 0.005; ***P < 0.0005) between the respective groups. Details of probiotics A and B are in Table 1
Feeding synbiotics A and B to layer chicks for a week did not significantly reduce (P > 0.05) ST load in the caeca, liver and spleen. The ST load significantly increased with day p.i. only in the caeca (Fig. 7a–f). There was a general trend of lower ST load in the liver and spleen of the synbiotics A and B supplemented groups; however, these differences were not statistically significant.
Effect of synbiotics A and B on mean load (log10 CFU/g of tissue) of Salmonella Typhimurium (ST) in caeca, liver and spleen tissues collected on days 3, 5 and 7 p.i. aST load in caecal tissue of synbiotic A supplemented chicks. bST load in liver tissue of synbiotic A supplemented chicks. cST load in spleen tissue of synbiotic A supplemented chicks. dST load in caecal tissue of synbiotic B supplemented chicks. eST load in liver tissue of synbiotic B supplemented chicks. fST load in spleen tissue of synbiotic B supplemented chicks. Superscripts (a,b) represent significant difference affected by day p.i. in positive control group. Superscripts (x,y) represent significant difference affected by day p.i. in probiotic supplemented group. In each graph, line across the bars represents significant differences (***P < 0.0005; **P <0.005) between the respective groups. Details of synbiotics A and B are in Table 1
Discussion
This study aimed to understand the effects of short-term feeding of four different commercial probiotic and synbiotic supplements on gut health in the presence or absence of Salmonella Typhimurium challenge in Isa-Brown layer chicks. During the last century, the incorporation of growth-promoting antibiotics into the feed of production animals has resulted in improvements to health conditions and productivity. Globally there are concerns about the use of in-feed antibiotics for growth promotion due to the development of antimicrobial resistance and the spread of resistance genes. Moreover, the use of antimicrobials can negatively alter gut microbiota. Probiotics and synbiotics are favoured in recent days due to their ability to induce a structural change in gut microbiota.
The rationale behind the discontinuation of the probiotics and synbiotics before Salmonella Typhimurium challenge was to study the effects of the Salmonella infection on developed gut microbiota and Salmonella invasion into internal organs. In this study, irrespective of the probiotic and synbiotic supplementation and Salmonella Typhimurium challenge, a total of 22 distinct genera were identified in layer chicks; however, we have only discussed the genera significantly affected by the probiotics, synbiotics or Salmonella Typhimurium challenge relative to positive and negative control groups.
In the current study, most of the genera that colonised the chicks’ caeca are involved in diverse physiological functions. For example, Bifidobacterium (Milani et al. 2015), Clostridium (Bayer et al. 2008), Enterococcus (Robert and Bernalier-Donadille 2003), Eubacterium (Montgomery 1988) and Ruminococcus (Moon et al. 2011) in the gut are involved in fibre digestion. Eubacterium is one of the dominant genera of the caecum in layers (Callaway et al. 2009). Some species of Eubacterium (for example Eubacterium hallii) in the gut are involved in metabolising glycerol to 3-hydroxypropionaldehyde that exists in a multi-compound system called reuterin (Fekry et al. 2016). Reuterin possesses antimicrobial activity against a range of pathogens (Vollenweider et al. 2010). Eubacterium hallii in the human gut has been linked with the formation of propionate (Engels et al. 2016). Therefore, the most abundant Eubacterium in chicks’ caeca could be linked with numerous functions ranging from short-chain fatty acids (SCFAs) production to metabolic balance. In this study, a reduction of Eubacterium abundance in the Salmonella Typhimurium positive control group compared with the probiotics (A and B) and synbiotic A supplemented and Salmonella Typhimurium challenged groups showed that these products were effective in maintaining the Eubacterium population in the gut even in the presence of Salmonella Typhimurium. This study also demonstrated some interaction between Eubacterium and Salmonella Typhimurium because, in the absence of challenge, the abundance of Eubacterium was not significantly different among the treatment groups.
Oscillospira is another genus of gut microbiota abundantly present in layer chickens’ caeca (Volf et al. 2016). Oscillospira species help in starch digestion in many different hosts (Mackie et al. 2003) and therefore are butyrate producers (Gophna et al. 2017). Butyrate is one of the three main types of SCFAs and is involved in ATP provision to enterocytes (Treem et al. 1994) and possesses anti-inflammatory (Vinolo et al. 2011) and antimicrobial properties (Cox et al. 1994). In this study, an increased abundance of Oscillospira in the probiotic A and synbiotic B supplemented groups without Salmonella Typhimurium challenge indicates that these products favoured starch digestion and SCFAs producing bacteria were favoured. It was also observed that, in the presence of the probiotic A and synbiotic B supplementation, Salmonella Typhimurium reduced the Oscillospira abundance compared with the Salmonella Typhimurium positive control group. It is possible that there is an interaction between Oscillospira and Salmonella, where Salmonella Typhimurium depletes its population. Ruminococcus is among other genera involved in SCFAs production in chickens (Huang et al. 2018). In this study, different probiotics and synbiotics affected Ruminococcus abundance differently. For example, synbiotic B supplementation reduced Ruminococcus abundance in the synbiotic supplemented groups with or without Salmonella Typhimurium challenge. Probiotic B and synbiotic A increased the Ruminococcus abundance both in the probiotic and synbiotic supplemented and Salmonella Typhimurium challenged groups. This shows that one particular probiotic or synbiotic may not favour the abundance of all beneficial bacterial genera in the gut. In this study, the predominant bacterial genera of Eubacterium, Oscillospira and Ruminococcus positively modulated by the probiotics and synbiotics indicate their role in the overall gut health in young chicks. No significant effect of Salmonella Typhimurium and Salmonella Enteritidis was observed on the composition of gut microbial communities of laying chickens (Azcarate-Peril et al. 2018; Nordentoft et al. 2011).
Probiotic A and synbiotics A and B supplementation increased the abundance of Trabulsiella, while Salmonella Typhimurium challenge decreased it. Trabulsiella is a member of the Enterobacteriaceae with no apparent role in chicken gut microbiota. Bifidobacterium is one of the dominant members of gut microbiota that plays a role in complex starch digestion (Milani et al. 2015), preventing the production of proinflammatory cytokines (Fanning et al. 2012) and stress reduction (Savignac et al. 2014). In this study, its abundance was affected mainly by the probiotic B and synbiotic A with or without Salmonella Typhimurium challenge. These probiotic and synbiotic were effective in maintaining Bifidobacterium abundance even in the presence of Salmonella Typhimurium. This shows that Bifidobacterium is one of the gut bacteria with a possible protective role against Salmonella Typhimurium infection. However, at least in the conditions applied in the current study, the protective environment produced by the Bifidobacterium was not sufficient to reduce the load of Salmonella Typhimurium significantly in caeca. We suggest further investigation to understand the interaction of Bifidobacterium with Salmonella Typhimurium as a probiotic candidate for the chicken gut. A reduced abundance of Klebsiella and Escherichia in the probiotic A and synbiotic B supplemented and no Salmonella Typhimurium challenge groups, respectively, showed the positive modulation of gut microbiota by these products. Important diseases of poultry attributed to Escherichia include cellulitis, septicaemia, colibacillosis, omphalitis and respiratory tract infection.
Different probiotics and synbiotics supplementation during Salmonella Typhimurium infection showed positive effects on the abundance of certain genera. For example, probiotic A supplementation increased the abundance of Butyricicoccus, Eubacterium, Coprococcus and Blautia in the Salmonella Typhimurium challenged compared to the positive control group. Similarly, synbiotic B affected the abundance of Escherichia, Coprococcus and Anaerotruncus, while synbiotic A affected Lactococcus, Lactobacillus, Eubacterium, Coprococcus, Bifidobacterium and Anaerotruncus. Probiotic B mainly influenced Paenibacillus, Eubacterium and Coprobacillus. This further strengthens the notion that, during the Salmonella Typhimurium infection, short-term feeding of probiotics or synbiotics has the potential to influence resident gut bacterial genera positively. However, this short-term feeding may not be sufficient to inhibit Salmonella Typhimurium from colonising caeca or invading internal organs. Overall, compared to the negative control group, the microbial diversity (measured at OTU level) was decreased with bird age (or sampling time-point) by synbiotic A supplementation. This shows that the synbiotic A reduced the genera of certain bacteria in the gut of layer chicks. The Shannon index of redundancy analysis (RDA+) showed that probiotic and synbiotic supplementation and Salmonella Typhimurium challenge shifted the gut microbiota diversity and therefore it clustered separately among different treatment groups, showing the importance of probiotic or synbiotic supplementation in Salmonella Typhimurium infected birds. These results were further supported by the presence of various biomarkers in different treatment groups. Probiotic and synbiotic supplementation increased the abundance of many genera of the gut microbiota in the presence or absence of Salmonella Typhimurium; however, these effects varied depending on the products.
The bacteriology results (counted as CFU/g of tissue) showed that feeding the probiotics and synbiotics for a week was not effective in significantly reducing ST load in the liver, spleen and caecal tissues of layer chicks. It seems that the early colonisation of caeca with probiotic bacterial strains may not competitively exclude Salmonella Typhimurium. Limited studies performed on probiotic or prebiotic supplementation and Salmonella Typhimurium challenge in layer chickens presented different results reflecting the importance of the nature and duration of the probiotic being supplemented, genetic strain, age of the bird and the bird rearing environment. For example, the inclusion of fructooligosaccharides (FOS) in an alfalfa moulting diet significantly decreased caecal Salmonella Enteritidis counts in laying hens (Donalson et al. 2008). In White Leghorn Hy-Line cockerels challenged with Salmonella Enteritidis, FOS supplementation (alone or in combination with probiotic) significantly decreased Salmonella load in caeca at days 1, 7 and 14 p.i. (Fukata et al. 1999). In 46-week old White Leghorn laying hens challenged with Salmonella Enteritidis, supplementation of Lactobacillus plantarum for 7 days post-infection did not significantly reduce Salmonella load in the caeca (Adhikari et al. 2018). Interestingly, synbiotic supplemented laying hens challenged with Salmonella Enteritidis showed no Salmonella in the caecal contents on day 10 p.i. (Luoma et al. 2017). Lactobacillus johnsonii strain R-17504 and Lactobacillus reuteri strain R-17485 supplementation into day-old layer chicks significantly reduced Salmonella Enteritidis load in caeca on day 6 p.i. (Van Coillie et al. 2007). In this study, no significant difference between the treatment groups for Salmonella Typhimurium load in caecal tissue indicate that the probiotics and synbiotics were not effective in reducing Salmonella count at days 3, 5 and 7 p.i. in Isa-Brown layer chicks. Further studies are required to investigate the long-term feeding of probiotic supplements on Salmonella Typhimurium colonisation, shedding and or invasion into vital organs of hens. Overall, a weeklong probiotic and synbiotic supplementation to layer chicks was effective in modulating positively the abundance of certain resident gut microbiota. Salmonella Typhimurium challenge decreased the abundance of many useful bacterial genera in chicks that were not supplemented with probiotics or synbiotic. On the other hand, there was an increase in the abundance of many useful bacterial genera in infected chicks that were supplemented with probiotics and synbiotics. These abundant genera play a pivotal role in maintaining overall gut health. Nevertheless, probiotic and synbiotic supplementation to chicks for 1 week did not competitively exclude Salmonella Typhimurium from caeca or prevent internal organ invasion. Further studies are required to understand the long-term feeding of probiotics on laying chicken gut microbiota and its effects on Salmonella Typhimurium shedding.
References
Adhikari P, Lee CH, Cosby DE, Cox NA, Kim WK (2018) Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult Sci 98(3):1235–1242. https://doi.org/10.3382/ps/pey443
Azcarate-Peril MA, Butz N, Cadenas MB, Koci M, Ballou A, Mendoza M, Ali R, Hassan H (2018) An attenuated Salmonella enterica serovar Typhimurium strain and galacto-oligosaccharides accelerate clearance of Salmonella infections in poultry through modifications to the gut microbiome. Appl Environ Microbiol 84(5):e02526–e02517. https://doi.org/10.1128/aem.02526-17
Babu US, Sommers K, Harrison LM, Balan KV (2012) Effects of fructooligosaccharide-inulin on Salmonella-killing and inflammatory gene expression in chicken macrophages. Vet Immunol Immunopathol 149(1):92–96. https://doi.org/10.1016/j.vetimm.2012.05.003
Ballou AL, Ali RA, Mendoza MA, Ellis JC, Hassan HM, Croom WJ, Koci MD (2016) Development of the chick microbiome: how early exposure influences future microbial diversity. Front Vet Sci 3(2). https://doi.org/10.3389/fvets.2016.00002
Barrow PA, Lovell MA (1991) Experimental infection of egg-laying hens with Salmonella Enteritidis phage type 4. Avian Pathol 20(2):335–348. https://doi.org/10.1080/03079459108418769
Bayer EA, Lamed R, White BA, Flint HJ (2008) From cellulosomes to cellulosomics. Chem Rec 8(6):364–377
Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 22(5):516–523
Bozkurt M, Bintaş E, Kırkan Ş, Akşit H, Küçükyılmaz K, Erbaş G, Cabuk M, Akşit D, Parın U, Ege G (2016) Comparative evaluation of dietary supplementation with mannan oligosaccharide and oregano essential oil in forced molted and fully fed laying hens between 82 and 106 weeks of age. Poult Sci 95(11):2576–2591
Bratburd JR, Keller C, Vivas E, Gemperline E, Li L, Rey FE, Currie CR (2018) Gut microbial and metabolic responses to Salmonella enterica serovar Typhimurium and Candida albicans. mBio 9(6):e02032–e02018. https://doi.org/10.1128/mBio.02032-18
Buts J-P, De Keyser N, De Raedemaeker L (1994) Saccharomyces boulardii enhances rat intestinal enzyme expression by endoluminal release of polyamines. Pediatr Res 36(4):522–527
Callaway TR, Dowd SE, Wolcott RD, Sun Y, McReynolds JL, Edrington TS, Byrd JA, Anderson RC, Krueger N, Nisbet DJ (2009) Evaluation of the bacterial diversity in cecal contents of laying hens fed various molting diets by using bacterial tag-encoded FLX amplicon pyrosequencing. Poult Sci 88(2):298–302
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Meth 7(5):335
Casey PG, Gardiner GE, Casey G, Bradshaw B, Lawlor PG, Lynch PB, Leonard FC, Stanton C, Ross RP, Fitzgerald GF, Hill C (2007) A five-strain probiotic combination reduces pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol 73(6):1858–1863. https://doi.org/10.1128/aem.01840-06
Chang CH, Teng PY, Lee TT, Yu B (2019) The effects of the supplementation of multi-strain probiotics on intestinal microbiota, metabolites and inflammation of young SPF chickens challenged with Salmonella enterica subsp. enterica. Anim Sci J 90(6):737–746. https://doi.org/10.1111/asj.13205
Chart H, Baskerville A, Humphrey T, Rowe B (1992) Serological responses of chickens experimentally infected with Salmonella enteritidis PT4 by different routes. Epidemiol Infect 109(2):297–302
Chen Y-S, Srionnual S, Onda T, Yanagida F (2007) Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett Appl Microbiol 45(2):190–193. https://doi.org/10.1111/j.1472-765X.2007.02167.x
Cox NA, McHan F, Bailey JS, Shotts EB (1994) Effect of butyric or lactic acid on the in vivo colonization of Salmonella typhimurium. J Appl Poult Res 3(4):315–318
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, Rychlik I (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect Immun 79(7):2755–2763
Cui Y, Wang Q, Liu S, Sun R, Zhou Y, Li Y (2017) Age-related variations in intestinal microflora of free-range and caged hens. Front Micro 8:1310–1310. https://doi.org/10.3389/fmicb.2017.01310
Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M (2013) Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14(1):26–37. https://doi.org/10.1016/j.chom.2013.06.007
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072
Ding XM, Li DD, Bai SP, Wang JP, Zeng QF, Su ZW, Xuan Y, Zhang KY (2017) Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult Sci 97(3):874–881
Donalson LM, McReynolds JL, Kim WK, Chalova VI, Woodward CL, Kubena LF, Nisbet DJ, Ricke SC (2008) The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella Enteritidis infection, and intestinal shedding in laying hens. Poult Sci 87(7):1253–1262
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Meth 10(10):996
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Engels C, Ruscheweyh H-J, Beerenwinkel N, Lacroix C, Schwab C (2016) The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Micro 7:713
Fanning S, Hall LJ, van Sinderen D (2012) Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 3(5):420–425
Fearnley E, Raupach J, Lagala F, Cameron S (2011) Salmonella in chicken meat, eggs and humans; Adelaide, South Australia, 2008. Int J Food Microbiol 146(3):219–227. https://doi.org/10.1016/j.ijfoodmicro.2011.02.004
Fekry MI, Engels C, Zhang J, Schwab C, Lacroix C, Sturla SJ, Chassard C (2016) The strict anaerobic gut microbe Eubacterium hallii transforms the carcinogenic dietary heterocyclic amine 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP). Environ Microbiol Rep 8(2):201–209
Forte C, Acuti G, Manuali E, Casagrande Proietti P, Pavone S, Trabalza-Marinucci M, Moscati L, Onofri A, Lorenzetti C, Franciosini M (2016) Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult Sci 95(11):2528–2535
Fukata T, Sasai K, Miyamoto T, Baba E (1999) Inhibitory effects of competitive exclusion and fructooligosaccharide, singly and in combination, on Salmonella colonization of chicks. J Food Prot 62(3):229–233
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66(5):365–378
Gole VC, Torok V, Sexton M, Caraguel CGB, Chousalkar KK (2014) Association between indoor environmental contamination by Salmonella enterica and contamination of eggs on layer farms. J Clin Microbiol 52(9):3250–3258. https://doi.org/10.1128/jcm.00816-14
Goodrich JK, Davenport ER, Waters JL, Clark AG, Ley RE (2016) Cross-species comparisons of host genetic associations with the microbiome. Science 352(6285):532–535
Gophna U, Konikoff T, Nielsen HB (2017) Oscillospira and related bacteria–From metagenomic species to metabolic features. Environ Microbiol 19(3):835–841
Haghighi HR, Abdul-Careem MF, Dara RA, Chambers JR, Sharif S (2008) Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet Microbiol 126(1):225–233. https://doi.org/10.1016/j.vetmic.2007.06.026
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291(5505):881–884
Huang P, Zhang Y, Xiao K, Jiang F, Wang H, Tang D, Liu D, Liu B, Liu Y, He X (2018) The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 6(1):211
Juricova H, Videnska P, Lukac M, Faldynova M, Babak V, Havlickova H, Sisak F, Rychlik I (2013) Influence of Salmonella enterica serovar Enteritidis infection on the development of the cecum microbiota in newly hatched chicks. Appl Environ Microbiol 79(2):745–747. https://doi.org/10.1128/aem.02628-12
Kubasova T, Kollarcikova M, Crhanova M, Karasova D, Cejkova D, Sebkova A, Matiasovicova J, Faldynova M, Pokorna A, Cizek A (2019) Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One 14(3):e0212446
Kurtoglu V, Kurtoglu F, Seker E, Coskun B, Balevi T, Polat E (2004) Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol. Food Addit Contam 21(9):817–823
Liu L, Lin L, Zheng L, Tang H, Fan X, Xue N, Li M, Liu M, Li X (2018) Cecal microbiome profile altered by Salmonella enterica, serovar Enteritidis inoculation in chicken. Gut Pathog 10(1):34–14. https://doi.org/10.1186/s13099-018-0261-x
Luoma A, Markazi A, Shanmugasundaram R, Murugesan GR, Mohnl M, Selvaraj R (2017) Effect of synbiotic supplementation on layer production and cecal Salmonella load during a Salmonella challenge. Poult Sci 96(12):4208–4216
Mackie RI, Aminov RI, Hu W, Klieve AV, Ouwerkerk D, Sundset MA, Kamagata Y (2003) Ecology of uncultivated Oscillospira species in the rumen of cattle, sheep, and reindeer as assessed by microscopy and molecular approaches. Appl Environ Microbiol 69(11):6808–6815
Marcq C, Cox E, Szalo IM, Thewis A, Beckers Y (2011) Salmonella Typhimurium oral challenge model in mature broilers: bacteriological, immunological, and growth performance aspects. Poult Sci 90(1):59–67
McWhorter AR, Chousalkar KK (2018) A long-term efficacy trial of a live, attenuated Salmonella Typhimurium vaccine in layer hens. Front Micro 9:1380–1380. https://doi.org/10.3389/fmicb.2018.01380
Mikulski D, Jankowski J, Naczmanski J, Mikulska M, Demey V (2012) Effects of dietary probiotic (Pediococcus acidilactici) supplementation on performance, nutrient digestibility, egg traits, egg yolk cholesterol, and fatty acid profile in laying hens. Poult Sci 91(10):2691–2700
Milani C, Lugli GA, Duranti S, Turroni F, Mancabelli L, Ferrario C, Mangifesta M, Hevia A, Viappiani A, Scholz M (2015) Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci Rep 5:15782
Mon KKZ, Saelao P, Halstead MM, Chanthavixay G, Chang H-C, Garas L, Maga EA, Zhou H (2015) Salmonella enterica serovars Enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front Vet Sci 2:61
Montgomery L (1988) Isolation of human colonic fibrolytic bacteria. Lett Appl Microbiol 6(3):55–57
Moon YH, Iakiviak M, Bauer S, Mackie RI, Cann IKO (2011) Biochemical analyses of multiple endoxylanases from the rumen bacterium Ruminococcus albus 8 and their synergistic activities with accessory hemicellulose-degrading enzymes. Appl Environ Microbiol 77(15):5157–5169
Naqid IA, Owen JP, Maddison BC, Gardner DS, Foster N, Tchórzewska MA, La Ragione RM, Gough KC (2015) Prebiotic and probiotic agents enhance antibody-based immune responses to Salmonella Typhimurium infection in pigs. Anim Feed Sci Technol 201:57–65. https://doi.org/10.1016/j.anifeedsci.2014.12.005
Ngunjiri JM, Taylor KJM, Abundo MC, Jang H, Elaish M, Kc M, Ghorbani A, Wijeratne S, Weber BP, Johnson TJ, Lee C-W (2019) Farm stage, bird age and body site dominantly affect the quantity, taxonomic composition, and dynamics of respiratory and gut microbiota of commercial layer chickens. Appl Environ Microbiol 85(9):e03137–e03118. https://doi.org/10.1128/aem.03137-18
Nordentoft S, Mølbak L, Bjerrum L, De Vylder J, Van Immerseel F, Pedersen K (2011) The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T-RFLP and 454 pyrosequencing. BMC Microbiol 11(1):187. https://doi.org/10.1186/1471-2180-11-187
Ocejo M, Oporto B, Hurtado A (2019) 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci Rep 9(1):2506. https://doi.org/10.1038/s41598-019-39323-x
O'Hara AM, O'Regan P, Fanning Á, O'Mahony C, MacSharry J, Lyons A, Bienenstock J, O'Mahony L, Shanahan F (2006) Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118(2):202–215. https://doi.org/10.1111/j.1365-2567.2006.02358.x
Park JW, Jeong JS, Lee SI, Kim IH (2016) Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult Sci 95(12):2829–2835
Penha Filho RAC, Díaz SJA, Fernando FS, Chang Y-F, Andreatti Filho RL, Berchieri Junior A (2015) Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus based probiotic. Vet Immunol Immunopathol 167(1):64–69. https://doi.org/10.1016/j.vetimm.2015.06.006
Pourabedin M, Xu Z, Baurhoo B, Chevaux E, Zhao X (2014) Effects of mannan oligosaccharide and virginiamycin on the cecal microbial community and intestinal morphology of chickens raised under suboptimal conditions. Can J Microbiol 60(5):255–266. https://doi.org/10.1139/cjm-2013-0899
Robert C, Bernalier-Donadille A (2003) The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol 46(1):81–89
Savignac HM, Kiely B, Dinan TG, Cryan JF (2014) Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 26(11):1615–1627
Shang HM, Hu TM, Lu YJ, Wu HX (2010) Effects of inulin on performance, egg quality, gut microflora and serum and yolk cholesterol in laying hens. Br Poult Sci 51(6):791–796. https://doi.org/10.1080/00071668.2010.531005
Shini S, Shini A, Blackall PJ (2013) The potential for probiotics to prevent reproductive tract lesions in free-range laying hens. Anim Prod Sci 53(12):1298–1308. https://doi.org/10.1071/AN12337
Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P (2017) The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 15:630–638. https://doi.org/10.1038/nrmicro.2017.58
Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J (2008) Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14(3):166–171. https://doi.org/10.1016/j.anaerobe.2008.02.001
Stanley D, Denman SE, Hughes RJ, Geier MS, Crowley TM, Chen H, Haring VR, Moore RJ (2012) Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol 96(5):1361–1369
Stappenbeck TS, Hooper LV, Gordon JI (2002) Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A 99(24):15451–15455
Tang SGH, Sieo CC, Ramasamy K, Saad WZ, Wong HK, Ho YW (2017) Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and synbiotic. BMC Vet Res 13(1):248
Treem WR, Ahsan N, Shoup M, Hyams JS (1994) Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 18(2):159–164
Van Coillie E, Goris J, Cleenwerck I, Grijspeerdt K, Botteldoorn N, Van Immerseel F, De Buck J, Vancanneyt M, Swings J, Herman L, Heyndrickx M (2007) Identification of Lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J Appl Microbiol 102(4):1095–1106. https://doi.org/10.1111/j.1365-2672.2006.03164.x
Videnska P, Sedlar K, Lukac M, Faldynova M, Gerzova L, Cejkova D, Sisak F, Rychlik I (2014) Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One 9(12):e115142
Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R (2011) Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22(9):849–855
Volf J, Polansky O, Varmuzova K, Gerzova L, Sekelova Z, Faldynova M, Babak V, Medvecky M, Smith AL, Kaspers B (2016) Transient and prolonged response of chicken cecum mucosa to colonization with different gut microbiota. PLoS One 11(9):e0163932
Vollenweider S, Evers S, Zurbriggen K, Lacroix C (2010) Unraveling the hydroxypropionaldehyde (HPA) system: an active antimicrobial agent against human pathogens. J Agric Food Chem 58(19):10315–10322
Williams Smith H, Tucker JF (1980) The virulence of Salmonella strains for chickens: their excretion by infected chickens. J Hyg (Lond) 84(3):479–488
Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion M-J, Berger B, Krause L (2016) Calypso: a user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 33(5):782–783
Zhang ZF, Kim IH (2013) Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J Anim Sci 91(10):4781–4787
Zhang JL, Xie QM, Ji J, Yang WH, Wu YB, Li C, Ma JY, Bi YZ (2012) Different combinations of probiotics improve the production performance, egg quality, and immune response of layer hens. Poult Sci 91(11):2755–2760
Zhang J, Kobert K, Flouri T, Stamatakis A (2013) PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30(5):614–620
Acknowledgements
Help in sample collection from Nitish Narendra Joat (The University of Adelaide) is acknowledged. Critical editorial comments on the manuscript by Juliet Roberts (University of New England, Australia) are highly acknowledged.
Funding
This study was financially supported by the Australian Eggs Limited under grant number 1FS802UA to Kapil K. Chousalkar. The funding body was not involved in the collection, analysis and interpretation of the data or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
K.K.C. developed the hypothesis and designed the experimental work. S.K. and K.K.C. performed the experimental work, analysed the data and wrote the manuscript. Both the authors reviewed and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethics statement
The Animal Ethics Committee at the University of Adelaide approved the work (approval number S-2017-080) in accordance with the guidelines specified in “Australian code for the care and use of animals for scientific purposes, 8th edition (2013).” Standard Operative Procedures were followed for caring and processing of the experimental chicks.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1156 kb).
Rights and permissions
About this article
Cite this article
Khan, S., Chousalkar, K.K. Short-term feeding of probiotics and synbiotics modulates caecal microbiota during Salmonella Typhimurium infection but does not reduce shedding and invasion in chickens. Appl Microbiol Biotechnol 104, 319–334 (2020). https://doi.org/10.1007/s00253-019-10220-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10220-7