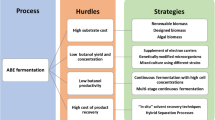
Abstract
There is a renewed interest in acetone-butanol-ethanol (ABE) fermentation from renewable substrates for the sustainable and environment-friendly production of biofuel and platform chemicals. However, the ABE fermentation is associated with several challenges due to the presence of heterogeneous components in the renewable substrates and the intrinsic characteristics of ABE fermentation process. Hence, there is a need to select optimal substrates and modify their characteristics suitable for the ABE fermentation process or microbial strain. This “designed biomass” can be used to establish the consolidated bioprocessing systems. As there are very few reports on designed biomass, the main objectives of this review are to summarize the main challenges associated with ABE fermentation from renewable substrates and to introduce feasible strategies for designing the substrates through pretreatment and hydrolysis technologies as well as through the establishment of consolidated bioprocessing systems. This review offers new insights on improving the efficiency of ABE fermentation from designed renewable substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2017, the International Energy Agency (IEA) predicted that there will be a 30% increase in global energy demand by 2040. More than 2.7 billion people still use wood and other solid fuels or kerosene for cooking and lighting. Additionally, fossil fuels are used as an energy source in power plants, industrial facilities, and vehicles. These traditional fuels cause air pollution. According to the World Health Organization (WHO), air pollution causes 6.5 million deaths every year (OECD/IEA 2016). Renewable resources can be an alternative energy source to overcome these challenges and to attain energy security. The renewable resources mainly include biomass energy, geothermal energy, hydropower energy, marine energy, solar energy, and wind energy. Biomass energy has several advantages, such as reduction in greenhouse gas emissions, high constancy and reliability through local distribution, and economic development of rural communities (Ellabban et al. 2014). Currently, energy from most biomasses, including agricultural wastes, is obtained by direct combustion, which causes air pollution. However, biomasses can be converted to biofuels, which can be transported, stored, and used as fossil fuels. This can aid in reducing the air pollution.
Compared to traditional biofuels, biobutanol is a more efficient biofuel as it has a higher heating value, lower volatility, less ignition problems, higher intersolubility with gasoline, and higher viscosity and lubricity (Trindade and Santos 2017). Biobutanol is reported to exhibit similar performance in engine ignition as gasoline (Trindade and Santos 2017). Biobutanol can be produced by acetone-butanol-ethanol (ABE) fermentation.
Currently, studies are ongoing to utilize cheap renewable substrates for the production of commercially viable biobutanol. The utilization of renewable substrates (mainly lignocellulose) requires complex processes and is associated with several challenges. The characteristics of the renewable substrates, such as rigid structure and heterogenous sugar components, are the major obstacles for biobutanol production. These obstacles can be addressed using advanced technologies. Most studies are focused on strain isolation and engineering, pretreatment technologies, and development of advanced ABE fermentation processes. The objective of these studies was to devise strategies for efficient substrate utilization based on substrate characteristics. However, there are some studies that have focused on the modification of substrate composition suitable for the strains and advanced ABE fermentation processes.
There are several reviews on the identification of suitable biomass, microorganism isolation and engineering, medium modification, butanol recovery, and advanced fermentation processes to overcome the challenges of ABE fermentation (Lee et al. 2016; Bharathiraja et al. 2017; Gottumukkala et al. 2017; Ibrahim et al. 2018). This review focuses on the selection and modification of substrates and consolidated systems for improving the efficiency of ABE fermentation. The application of the “designed biomass” is discussed in this review. The process of designing biomass includes substrate selection and modification customized for the microbial strains and advanced fermentation processes. This review summarizes the characteristics of the renewable substrates, microbial strains, and the advanced fermentation processes. Subsequently, the methods and tools for the application of designed biomasses are reviewed, including substrate selection, appropriate pretreatment, and enzymatic hydrolysis methods applicable for substrate modification and establishment of consolidated systems using the designed biomasses. There are very few reports on the designed biomass for ABE fermentation. Hence, this review has a broad prospect in this area.
Designed biomass study
In traditional studies on fermentative production from biomasses, the targeted biomass is initially selected, and mainly two methods are applied (Abdel-Rahman et al. 2011). The first method is the mutagenesis method or molecular breeding method for the microbial strain, which can improve or promote the biomass degradation and utilization by the microbial strain (Lu et al. 2017). The second method is a screening method to isolate a strain that can ferment the biomass components to produce a value-added substance, and subsequently the establishment of an efficient process (Youn et al. 2016). In the fermentation process of designed biomass, a well-known robust microbial strain or an established highly efficient process obtained in the traditional study can be applied (Zhao et al. 2018). Furthermore, the optimal substrate can be designed for the candidate microbial strains or fermentation process through modification of substrate characteristics to establish an adaptive process (Zhao et al. 2019). The designed biomass includes not only saccharides but also organic acids and glycerol.
General characteristics of ABE fermentation
ABE-producing clostridial strains and metabolism
The production of biobutanol through ABE fermentation started in 1912 and peaked in 1950, when approximately two thirds of the world’s butanol (about 18,168 m3) was produced through ABE fermentation process. Subsequently, the production of biobutanol decreased because of the high substrate costs and cheaper production of biobutanol through chemical synthesis. However, the increased concerns on air pollution, global warming, energy demand, and energy security have resulted in renewed interest in ABE fermentation process (Dürre 2007).
Four wild-type strains are widely used in the ABE fermentation process: Clostridium acetobutylicum, C. beijerinckii, C. saccharoperbutylacetonicum, and C. saccharobutylicum. C. acetobulicum ATCC 824 is the most well-studied ABE-producing strain. This strain produces acetone, butanol, and ethanol in the ratio of 3:6:1 through two distinctive phases of metabolic pathways: acidogenesis and solventogenesis (Jones and Woods 1986). During the acidogenesis, an ABE-producing strain ferments carbon sources to organic acids, such as acetic acid and butyric acid with the release of energy and production of reducing equivalents, which decreases the pH of the medium. When the cells enter stationary phase, the metabolism switches from acidogenesis to solventogenesis, where organic acids are utilized for the production of organic solvents, such as acetone, butanol, and ethanol, which subsequently increases the pH of the medium (Tashiro et al. 2013). The ABE-producing strain can metabolize several substrates, including hexoses (glucose, mannose, galactose, and fructose), pentoses (xylose and arabinose), disaccharides (sucrose, lactose, and cellobiose), and starch (Jones and Woods 1986; Patakova et al. 2013; Raganati et al. 2015). These substrates can be transported through the PEP-dependent phosphotransferase system (PTS) and non-PTS mechanisms, such as H+-symport, Na+-symport, and ATP-binding cassette (ABC) systems (Mitchell 2016). Subsequently, hexoses are metabolized via the Embden-Meyerhof-Parnas (EMP) pathway. The pentose sugars are metabolized in the pentose phosphate pathway (PPP) resulting in the production of fructose 6-phosphate and glyceraldehyde 3-phosphate, which enter the EMP pathway. Hence, converting 1 mol of hexose to 2 mol of pyruvate generates 2 mol of ATP and 2 mol of NADH, and converting 3 mol of pentose generates 5 mol of ATP and 5 mol of NADH (Jones and Woods 1986).
Changes of metabolic fluxes by the ABE-producing clostridial strains
Typically, acetone, butanol, and ethanol are produced in the ratio of 3:6:1 (Jones and Woods 1986). However, the formation of by-products can result in a butanol yield of less than 0.3 g/g-sugar (Gao et al. 2015). Jiang et al. mutated the acetoacetate decarboxylase gene (adc) in C. acetobutylicum to decrease the production of acetone. The production of acetone in the adc mutant of C. acetobutylicum decreased from 2.83 to 0.21 g/L. In addition to decreased acetone production, accumulation of acetic acid was observed. Additionally, the butanol production decreased from 13.6 to 7.4 g/L. However, the butanol production in the adc mutant recovered upon supplementation of methyl viologen (MV), an artificial electron carrier, with decreased accumulation of acetic acid and acetone (Jiang et al. 2009). Additionally, overexpression of aldehyde/alcohol dehydrogenase (ald) and CoA-transferase (ctfAB) in C. beijerinckii increased the concentration of butanol and acetone by 15.3% and 108%, respectively. The overexpression of ald and ctfAB also stimulated the accumulation of acids, which decreased the butanol/acetone ratio from 8.17 to 4.52 (Lu et al. 2017). Liu et al. introduced a synthetic 2,3-butanediol synthesis pathway in C. acetobutylicum to compensate for the reducing power (NADH), which increased the butanol concentration from 11.4 to 12.1 g/L and decreased the acetone concentration to less than 0.3 g/L (Liu et al. 2018). These studies suggested that the butanol/acetone ratio is determined not only by the expression levels or activities of modified genes related to ABE production but also by the NADH/NAD+ ratio. Additionally, the NADH/NAD+ ratio decreases in the presence of soluble lignin compounds of corn stover hydrolysate derived from pretreatment and hydrolysis processes (Liu et al. 2017), while the ratio increases when glycerol is used as a substrate (Ujor et al. 2014). Thus, it is important to select a suitable substrate that can improve the NADH/NAD+ ratio.
Sources and characterization of renewable substances for ABE production
Traditional ABE fermentation process uses first-generation substrates, such as molasses, whey permeate, corn, cassava, potato, and Jerusalem artichokes. These substrates are also used for consumption, and thus, the use of these substrates increases the cost of butanol production (Zheng et al. 2015b). The high cost of butanol discourages investment in the fuel market (Mariano et al. 2013). Thus, there is a continued search for cheaper and more suitable alternative substrates for ABE fermentation. The potential sources for second-generation substrates include short-rotation coppice (poplar and eucalyptus), perennial cultivation (switchgrass and reed canary grass), agriculture (straw and stover), forestry and logging (treetops and branches), crop processing (coffee and corn), sugar and first-generation bioethanol production process (sugar cane and sweet sorghum), vegetable oil production (canola and oil palm), forestry processing (sawdust and bark), and municipal solid waste (palettes and furniture) (Eisentraut 2010).
Most of the second-generation substrates are lignocellulosic biomasses, mainly consisting of cellulose, hemicellulose, and lignin. Cellulose is a linear chain of several hundred to thousand D-glucose units linked together by β-(l→4)-glycosidic bonds. Several cellulose chains form microfibrils, which are stabilized by the hydrogen bonds. The microfibrils are assembled into macrofibrils to form the crystalline cellulose. The low permeability of crystalline cellulose does not allow the penetration of even water molecules. Moreover, cellulose also forms amorphous region. Contrastingly, hemicellulose is a short-chain branched heteropolymer, consisting of D-xylose, L-arabinose, D-mannose, D-glucose, D-galactose, and D-glucuronic acid. The compositions, glycosidic linkages, side-chain, and degree of polymerization of cellulose and hemicellulose vary depending on the types of plants and tissues. Unlike cellulose and hemicellulose, lignin does not contain sugar units and is composed of three major monomeric units: p-hydroxyphenyl (H), guaicyl (G), and syringyl (S). The ratio of these components vary between different plants and tissues (Menon and Rao 2012; Zhao et al. 2012). In nature, these components are attached to each other as well as to other polymers, such as pectin in a matrix, which prevents the degradation of biomass and forms the crystalline structure. Hence, utilization of biomasses requires pretreatment and enzymatic hydrolysis before fermentation process.
Challenges in ABE fermentation from renewable substrates
Insufficient utilization of substrate
The raw materials required for lignocellulosic butanol production include lignocellulosic biomasses, pretreatment liquor, water, enzymes, and medium. The raw materials are estimated to account for 26.82% of the annual operating cost even though the lignocellulosic biomasses are cheap substrates (Qureshi et al. 2013). Thus, the efficiency of butanol production is dependent on the efficient utilization of raw materials.
The hydrolysis methods and fermentation process does not efficiently utilize sugars in the lignocellulosic biomasses. In batch enzymatic hydrolysis, cellulose degradation is initiated from the accessible fraction with fast hydrolysis rate. However, hydrolysis rate subsequently decreases because the residual recalcitrant fraction is difficult to hydrolyze. This results in incomplete digestion of cellulose (Arantes and Saddler 2011; Jin et al. 2012). However, hemicellulose, which is the second most abundant polysaccharide of biomasses (Menon and Rao 2012), can be degraded at a lower temperature than cellulose (Bahrin et al. 2012) and can be completely degraded during pretreatment (Zhao et al. 2009; Leu and Zhu 2013). Additionally, pentose sugars in hemicellulose can be utilized by most ABE-producing Clostridia (Tashiro et al. 2013). Thus, efficient ABE fermentation from biomasses requires utilization of sugars from both cellulose and hemicellulose. However, as shown in Table 1, most studies mainly focus on the cellulose hydrolysis because glucose is the most preferred sugar for most microorganisms (Mitchell 2016). Moreover, the consumption of pentose sugars is readily inhibited by carbon catabolite repression (CCR) in the presence of glucose (Tashiro et al. 2013) in both batch mode (Noguchi et al. 2013) and continuous mode (Kihara et al. 2019).
In addition to lignocellulosic biomasses, enzymes are also important raw materials. The production of glucose from biomasses requires high enzyme load due to the insufficient activity of β-glucosidase in commercial cellulases and product inhibition of enzyme activity by glucose during the hydrolytic process (Rani et al. 2014; Payne et al. 2015). However, loading enzymes over the saturation level leads to competition for the binding site of biomasses between the enzymes, which reduces the synergistic action of different enzymes (Van Dyk and Pletschke 2012). Additionally, the residual recalcitrant fractions and lignin of lignocellulose absorb some enzymes, which prevents the enzyme activity on fresh substrate (Arantes and Saddler 2011; Lü et al. 2017). Thus, the hydrolysis and fermentation processes must be designed carefully.
Furthermore, medium also influences the efficiency of fermentation from biomass hydrolysate. Absence of essential nitrogen sources decreases the butanol concentration (Arifin et al. 2014). However, fermentation with costly medium used in the laboratory cannot be scaled up for industrial fermentation with biomasses (Pfromm et al. 2010). Moreover, the nitrogen resources may react with carbohydrates through Maillard reaction, which decreases the enzyme activities (Bruins et al. 2003; Xiong et al. 2005).
Inhibitors
Some inhibitors are formed during the pretreatment of lignocellulose and ABE fermentation, such as the phenolic compounds, aliphatic acids, furan aldehydes, inorganic ions, and bioalcohols (Qureshi et al. 2013). The phenolic compounds are reported to be the most toxic inhibitors. The phenolic compounds interfere with the metabolic pathway generating butanol (Cho et al. 2009). To detoxify the inhibitors, both bacteria and yeast use NADH/NADPH to convert the inhibitors to less toxic compounds and consume ATP to expel the inhibitors (Piotrowski et al. 2014). Similarly, butanol is an intrinsic inhibitor, which is reported to be toxic to the bacteria at concentration range of 15–20 g/L (Ibrahim et al. 2017). Butanol destabilizes the internal pH, lowers ATP, and inhibits sugar uptake during ABE fermentation (Baral and Shah 2014). Thus, the candidate substrate should have low lignin content and high reducing power.
Limitation of substrate loading
In general batch hydrolysis with low substrate loading, sugar concentrations are too low, which limits butanol concentration in ABE fermentation (Ibrahim et al. 2017). This limitation can be overcome through high substrate loading. However, the accumulation of residual solid in the reactor affects the hydrolysis rate (Gupta et al. 2012). Hydrolysis rate can also be decreased when the agitation efficiency is low (Kadić and Lidén 2017). The negative effect of high solid loading is dependent on both enzyme and substrate used in the fermentation process and can be reduced by modifying the biomass (Weiss et al. 2019).
Development of designed biomass to address the challenges of ABE fermentation
Substrate selection
Lignocellulosic substrates
Numerous lignocellulosic biomasses have been widely used for ABE fermentation, including woody biomasses, such as eucalyptus (Zheng et al. 2017), pine, and elm woods (Amiri and Karimi 2015), and grass biomasses, such as Napier grass (He et al. 2017), rice straw (Amiri et al. 2014), corn stover (Baral et al. 2018), and switchgrass (Taylor et al. 2018). Generally, woody biomass contains 40–55% cellulose, 8–25% hemicellulose, and 18–35% lignin, whereas grass biomass contains 25–50% cellulose, 20–50% hemicellulose, and 10–30% lignin (Zhao et al. 2012).
The varying contents of cellulose, hemicellulose, and lignin in the biomass result in differential performances in enzymatic hydrolysis and ABE fermentation. Napier grass contains 36.8% cellulose and 23.4% lignin (He et al. 2017), while rice straw contains 49.2% cellulose and 17% lignin (Amiri et al. 2014). Enzymatic hydrolysis of NaOH-pretreated Napier grass yielded 82% glucose (He et al. 2017), whereas NaOH-pretreated rice straw yielded 46.2% glucose (Amiri et al. 2014). Gao et al. (2014) used NaOH to pretreat phragmites (34.40% cellulose and 28.83% lignin) and switchgrass (30.05% cellulose and 24.49% lignin) and obtained a glucose yield of 28% and 33%, respectively. Additionally, the butanol yield from phragmites (0.200 g/g) was lower than that from switchgrass (0.229 g/g). The high lignin content negatively affects the pretreatment, enzymatic hydrolysis, and fermentation as it physically hinders the enzyme activity on cellulose, limits hemicellulose degradation, and generates inhibitors (Davison et al. 2006; Zeng et al. 2014). Hence, biomasses with low lignin content are preferred for butanol production.
Low lignin content in biomasses is favorable for ABE fermentation. However, the substrate choice is also influenced by the cost and availability of the substrate. Even though the lignin content of barley straw (7–14%) was lower than that of wheat straw (17–24%) (Merino and Cherry 2007; Kumar et al. 2012), the butanol production cost using barley straw (0.7476 $/kg butanol) was higher than that using wheat straw (0.6856 $/kg butanol) (Kumar et al. 2012). The availability of biomasses is district dependent. In the USA, 8.8 million tons of barley straw and 3 million tons of wheat straw are produced. In Mexico, 0.895 million tons of barley straw and 96.8 million tons of wheat straw are produced (Eisentraut 2010; Balan 2014). The availability of biomass influences the cost of butanol production. The cost of butanol production in a production plant with a processing capacity of 751 × 106 kg wheat straw/year was $1.30/kg, which was lower than that in a production plant with a processing capacity of 150 × 106 kg wheat straw/year ($2.73/kg) (Qureshi et al. 2013). Therefore, the biomasses must be selected considering their availability and low lignin content.
To meet the availability of biomasses, the biomasses can be mixed with each other or other resources. Yang et al. mixed salix hydrolysate with starchy slurry, which improved the xylose consumption from 29 to 81%. Additionally, the ABE yield increased from 0.26 to 0.33 g/g sugars (Yang et al. 2017). The starchy slurry not only provides nutrients for ABE fermentation but also improves butanol production. However, efforts are ongoing to discover more mixtures of different substrates.
In addition to the raw biomasses, industry products of lignocellulosic biomass origin are an alternative choice, such as waste office paper (Ikeda et al. 2006) and paper mill sludge (Gogoi et al. 2018). Previously, Zhao et al. have used paper pulp (93.2% glucan) for ABE fermentation (Zhao et al. 2019). Zhao et al. demonstrated the enzymatic hydrolysis of paper pulp without pretreatment. Additionally, ABE fermentation was not inhibited using paper pulp hydrolysate as observed when commercial sugars are used as substrates. This indicated that the paper pulp hydrolysate does not contain inhibitors. The industry products are expected to reduce biomass collection cost as they are placed in collections. Additionally, the use of industry products can decrease the pretreatment cost. The construction of a production plant with a processing capacity of 751 × 106 kg wheat straw/year was estimated to be as high as 104,931,000 dollars (Qureshi et al. 2013).
Non-lignocellulosic substrates
Some non-cellulosic substrates are suitable substrates for ABE fermentation. Ujor et al. supplemented glycerol during ABE fermentation, which increased the butanol production by 2.3-fold and mitigated the negative effect of furfural (Ujor et al. 2014). Organic acids, such as lactic acid and acetic acid, are also suitable substrates for ABE fermentation. The organic acids are used in ABE fermentation along with commercial sugars (Tashiro et al. 2004; Oshiro et al. 2010; Yoshida et al. 2012; Gao et al. 2015). The concentration and yield of butanol increased when 5 g/L lactic acid was used along with 20 g/L glucose (Oshiro et al. 2010). Exogenous acetic acid altered the metabolic flux and stimulated solventogenesis in the early acidogenic growth phase (Gao et al. 2015). However, consumption of acetic acid increased acetone concentration by 90.5% (Gao et al. 2015), which decreased the butanol to acetone ratio by 27.1%, whereas high lactic acid concentration resulted in insufficient substrate consumption (Oshiro et al. 2010). The co-consumption of mixed organic acids (2.2 g/L acetic acid, 1.8 g/L lactic acid, and 0.6 g/L formic acid) offset the inhibitory effect of phenolics (Bellido et al. 2018). In the presence of 1.33 g/L lactic acid and 0.31 g/L glycerol, the butanol to ABE ratio improved from 0.6 to 0.85 (Hou et al. 2017).
The organic acids can be produced by lactic acid fermentation or can be recovered from the industry wastes (Demiral and Yildirim 2003; Oshiro et al. 2010). Thus, they are an ideal substrate for ABE fermentation. The organic acids are also present in the lignocellulosic biomasses and organic acid pretreatment effluent (Baral and Shah 2014; Taylor et al. 2018). However, the acids are always washed away and not used for ABE fermentation (Zabihi et al. 2010; Taylor et al. 2018). Hence, strategies must be devised for utilizing the waste acids.
Medium components supplemented to substrates
The components and dosage of medium used in fermentation also influence the fermentation performances and cost. Glucose, tryptone, yeast extract, peptone, K2HPO4, Na2CO3, and MgSO4 markedly affects the biobutanol production during fermentation of commercial sugars (Al-Shorgani et al. 2013). Yeast extract, tryptone, and FeSO4∙7H2O are reported to be a good source of nutrients for ABE production from eucalyptus hydrolysate (Zheng et al. 2017). In addition to the common nutrients, other metal ions and nitrogen sources could also improve ABE fermentation. Wu et al. added 4 g/L CaCO3 and 1 mg/L ZnSO4·7H2O to the fermentation broth, which improved the xylose consumption from 38.1 to 49.7 g/L and butanol concentration from 6.3 to 10.5 g/L (Wu et al. 2016). The metal ions are also present in lignocellulosic biomasses. The concentration of Zn in rice straw and sewage sludge is 77.88 and 88.87 mg/kg, respectively (Xin et al. 2019). Liao et al. reported that addition of proline mitigated the effect of lignocellulose-derived inhibitors (formic acid and phenolic compounds) (Liao et al. 2019). Proline is an important amino acid that protects the cells under stress conditions. Improved production of L-proline can be achieved in Chlorella sp. 580 algae (Leavitt 1983). Therefore, these biomasses containing special nutrients are also potential candidate substrate for ABE fermentation.
Substrate modification
Pretreatment
Cellulose accessibility is dependent on the accessible surface area of cellulose, chemical composition (lignin/hemicellulose content), and physical properties of biomass (particle size and porosity) (Meng and Ragauskas 2014). Organosolv pretreatment is one of the environment-friendly and efficient pretreatment methods for improving the cellulose accessibility. The organosolv pretreatments can cleave the internal bonds in lignin and those between lignin and hemicellulose and promote hydrolysis of glycosidic bonds in hemicelluloses and cellulose (Zhao et al. 2009; Zhang et al. 2016). Harrison et al. reported that glycerol pretreatment was more efficient in reducing the levels of sugar degradation products (furfural and 5-hydroxymethylfurfural (5-HMF)) than dilute acid pretreatment (Harrison et al. 2013). Organic acid pretreatment is another environment-friendly pretreatment method. The wheat straw lignin extracted by mixed formic acid and acetic acid is used as a phenol substitute for green phenolic resins (Tachon et al. 2016). However, this pretreatment method is not used for ABE fermentation. As the pretreatment agents are potential substrates for ABE fermentation, the effect of these agents on ABE fermentation must be investigated.
Semi-hydrolysis
Consumption of pentose sugar during ABE fermentation is readily inhibited by high glucose concentration due to carbon catabolite repression (CCR) (Tashiro et al. 2013). CCR can be inhibited by co-fermentation of xylose and cellobiose instead of glucose (Noguchi et al. 2013). Semi-hydrolysis results in the production of a mixture of cellobiose and glucose from pentose sugars using cellulase with low β-glucosidase activity (Zhao et al. 2018). The semi-hydrolysate inhibits CCR, reduces enzyme loading, and improves butanol production. However, the disadvantage of semi-hydrolysis is product inhibition of cellulase by cellobiose. This can be addressed using multifunctional cellulases. Some multifunctional cellulases (Umcel9y-1, Td2F2, and CoGH1A) can mitigate the product inhibition through transglycosylation (Wang et al. 2017a). The Umcel9y-1 enzyme, which has endo-/exoglucanase activities, can convert cellobiose and glucose to sophorose, laminaribiose, and gentiobiose and mitigate product inhibition (Zhou et al. 2016). However, further studies are needed to achieve commercial application of these enzymes.
High solid loading
High solid loading for generating satisfactory amount of sugar is essential for economic feasibility of ABE fermentation. However, high solid loading exhibits negative effect on enzymatic hydrolysis. Weiss et al. revealed that the cellulose conversion yield decreased when solid loading was over 15% (Weiss et al. 2019). The cellulose conversion yield of high solid loading could be improved through a combination of pretreatment. Papayannakos et al. compared different pretreatment methods using 15% solid loading. The highest concentration of glucose was obtained with a combination of organosolv and hydrothermal treatment, followed by organosolv pretreatment, alkali pretreatment, microwave-assisted acid pretreatment, and hydrothermal treatment (Papayannakos et al. 2019). Weiss et al. improved the cellulose hydrolysis yield using a combination of steam-exploded pretreatment and physical mill or chlorite pretreatment (Weiss et al. 2019). However, the combined pretreatment still could not improve the cellulose hydrolysis yield. The cellulose hydrolysis yield after 30% solid loading was around 60%, which was lower than cellulose hydrolysis yield of 80% obtained after 5% solid loading. The cellulose conversion yield can be improved by recycling the partially hydrolyzed material of fed batch mode or supplementation of surfactant (Weiss et al. 2019). Therefore, further studies are needed for using high solid loading.
Transgenic biomasses
To reduce severe pretreatments and high enzyme loading, transgenic biomasses can be used as substrates (Xiao et al. 2016). Shen et al. downregulated the expression of AC-I, AC-II, and AC-III elements of monolignol pathway genes, which resulted in decreased lignin content in switchgrass (Shen et al. 2012). Nigorikawa et al. enhanced the saccharification of rice straw by overexpression of exo-glucanase in planta (Nigorikawa et al. 2012). Although in planta expression of enzymes could lead to phenotypic defects, expression of hyperthermophilic enzymes and using a senescence-inducible promoter can mitigate the phenotypic defects (Park et al. 2016). Phenotypic defects can also be mitigated through overexpression of exo-glucanase under the control of a senescence-inducible promoter, which improves the saccharification of rice straw (Furukawa et al. 2014).
Transgenic plants can also be used as a molecular biofactory of cellulolytic enzymes, which can largely reduce the enzyme cost. In the production of cellulolytic enzymes, such as endoglucanase, exo-glucanase, and pectate lyases, the production cost of these enzymes from plants is 1057- to 3100-fold lower than that from fermentation (Wade et al. 2013).
Process design
Parameters to evaluate butanol production process
Many parameters have been used for studies on butanol production from renewable substrates as described below. Conventionally, sugar concentration (g), sugar yield to enzyme loading (g/U), and sugar (glucan, xylan, and arabinan) conversion (%) have been used to evaluate the hydrolysis process, while butanol concentration (g), butanol productivity (g/L/h), and butanol yield to consumed sugar (g/g, mol/mol, and C-mol/C-mol) are used to assess the fermentation efficiency (Andrić et al. 2010; Tashiro et al. 2013; Wang et al. 2017b; Zhao et al. 2018). It is important to note that these parameters should be used to evaluate the individual performances of hydrolysis and fermentation processes. Therefore, butanol yield to enzyme loading (g/U) and overall butanol productivity (g/L/h) have been proposed to comprehensively appraise the two processes along with butanol yield to biomass loading (g/g). These parameters could intuitively evaluate the efficiency of butanol production, especially for integrated and consolidated processes (Zhao et al. 2018; Zhao et al. 2019).
Parameters to evaluate hydrolysis process:
Sugar concentration (g) = Wsugar/Vhydrolysis
Sugar yield to enzyme loading (g/U) = Wsugar/Uenzymes
Sugar conversion (%) = Wsugar × ε/total polysaccharide × 100
Parameters to evaluate fermentation process:
Butanol concentration (g/L) = Wbutanol/Vfermentation
Butanol productivity (g/L/h) = Cbutanol/Tfermentation
Butanol yield to consumed sugar (g/g or mol/mol) = Wbutanol/Wconsumed sugar
Butanol yield to consumed sugar (C-mol/C-mol) = Mbutanol × 4/Mconsumed sugar × Ncarbon of sugar
Parameters to evaluate integrated and consolidated fermentation processes:
Butanol yield to biomass loading (g/g) = Wbutanol/Wbiomass loading
Butanol yield to enzyme loading (g/U) = Wbutanol/Uenzymes
Overall butanol productivity (g/L/h) = Cbutanol/(Thydrolysis + Tfermentation)
where W is the weight of each compound (g), C is the concentration of each compound (g/L), U is the total units loaded with cellulase and β-glucosidase, T is the time (h) of hydrolysis and fermentation, N is the number of carbon, and ε is anhydro corrections (0.88, 0.9, and 0.95 for xylose or arabinose, glucose, and cellobiose, respectively) (Zhao et al. 2018).
Separate hydrolysis and fermentation
In separate hydrolysis and fermentation (SHF), enzymatic hydrolysis, butanol fermentation, and butanol recovery are performed in different reactors. The advantage of SHF is that the processes can be performed separately under the respective optimal temperature conditions (45–60 °C for enzymatic hydrolysis and 30–37 °C for ABE fermentation) (Ibrahim et al. 2017). However, the disadvantages of SHF include end-product inhibition of cellulase and long reaction time, which leads to low butanol yield to enzyme loading and overall butanol productivity (Zhao et al. 2019). Moreover, the SHF requires preparation of new inoculum each time.
Simultaneous saccharification and fermentation
To avoid loss of cellulosic hydrolases, simultaneous saccharification and fermentation (SSF) has been investigated. In SSF, enzymatic hydrolysis and fermentation are conducted simultaneously in the same reactor. SSF can reduce the operation time and decrease the overall operational cost. SSF was expected to produce higher biobutanol yield and productivity from biomasses as they mitigate sugar product inhibition of cellulase system (Ibrahim et al. 2017). However, SSF of wheat straw was reported to result in lower ABE production (11.93 g/L) than separate hydrolysis and fermentation (SHF) (13.12 g/L) (Qureshi et al. 2008). This is because the optimal temperature of SSF process is different from that of cellulase activity (45–60 °C) for ABE fermentation (30–37 °C) (Ibrahim et al. 2017). To overcome this drawback, more efficient SSF process can be conducted at higher temperature or lower temperature using a thermotolerant ABE-producing strain or a cold-active hydrolytic enzymes, respectively (Wang et al. 2015).
Repeated enzymatic hydrolysis and fermentation
Most cellulosic hydrolases absorb the solid residue of lignocellulosic biomasses, which facilitates reutilization of the enzymes in repeated enzymatic hydrolysis and fermentation. Vanderghem et al. used repeated enzymatic hydrolysis and improved the conversion of cellulose to cellobiose by 4.4% compared to batch hydrolysis (Vanderghem et al. 2010). Zhao et al. designed the simultaneously repeated hydrolysis and fermentation (SRHF) as shown in Fig. 1. The SRHF process improves butanol yield to enzyme loading by 183% and overall butanol productivity by 6.04% (Zhao et al. 2018). However, conversion of cellulose to glucose decreases due to the loss of β-glucosidase activities. Although the β-glucosidase activities can be preserved using ultrafiltration recycling method, some enzymes lose their activity due to inefficient binding to the residual solid and lignin (Qi et al. 2011). The inefficient binding can be mitigated by feeding fresh substrate in a novel integrated process, which not only recycle the enzymes but also improve the hydrolysis of residual recalcitrant solid (Jin et al. 2012).
Non-isothermal simultaneous saccharification and fermentation
To address the limitations of SSF, non-isothermal simultaneous saccharification and fermentation (NSSF) has been developed. In NSSF, enzymatic hydrolysis and fermentation are performed simultaneously but in two separate reactors at respective optimum temperature (Jouzani and Taherzadeh 2015). NSSF has been used in ethanol fermentation (Chilari et al. 2017). A novel non-isothermal simultaneous saccharification and fermentation with in situ butanol recovery (NSSFR) has been developed, which improves butanol production (20.6 g/L), butanol yield to consumed sugar (0.847 C-mol/C-mol), butanol yield to solid loading (197 g/kg-pulp), butanol yield to enzyme loading (0.0360 g/U-cellulase), butanol yield to medium (20.6 g/L-medium), butanol productivity (0.343 g/L/h), and overall butanol productivity (0.286 g/L/h) (Zhao et al. 2019).
Conclusion
Biobutanol is an efficient biofuel as its chemical and physical characteristics are better than traditional biofuels. Biobutanol can be produced from acetone-butanol-ethanol (ABE) fermentation using inedible substrates, such as lignocellulosic materials and organic acids. Currently, several studies are focused on identification of biomass, microorganism isolation and engineering, medium modification, butanol recovery, and advanced fermentation modes. However, the fermentation process has several disadvantages, such as insufficient utilization of substrate, high production of acetone by-product and inhibitors, and limitation of substrate loading. To overcome the limitation of ABE fermentation, it is important to identify and modify substrates based on the candidate strain or process. The choice of lignocellulosic biomasses should ensure availability and low lignin content. Additionally, the use of biomasses containing special nutrients, transgenic biomasses exhibiting high cellulose accessibility, and non-cellulosic substrates, such as glycerol, organic acids, must be considered. Moreover, these substrates should be modified by pretreatment and enzymatic hydrolysis to enhance the production of favorable sugars during ABE fermentation. Finally, efficient modern fermentation processes using these designed biomass needs to be developed.
Perspectives and directions for future research
Although many studies have reported on ABE fermentation processes from several types of lignocellulosic biomasses as reviewed above, biobutanol is still not economically viable. There would be still drawbacks and challenges in each process as follows:
- 1.
Substrates: More available inedible substrate which consists of high fermentable sugar and low lignin content and inhibitors and which can be pretreated easily by an existing process would be designed. And then, a stable and sufficient supplementation of the substrates would be further required to be established.
- 2.
Hydrolytic enzymes: Multifunctional enzymes to hydrolyze several types of substrate simultaneously and cold-active enzymes to improve the hydrolysis efficiency in SSF system at the optimal temperature of fermentation would be discovered. In addition, cost-effective supplementation of the commercial enzymes would be achieved.
- 3.
ABE-producing strains: More thermotolerant ABE-producing strains to produce more butanol in SSF system at the optimal temperature of hydrolysis needs to be studied. In addition, breeding a hyper-butanol producing strain higher than 30 g/L would be necessary to improve a performance in separation and purification process for butanol followed by fermentation process.
These research efforts would contribute to construct an economically feasible consolidated bioprocessing system of biobutanol.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2011) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156:286–301
Al-Shorgani NK, Hamid AA, Yusoff WMW, Kalil MS (2013) Pre-optimization of medium for biobutanol production by a new isolate of solvent-producing Clostridium. BioResources 8:1420–1430
Amiri H, Karimi K (2015) Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst Eng 38:1959–1972
Amiri H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol 152:450–456
Andrić P, Meyer AS, Jensen PA, Dam-Johansen K (2010) Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol Adv 28:308–324
Arantes V, Saddler JN (2011) Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels 4:3
Arifin Y, Tanudjaja E, Dimyati A, Pinontoan R (2014) A second generation biofuel from cellulosic agricultural by-product fermentation using Clostridium species for electricity generation. Energy Procedia 47:310–315
Bahrin EK, Baharuddin AS, Ibrahim MF, Abdul Razak MN, Sulaiman A, Abd-Aziz S, Hassan MA, Shirai Y, Nishida H (2012) Physicochemical property changes and enzymatic hydrolysis enhancement of oil palm empty fruit bunches treated with superheated steam. BioResources 7:1784–1801
Balan V (2014) Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol 2014:1–31
Baral NR, Quiroz-Arita CE, Bradley TH (2018) Probabilistic lifecycle assessment of butanol production from corn stover using different pretreatment methods. Environ Sci Technol 52:14528–14537
Baral NR, Shah A (2014) Microbial inhibitors: formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl Microbiol Biotechnol 98:9151–9172
Bellido C, Lucas S, González-Benito G, García-Cubero MT, Coca M (2018) Synergistic positive effect of organic acids on the inhibitory effect of phenolic compounds on acetone-butanol-ethanol (ABE) production. Food Bioprod Process 108:117–125
Bharathiraja B, Jayamuthunagai J, Sudharsanaa T, Bharghavi A, Praveenkumar R, Chakravarthy M, Yuvaraj D (2017) Biobutanol – an impending biofuel for future: a review on upstream and downstream processing tecniques. Renew Sustain Energy Rev 68:788–807
Bruins ME, Van Hellemond EW, Janssen AEM, Boom RM (2003) Maillard reactions and increased enzyme inactivation during oligosaccharide synthesis by a hyperthermophilic glycosidase. Biotechnol Bioeng 81:546–552
Chilari D, Dimos K, Georgoula G, Paschos T, Mamma D, Louloudi A, Papayannakos N, Kekos D (2017) Bioethanol production from alkali-treated cotton stalks at high solids loading applying non-isothermal simultaneous saccharification and fermentation. Waste and Biomass Valorization 8:1919–1929
Cho DH, Lee YJ, Um Y, Sang BI, Kim YH (2009) Detoxification of model phenolic compounds in lignocellulosic hydrolysates with peroxidase for butanol production from Clostridium beijerinckii. Appl Microbiol Biotechnol 83:1035–1043
Davison BH, Drescher SR, Tuskan GA, Davis MF, Nghiem NP (2006) Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl Biochem Biotechnol 130:427–435
Demiral H, Yildirim ME (2003) Recovery of acetic acid from waste streams by extractive distillation. Water Sci Technol 47:183–188
Dürre P (2007) Biobutanol: An attractive biofuel. Biotechnol J 2:1525–1534
Eisentraut A (2010) Sustainable production of second-generation biofuels: potential and perspectives in major economies and developing countries. Int Energy Agency 221
Ellabban O, Abu-Rub H, Blaabjerg F (2014) Renewable energy resources: current status, future prospects and their enabling technology. Renew Sustain Energy Rev 39:748–764
Furukawa K, Ichikawa S, Nigorikawa M, Sonoki T, Ito Y (2014) Enhanced production of reducing sugars from transgenic rice expressing exo-glucanase under the control of a senescence-inducible promoter. Transgenic Res 23:531–537
Gao K, Boiano S, Marzocchella A, Rehmann L (2014) Cellulosic butanol production from alkali-pretreated switchgrass (Panicum virgatum) and phragmites (Phragmites australis). Bioresour Technol 174:176–181
Gao K, Rehmann L (2014) ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass and Bioenergy 66:110–115
Gao M, Tashiro Y, Yoshida T, Zheng J, Wang Q, Sakai K, Sonomoto K (2015) Metabolic analysis of butanol production from acetate in Clostridium saccharoperbutylacetonicum N1-4 using 13C tracer experiments. RSC Adv 5:8486–8495
Gogoi H, Nirosha V, Jayakumar A, Prabhu K, Maitra M, Panjanathan R (2018) Paper mill sludge as a renewable substrate for the production of acetone-butanol-ethanol using Clostridium sporogenes NCIM 2337. Energy Sources, Part A Recover Util Environ Eff 40:39–44
Gottumukkala LD, Haigh K, Görgens J (2017) Trends and advances in conversion of lignocellulosic biomass to biobutanol: microbes, bioprocesses and industrial viability. Renew Sustain Energy Rev 76:963–973
Gupta R, Kumar S, Gomes J, Kuhad RC (2012) Kinetic study of batch and fed-batch enzymatic saccharification of pretreated substrate and subsequent fermentation to ethanol. Biotechnol Biofuels 5:16
Harrison MD, Zhang Z, Shand K, O’Hara IM, Doherty WOS, Dale JL (2013) Effect of pretreatment on saccharification of sugarcane bagasse by complex and simple enzyme mixtures. Bioresour Technol 148:105–113
He CR, Kuo YY, Li SY (2017) Lignocellulosic butanol production from Napier grass using semi-simultaneous saccharification fermentation. Bioresour Technol 231:101–108
Hou X, From N, Angelidaki I, Huijgen WJJ, Bjerre AB (2017) Butanol fermentation of the brown seaweed Laminaria digitata by Clostridium beijerinckii DSM-6422. Bioresour Technol 238:16–21
Ibrahim MF, Kim SW, Abd-Aziz S (2018) Advanced bioprocessing strategies for biobutanol production from biomass. Renew Sustain Energy Rev 91:1192–1204
Ibrahim MF, Ramli N, Kamal Bahrin E, Abd-Aziz S (2017) Cellulosic biobutanol by Clostridia: challenges and improvements. Renew Sustain Energy Rev 79:1241–1254
Ikeda Y, Park EY, Okuda N (2006) Bioconversion of waste office paper to gluconic acid in a turbine blade reactor by the filamentous fungus Aspergillus niger. Bioresour Technol 97:1030–1035
Jiang Y, Xu C, Dong F, Yang Y, Jiang W, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Jin M, Gunawan C, Uppugundla N, Balan V, Dale BE (2012) A novel integrated biological process for cellulosic ethanol production featuring high ethanol productivity, enzyme recycling and yeast cells reuse. Energy Environ Sci 5:7168
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Jouzani GS, Taherzadeh MJ (2015) Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review. Biofuel Res J 5:152–195
Kadić A, Lidén G (2017) Does sugar inhibition explain mixing effects in enzymatic hydrolysis of lignocellulose? J Chem Technol Biotechnol 92:868–873
Kihara T, Noguchi T, Tashiro Y, Sakai K, Sonomoto K (2019) Highly efficient continuous acetone–butanol–ethanol production from mixed sugars without carbon catabolite repression. Bioresour Technol Reports 7:100185
Kumar M, Goyal Y, Sarkar A, Gayen K (2012) Comparative economic assessment of ABE fermentation based on cellulosic and non-cellulosic feedstocks. Appl Energy 93:193–204
Leavitt RI (1983) Preparation of proline from algae. U.S. Patent No. 4:390,624
Lee SH, Yun EJ, Kim J, Lee SJ, Um Y, Kim KH (2016) Biomass, strain engineering, and fermentation processes for butanol production by solventogenic Clostridia. Appl Microbiol Biotechnol 100:8255–8271
Leu SY, Zhu JY (2013) Substrate-related factors affecting enzymatic saccharification of lignocelluloses: Our recent understanding. Bioenergy Res 6:405–415
Liao Z, Guo X, Hu J, Suo Y, Fu H, Wang J (2019) The significance of proline on lignocellulose-derived inhibitors tolerance in Clostridium acetobutylicum ATCC 824. Bioresour Technol 272:561–569
Liu D, Yang Z, Wang P, Niu H, Zhuang W, Chen Y, Wu J, Zhu C, Ying H, Ouyang P (2018) Towards acetone-uncoupled biofuels production in solventogenic Clostridium through reducing power conservation. Metab Eng 47:102–112
Liu Z-Y, Yao X, Zhang Q, Liu Z, Wang Z, Zhang Y, Li F (2017) Modulation of the acetone/butanol ratio during fermentation of corn stover-derived hydrolysate by Clostridium beijerinckii strain NCIMB 8052. Appl Environ Microbiol 83:e03386–e03316
Lu C, Yu L, Varghese S, Yu M, Yang S-T (2017) Enhanced robustness in acetone-butanol-ethanol fermentation with engineered Clostridium beijerinckii overexpressing adh E2 and ctf AB. Bioresour Technol 243:1000–1008
Lü F, Chai L, Shao L, He P (2017) Precise pretreatment of lignocellulose: relating substrate modification with subsequent hydrolysis and fermentation to products and by-products. Biotechnol Biofuels 10:88
Mariano AP, Dias MOS, Junqueira TL, Cunha MP, Bonomi A, Filho RM (2013) Butanol production in a first-generation Brazilian sugarcane biorefinery: technical aspects and economics of greenfield projects. Bioresour Technol 135:316–323
Meng X, Ragauskas AJ (2014) Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr Opin Biotechnol 27:150–158
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals biorefinery concept. Prog Energy Combust Sci 38:522–550
Merino ST, Cherry J (2007) Progress and challenges in enzyme development for biomass utilization. In: Olsson L (ed) Advances in biochemical engineering/biotechnology. 108:95–120
Mitchell WJ (2016) Sugar uptake by the solventogenic Clostridia. World J Microbiol Biotechnol 32:1–10
Moradi F, Amiri H, Soleimanian-Zad S, Ehsani MR, Karimi K (2013) Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 112:8–13
Nigorikawa M, Watanabe A, Furukawa K, Sonoki T, Ito Y (2012) Enhanced saccharification of rice straw by overexpression of rice exo-glucanase. Rice 5:14
Noguchi T, Tashiro Y, Yoshida T, Zheng J, Sakai K, Sonomoto K (2013) Efficient butanol production without carbon catabolite repression from mixed sugars with Clostridium saccharoperbutylacetonicum N1-4. J Biosci Bioeng 116:716–721
OECD/IEA (2016) International Energy Agency. Energy and Air Pollution, World Energy Outlook-Spec Rep
Oshiro M, Hanada K, Tashiro Y, Sonomoto K (2010) Efficient conversion of lactic acid to butanol with pH-stat continuous lactic acid and glucose feeding method by Clostridium saccharoperbutylacetonicum. Appl Microbiol Biotechnol 87:1177–1185
Papayannakos N, Kekos D, Paschos T, Dimos K, Lappas A, Mamma D, Kalogiannis K, Louloudi A (2019) Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation 5:5
Park SH, Ong RG, Sticklen M (2016) Strategies for the production of cell wall-deconstructing enzymes in lignocellulosic biomass and their utilization for biofuel production. Plant Biotechnol J 14:1329–1344
Patakova P, Linhova M, Rychtera M, Paulova L, Melzoch K (2013) Novel and neglected issues of acetone-butanol-ethanol (ABE) fermentation by clostridia: Clostridium metabolic diversity, tools for process mapping and continuous fermentation systems. Biotechnol Adv 31:58–67
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Ståhlberg J, Beckham GT (2015) Fungal cellulases. Chem Rev 115:1308–1448
Pfromm PH, Amanor-Boadu V, Nelson R, Vadlani P, Madl R (2010) Bio-butanol vs. bio-ethanol: A technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium acetobutylicum. Biomass and Bioenergy 34:515–524
Piotrowski JS, Zhang Y, Bates DM, Keating DH, Sato TK, Ong IM, Landick R (2014) Death by a thousand cuts: The challenges and diverse landscape of lignocellulosic hydrolysate inhibitors. Front Microbiol 5:1–8
Qi B, Chen X, Su Y, Wan Y (2011) Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose. Bioresour Technol 102:2881–2889
Qureshi N, Saha BC, Cotta MA, Singh V (2013) An economic evaluation of biological conversion of wheat straw to butanol: A biofuel. Energy Convers Manag 65:456–462
Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA (2008) Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I-Batch fermentation. Biomass and Bioenergy 32:168–175
Raganati F, Procentese A, Olivieri G, Götz P, Salatino P, Marzocchella A (2015) Kinetic study of butanol production from various sugars by Clostridium acetobutylicum using a dynamic model. Biochem Eng J 99:156–166
Rani V, Mohanram S, Tiwari R, Nain L, Arora A (2014) Beta-glucosidase : key enzyme in determining efficiency of cellulase and biomass hydrolysis. Bioprocess Biotech 5:1–8
Shen H, He X, Poovaiah CR, Wuddineh WA, Ma J, Mann DGJ, Wang H, Jackson L, Tang Y, Neal Stewart J, Chen F, Dixon RA (2012) Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol 193:121–136
Tachon N, Benjelloun-Mlayah B, Delmas M (2016) Organosolv wheat straw lignin as a phenol substitute for green phenolic resins. BioResources 11:5797–5815
Tashiro Y, Takeda K, Kobayashi G, Sonomoto K, Ishizaki A, Yoshino S (2004) High butanol production by Clostridium saccharoperbutylacetonicum N1-4 in fed-batch culture with pH-stat continuous butyric acid and glucose feeding method. J Biosci Bioeng 98:263–268
Tashiro Y, Yoshida T, Noguchi T, Sonomoto K (2013) Recent advances and future prospects for increased butanol production by acetone-butanol-ethanol fermentation. Eng Life Sci 13:432–445
Trindade WR, Santos RG (2017) Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew Sustain Energy Rev 69:642–651
Ujor V, Agu CV, Gopalan V, Ezeji TC (2014) Glycerol supplementation of the growth medium enhances in situ detoxification of furfural by Clostridium beijerinckii during butanol fermentation. Appl Microbiol Biotechnol 98:6511–6521
Van Dyk JS, Pletschke BI (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Vanderghem C, Boquel P, Blecker C, Paquot M (2010) A multistage process to enhance cellobiose production from cellulosic materials. Appl Biochem Biotechnol 160:2300–2307
Wade M, Li Y-C, Wahl G (2013) NIH Public Access. Nat Rev Cancer 13:83–96
Wang Y, Gao C, Zheng Z, Liu FM, Zang JY, Miao JL (2015) Immobilization of cold-active cellulase from antarctic bacterium and its use for kelp cellulose ethanol fermentation. BioResources 10:1757–1772
Wang X, Wu Y, Zhou Y (2017a) Transglycosylation, a new role for multifunctional cellulase in overcoming product inhibition during the cellulose hydrolysis. Bioengineered 8:129–132
Wang Y, Ho SH, Yen HW, Nagarajan D, Ren NQ, Li S, Hu Z, Lee DJ, Kondo A, Chang JS (2017b) Current advances on fermentative biobutanol production using third generation feedstock. Biotechnol Adv 35:1049–1059
Weiss ND, Felby C, Thygesen LG (2019) Enzymatic hydrolysis is limited by biomass–water interactions at high-solids: improved performance through substrate modifications. Biotechnol Biofuels 12:1–13
Wu Y, Xue C, Chen L, Yuan W, Bai F (2016) Synergistic effect of calcium and zinc on glucose/xylose utilization and butanol tolerance of Clostridium acetobutylicum. FEMS Microbiol Lett 363:1–7
Xiao Y, Poovaiah C, Coleman HD (2016) Expression of glycosyl hydrolases in lignocellulosic feedstock: An alternative for affordable cellulosic ethanol production. Bioenergy Res 9:1290–1304
Xin L, Guo Z, Xiao X, Peng C, Zeng P, Feng W, Xu W (2019) Feasibility of anaerobic digestion on the release of biogas and heavy metals from rice straw pretreated with sodium hydroxide. Environ Sci Pollut Res 26:19434–19444
Xiong H, Von Weymarn N, Turunen O, Leisola M, Pastinen O (2005) Xylanase production by Trichoderma reesei Rut C-30 grown on L-arabinose-rich plant hydrolysates. Bioresour Technol 96:753–759
Yang M, Kuittinen S, Vepsäläinen J, Zhang J, Pappinen A (2017) Enhanced acetone-butanol-ethanol production from lignocellulosic hydrolysates by using starchy slurry as supplement. Bioresour Technol 243:126–134
Yoshida T, Tashiro Y, Sonomoto K (2012) Novel high butanol production from lactic acid and pentose by Clostridium saccharoperbutylacetonicum. J Biosci Bioeng 114:526–530
Youn SH, Lee KM, Kim KY, Lee SM, Woo HM, Um Y (2016) Effective isopropanol-butanol (IB) fermentation with high butanol content using a newly isolated Clostridium sp. A1424. Biotechnol Biofuels 9:1–15
Zabihi S, Alinia R, Esmaeilzadeh F, Kalajahi JF (2010) Pretreatment of wheat straw using steam, steam/acetic acid and steam/ethanol and its enzymatic hydrolysis for sugar production. Biosyst Eng 105:288–297
Zeng Y, Zhao S, Yang S, Ding SY (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:98–45
Zhang WL, Liu ZY, Liu Z, Li FL (2012) Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett Appl Microbiol 55:240–246
Zhang Z, Harrison MD, Rackemann DW, Doherty WOS, O’Hara IM (2016) Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem 18:360–381
Zhao T, Tashiro Y, Zheng J, Sakai K, Sonomoto K (2018) Semi-hydrolysis with low enzyme loading leads to highly effective butanol fermentation. Bioresour Technol 264:335–342
Zhao T, Yasuda K, Tashiro Y, Darmayanti RF, Sakai K, Sonomoto K (2019) Semi-hydrolysate of paper pulp without pretreatment enables a consolidated fermentation system with in situ product recovery for the production of butanol. Bioresour Technol 278:57–65
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels. Bioprod Biorefining 6:465–482
Zheng J, Tashiro Y, Wang Q, Sakai K, Sonomoto K (2015a) Feasibility of acetone-butanol-ethanol fermentation from eucalyptus hydrolysate without nutrients supplementation. Appl Energy 140:113–119
Zheng J, Tashiro Y, Wang Q, Sonomoto K (2015b) Recent advances to improve fermentative butanol production: Genetic engineering and fermentation technology. J Biosci Bioeng 119:1–9
Zheng J, Tashiro Y, Zhao T, Wang Q, Sakai K, Sonomoto K (2017) Enhancement of acetone-butanol-ethanol fermentation from eucalyptus hydrolysate with optimized nutrient supplementation through statistical experimental designs. Renew Energy 113:580–586
Zhou Y, Wang X, Wei W, Xu J, Wang W, Xie Z, Zhang Z, Jiang H, Wang Q, Wei C (2016) A novel efficient β-glucanase from a paddy soil microbial metagenome with versatile activities. Biotechnol Biofuels 9:36
Funding
This work was partially supported by JST SICORP Grant Number JPMJSC16E5, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, T., Tashiro, Y. & Sonomoto, K. Smart fermentation engineering for butanol production: designed biomass and consolidated bioprocessing systems. Appl Microbiol Biotechnol 103, 9359–9371 (2019). https://doi.org/10.1007/s00253-019-10198-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10198-2