Abstract
Bacteria and fungi were isolated from eight different soil samples from different regions in Kazakhstan contaminated with oil or salt or aromatic compounds. For the isolation of the organisms, we used, on the one hand, typical hydrocarbons such as the well utilizable aliphatic alkane tetradecane, the hardly degradable multiple-branched alkane pristane, and the biaromatic compound biphenyl as enrichment substrates. On the other hand, we also used oxygenated derivatives of alicyclic and monoaromatic hydrocarbons, such as cyclohexanone and p-tert-amylphenol, which are known as problematic pollutants. Seventy-nine bacterial and fungal strains were isolated, and 32 of them that were clearly able to metabolize some of these substrates, as tested by HPLC-UV/Vis and GC-MS analyses, were characterized taxonomically by DNA sequencing. Sixty-two percent of the 32 isolated strains from 14 different genera belong to well-described hydrocarbon degraders like some Rhodococci as well as Acinetobacter, Pseudomonas, Fusarium, Candida, and Yarrowia species. However, species of the bacterial genus Curtobacterium, the yeast genera Lodderomyces and Pseudozyma, as well as the filamentous fungal genera Purpureocillium and Sarocladium, which have rarely been described as hydrocarbon degrading, were isolated and shown to be efficient tetradecane degraders, mostly via monoterminal oxidation. Pristane was exclusively degraded by Rhodococcus isolates. Candida parapsilosis, Fusarium oxysporum, Fusarium solani, and Rhodotorula mucilaginosa degraded cyclohexanone, and in doing so accumulate ε-caprolactone or hexanedioic acid as metabolites. Biphenyl was transformed by Pseudomonas/Stenotrophomonas isolates. When p-tert-amylphenol was used as growth substrate, none of the isolated strains were able to use it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kazakhstan is one of the fifteens most important oil-producing countries worldwide with around 1956 thousand barrel a day (Trading Economics 2019) and it is the twelfth largest oil-exporting nation with 68.1 million tons a year (Statista 2019)—a value that is still rising. Oil production and oil transport are frequently accompanied by spills that pollute the environment. To respond in an appropriate way, much effort worldwide has been—and is still being—invested in remediation, particularly in bioremediation (Bento et al. 2005; Juwarkar et al. 2010; Varjani et al. 2017). Among the various options, biodegradation with microorganisms seems to be one of the most used and cost-effective procedures for remediation of oil pollution. In Kazakhstan, we isolated various highly potent microbial oil degraders from contaminated soils of the Uzen deposit (Mangystau region) and of the Kumkol deposit (Kyzylorda region). These isolates utilized a large number of oil components and by degrading pollutants had beneficial effects on the growth of barley seeds in the presence of crude oil (Mikolasch et al. 2015, 2016). The question has also been raised whether microorganisms of the same quality and quantity can be isolated from areas with different oil pollutants and whether correlations concerning biodiversity and metabolic activity can be drawn. Furthermore, very little is known about the diversity of species and the degradation or transformation potential of microorganisms from other than heavily oil-polluted deposits in Kazakhstan. For this reason, samples from different contaminated soils were taken during an expedition from the north bank of the Lake Balkhash to the Talgar Pass (Tien Shan Mountains). These samples were characterized as to their biodiversity and metabolic activity. So that suitable strains would be available for further biotechnological and biochemical purposes, characterization was carried out not by analysis of the microbiome by extraction of rRNA/DNA, but rather by isolation and analysis of highly effective degraders in culture. For this reason, samples were grown on mineral media containing either typical hydrocarbons such as the well utilizable aliphatic alkane tetradecane, the hardly utilizable multiple-branched alkane pristane, and the biaromatic compound biphenyl or the oxygenated derivatives of alicyclic and monoaromatic hydrocarbons such as cyclohexanone and p-tert-amylphenol, which are known to be problematic pollutants. Many microorganisms can oxidize cyclohexane to cyclohexanone, but further metabolism of the oxygenated alicyclic cyclohexanone is difficult for many organisms (Dallinger et al. 2016). Furthermore, the branched side chain of p-tert-amylphenol prevents a ready further oxidation of this phenol derivative. An additional aim of this study was therefore to identify effective new degraders for pollutants that are otherwise difficult to degrade.
Materials and methods
Sampling
Soil samples were collected at eight different locations in Kazakhstan (samples E1–E8 in Table 1) in order to have different samples of material contaminated with oil or salt or aromatics.
Media
Nutrient broth II (SIFIN, Berlin) (NB) and malt agar (2.5% malt extract) (MA) served as cultivation media on plates.
Mineral salts medium for bacteria (MSMB) pH 6.3 (Hundt et al. 1998) and mineral salts medium for fungi (MSMF) pH 5.4 supplemented with 1% vitamin solution (Awe et al. 2008) were used for the isolation of microorganisms from various polluted soils and, without agar, in liquid form for metabolic experiments.
Enrichment and isolation
Bacteria and fungi from the contaminated soil samples were enriched on the substrates tetradecane, pristane, cyclohexanone, biphenyl, and 4-tert-amylphenol (Supplementary Material Table S1) each with MSMB and MSMF according to a previously described method (Nhi-Cong et al. 2010).
After enrichment, fungi were obtained by plating 0.1 mL of the enriched cultures in MSMF on MA plates. Bacteria enriched in MSMB were plated on NB plates. Pure cultures from MSMB were cultivated on NB and from MSMF on MA slants.
Evaluation of growth on isolation substrates
For growth tests in liquid medium, microorganisms were cultivated in 500-mL flasks containing 100 mL of MSMB or MSMF supplemented with the isolation substrates (see concentrations in Supplementary Material Table S1) at 30 °C and 130 rpm, harvested, dried, and analyzed. Strains, which showed satisfactory growth after 6 days, were then used for further treatment. Cultures with slower growth on the substrates were harvested after 12 days. The experiments with the substrate cyclohexanone were carried out with glass in place of cellulose stoppers, due to the excessive evaporation of this substrate.
Because of difficulties in quantification of cell densities by optical density (OD) measurements, we analyzed cell growth by the dry weight method according to Mikolasch et al. (2015). For this, the final cell weight minus the cell weight at the beginning was divided by the cell weight at the beginning. The growth values are therefore dimensionless.
Growth value = (cell weight end [mg] − cell weight start [mg]) (cell weight start [mg])−1
Identification of isolated microorganisms
Isolates were first identified as bacteria, yeasts, or fungi using a phase-contrast microscope (Axiolab, Zeiss). Initial assignments were made on the basis of shape, mobility, formation of endospores, pullulation, and colony morphology of the isolates.
Identification of bacteria
The KOH test was used to determine the Gram characteristics of all bacteria (Suslow et al. 1982). To distinguish between aerobic and facultative anaerobic bacteria, we used the oxidative/fermentative test according to Hugh and Leifson (1953). Furthermore, all bacteria were grown on plates for 48 h and chromosomal DNA was isolated in triplicate by using cell material of one colony in 20 μL ddH2O and the maximal power of a microwave (Severin, Germany) for 3 min. After centrifugation for 1 min at 12,000 rpm, 1 μL supernatant was used as template in PCR. This isolation method is referred to as method 1. For the cells of strains that could not be disrupted by this method, we used the innuSPEED soil DNA-Kit (Analytic Jena AG, Germany) according to the manufacturer’s protocol. This method is referred to as method 2. For strains whose cells could not be disrupted by either of these methods, we disrupted the cells using a Fastprep24 instrument (MP Biomedicals, Germany) in combination with the DNeasy PowerSoil Kit (Qiagen, US)—referred to here as method 3.
Bacterial almost full-length 16S rRNA genes were amplified from 1 μL DNA extracts as template with the oligonucleotide primers 616V (AGAGTTTGATYMTGGCTC, 0.5 μM) (Juretschko et al. 1998) and 1492R (GGTTACCTTGTTACGACTT, 0.5 μM) (Kane et al. 1993) and with MgCl2 2 mM, dNTPs mix 0.2 mM, and BSA 0.2 mg mL−1 (Eurofins Genomics, Germany). Reactions were performed in an Analytik Jena thermocycler using GoTaq-Polymerase (Eurofins Genomics, Germany) with the following conditions: step 1 denaturation 95 °C 5 min, step 2 denaturation 95 °C 30 s, step 3 annealing 47 °C 45 s, step 4 elongation 72 °C 90 s. Steps 2 to 4 were repeated 34 times, followed by elongation at 72 °C for 5 min. The resulting PCR products were purified using the DNA Clean & Concentrator Kit D4003 (Zymo Research, Germany). DNA concentrations were determined by nanodrop spectrophotometer (PEQLAB Biotechnologie GmbH). Sanger sequencing was performed by Eurofins Genomics (Germany) with 616V and 1492R primers, respectively. The resulting forward and reverse sequences were assembled using the program Geneious (geneious, US). The resulting almost full-length 16S rRNA sequences were compared with the NCBI nr database using the blastn algorithm (Altschul et al. 1990).
Identification of yeasts and filamentous fungi
The urease test with Christensen’s medium (Seeliger 1956) was used to determine ascomycetous and basidiomycetous yeasts. All yeasts were grown for 48 h and chromosomal DNA was isolated by using methods 1, 2, and/or 3 as described above for bacteria. Methods 2 and 3 were used for filamentous fungi.
Fungal almost full-length ITS genes were amplified using 1 μL DNA extract as template with oligonucleotides ITS1 (TCCGTAGGTGAACCTGCGG, 0.5 μM) and ITS4 (TCCTCCGCTTATTGATATGC, 0.5 μM) (White et al. 1990) as primers and with MgCl2 2 mM, dNTPs mix 0.2 mM, and BSA 0.2 mg mL−1 (Eurofins Genomics, Germany). Reactions were performed in an Analytik Jena thermocycler using GoTaq-Polymerase (Eurofins Genomics, Germany) with the following conditions: step 1 denaturation 95 °C 10 min, step 2 denaturation 95 °C 60 s, step 3 annealing 55 °C 60 s, step 4 elongation 72 °C 90 s. Steps 2 to step 4 were repeated 29 times, followed by elongation at 72 °C for 10 min. The resulting PCR products were purified, sent for Sanger sequencing, and analyzed as described above for bacteria.
Metabolic experiments
Pre-cultivated cells of the isolated strains were shaken in 100-mL Erlenmeyer flasks containing 10 mL medium plus the isolation substrates as carbon and energy source at 30 °C and 130 rpm. Assays without substrates and without cells were used as controls.
Bacterial cells were pre-grown on NB plates, for yeasts and filamentous fungi on MA plates. For each strain, the cell material from five well-grown plates was used to prepare an inoculation suspension of 6 mL. In each case, 2 mL of this was used to inoculate the parallel transformation assays and the control flasks. For degradation experiments, fungi were cultivated in MSMF supplemented with 1% vitamin solution and bacteria in MSMB. As sole source of carbon and energy, 25 μL of the isolation substrates tetradecane, pristane, and cyclohexanone was used in 10 mL assays (substrate concentration 0.25% v/v). Biphenyl was used as substrate according to the method described by Sietmann et al. (2000) at a final concentration of 100 μg mL−1.
After 5 (for bacteria and yeasts) and 7 (for filamentous fungi) days of incubation, the supernatant of each transformation assay was extracted according to Mikolasch et al. (2015). The extracts obtained were analyzed by high-performance liquid chromatography (HPLC) and gas chromatography mass spectrometry (GC-MS). The data are reported as means for parallel experiments. The deviation of the parallel values was no more than 10%.
Chemical analysis and identification of products
The HPLC-UV/Vis and GC-MS analyses were carried out as described previously (Mikolasch et al. 2016). The elution profile of the GC-MS method was changed as follows: the column temperature started at 80 °C and increased to 310 °C at 10 °C min−1 and was finally maintained at 310 °C for 5 min.
Results
To investigate the diversity of species and the degradation or transformation potential of microorganisms, samples from eight different contaminated soils in Kazakhstan were collected during an expedition from the north bank of the Lake Balkhash to the Talgar Pass (Tien Shan Mountains). Five soil samples were taken from the ground of abandoned petrol stations along the route (E3–E7) and one from an old filling station that was still operating (E1). Tetradecane, pristane, and cyclohexanone—all components of crude oil—were used as isolation substrates for samples E1 and E3–E7. One sample was of salty soil from the heavily polluted Lake Balkhash region (E2) (Krupa et al. 2017). The final sample came from the Talgar Pass at a height of 3162 m where there was serious soil pollution with wood preservatives in connection with the construction of wooden cable stations. From this soil sample (E8), we expected microorganisms able to degrade aromatic hydrocarbons; therefore, we used biphenyl and 4-tert-amylphenol as isolation substrates. Because of the interest in a biotechnological application of strains effectively degrading pollutants, we concentrated in general on the best-growing microorganisms.
Isolation of and screening for degraders of pollutants
Seventy-nine strains of microorganisms were isolated from 32 enrichment experiments using the five substrates, tetradecane (1), pristane (2), cyclohexanone (3), biphenyl (4), 4-tert-amylphenol (5), and the eight soil samples described above (Table 2 and Supplementary Material Table S2).
Thirty-eight cultures enriched on MSMB and 41 strains obtained from MSMF were cultivated in liquid media on the substrates, tetradecane, pristane, cyclohexanone, biphenyl, and 4-tert-amylphenol. Due to difficulties in quantification of cell densities via OD measurements, we analyzed the cell growth by a dry weight method (Mikolasch et al. 2015).
Twenty-one of the 37 microorganisms isolated on tetradecane were able to grow on this substrate (Table 3 and Supplementary Material Table S3 and S4). Four of the 21 strains showed good growth values (three from MSMB and one from MSMF), six moderate (one from MSMB and five from MSMF), and 11 weak growth (five from MSMB and six from MSMF). However, more than half of the isolates were able to use tetradecane as the sole source of carbon and energy. The highest growth value 469 (the final cell weight minus the cell weight at the beginning was divided by the cell weight at the beginning) was determined for the strain SBUG 2074, whereas the largest final yield of cell material was measured for SBUG-Y 2192 with 646 mg/100 mL (growth value 134; Supplementary Material Table S2 and S3). In general, the growth values of strains isolated on MSMF were smaller than the ones of strains isolated on MSMB.
The growth on cyclohexanone was much weaker than on tetradecane (Table 3 and Supplementary Material Table S5 and S6). Only six of the 18 strains showed weak growth (one from MSMB and five from MSMF). No strain had better than weak growth values. However, one third of the isolates are able to use cyclohexanone as the sole source of carbon and energy. The highest growth value 37 and the largest final yield of cell material with 159 mg/100 mL were determined for the strain SBUG-M 1735 (Supplementary Material Table S2 and S6). The growth values of the strains isolated on MSMF are similar (three strains) or higher (two strains) than the value of the strain isolated on MSMB.
Three of the nine strains isolated on pristane (all from MSMB) are able to use this substrate as the sole source of carbon and energy (Table 3 and Supplementary Material Table S7 and S8). No strain isolated from MSMF could really grow on the isolation substrate (growth values between four and nine). The strain SBUG 2033 from MSMB showed a moderate growth value of 83 and the largest final yield of cell material with 152 mg/100 mL (Supplementary Material Table S2 and S7).
4-tert-Amylphenol was neither used as growth substrate by the six strains isolated in MSMB nor by the seven isolates from MSMF (Supplementary Material Table S9 and S10). The growth values are less than 1.
The two strains isolated on biphenyl showed growth values of 6.6 and 10, which indicates a poor and weak growth (Table 3 and Supplementary Material Table S11).
In order to determine and compare the number of enriched strains with the number of strains which are able to use the isolation substrates as growth substrate, we used the number of isolated pure cultures per soil sample and substrate (Fig. 1a, Supplementary Material Table S12).
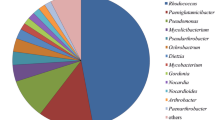
(a, bar chart) Number of isolated microorganisms and number of strains able to grow on the isolation substrate per soil sample and substrate, and the sum of strains per substrate. (b, circular charts) Percentage distribution of phyla and genera. The diversity of genera within the phyla is illustrated by genus names and percent values. Each circular chart represents the strains able to grow on the corresponding isolation substrate. (No strain was able to grow on 4-tert-amylphenol)
By far the highest number of organisms were recovered on tetradecane. This reflects that seven of the eight soil samples were used for enrichment on this substrate whereas only one to three soil samples served as basis for enrichment on the other substrates. However, though two to seven pure cultures were isolated from each enrichment experiment, only one to five strains were able to use the isolation substrate as a sole source of carbon and energy. An exception is 4-tert-amylphenol, which could not serve as substrate. Four or five pure cultures were obtained on tetradecane and cyclohexanone, whereas no more than 3 pure strains were isolated on pristane and biphenyl.
Identification of degraders of pollutants
All bacterial isolates able to grow on the isolation substrates were assigned as Gram positive or negative and identified by 16S rRNA sequence analysis, and the oxidative/fermentative test (Hugh and Leifson 1953). The fungi were characterized by ITS gene sequence analyses and the urease test with Christensen’s medium (summary in Table 3 and identification details in Supplementary Material Table S13, S14, S15). Bacterial 16S rRNA gene sequences and fungal ITS sequences were deposited in Genbank under the accession numbers listed in Table 3. As is current practice in the literature, we considered only those microorganisms having the desired characteristics (Martorell et al. 2017).
The 32 identified strains belong to 14 different genera. Forty-four percent of the strains are bacteria and 56% are fungi. The bacterial species belong to the Firmicutes (21%), Proteobacteria (36%; 80% Gamma- and 20% Betaproteobacteria), and Actinobacteria (43%). The fungi are divided into 22% Basidiomycota and 78% Ascomycota. The percentage of Firmicutes, Proteobacteria, Actinobacteria, Ascomycota, and Basidiomycota for the strains able to grow on the isolation substrates can be inferred from the circular charts of Fig. 1b. The highest number of different strains was found with the more easily useable substrate tetradecane followed by cyclohexanone. Only few bacterial strains belonging to the genera Pseudomonas and Rhodococcus were isolated and able to grow on the more recalcitrant substrates pristane and biphenyl, whereas 4-tert-amylphenol was not a growth substrate.
Verification of the degradation potentials by analyses of intermediates
To confirm that the identified pollutant degraders have a high metabolic activity toward the isolation substrates, transformation experiments in liquid medium were conducted on the isolation substances, and the metabolites were detected by GC-MS or HPLC (Table 4).
Most of the tetradecane degraders were able to transform 50% or more of the substrate, six of the 21 transforming 100% of it. Two or more monocarboxylic acids, mostly myristic (1-tetradecanoic) and lauric (1-dodecanoic) acid, were detected in each assay. Metabolism of strains utilizing tetradecane was confirmed by their formation of monocarboxylic acids. In contrast, a dicarboxylic acid was formed only by the two species, Purpureocillium lilacinus SBUG-M 1730 and Yarrowia lipolytica SBUG-Y 2204. Three other species, Fusarium oxysporum SBUG-M 1727, Fusarium sp. SBUG-M, and Sarocladium sp. SBUG-M1728, also formed ketones during the transformation of tetradecane.
Two of the cyclohexanone degraders were able to metabolize more than 50% of the substrate, another two metabolized more than 40%, and the remaining three metabolized less than 20%. Metabolites were detected from five of the seven species.
Two of the three pristane degraders utilized more than 80% of the substrate, and Rhodococcus sp. SBUG 2031 metabolized more than 95% of it. In both cases, monomethyl substituted alkanedioic and alkenedioic acids were produced.
More than 50% of the aromatic substrate biphenyl was transformed by Pseudomonas/Stenotrophomonas SBUG 2091 and Pseudomonas sp. SBUG 2067. Phenyl-succinic acid and hydroxylated benzoic acids were detected as metabolites by HPLC.
Discussion
From the eight samples of different contaminated soils collected between the north bank of the Lake Balkhash to the Talgar Pass in the Tien Shan Mountains, we isolated 32 oil component degraders belonging to the bacterial phyla Firmicutes, Proteobacteria, and Actinobacteria and to the fungal divisions Basidiomycota and Ascomycota. Fifteen percent of these strains, two Curtobacterium, two Purpureocillium, and one Sarocladium, have not been extensively described in literature, and isolated pure cultures have not so far been shown to be hydrocarbon degraders, though Curtobacterium and Sarocladium have occasionally been described in oil-contaminated soils (Mohammadian et al. 2017; Rajaei et al. 2013). It is now clear that they are powerful tetradecane degraders (Table 4).
The genus Curtobacterium has been defined for several brevibacteria and some coryneform bacteria (Collins and Jones 1983; Funke et al. 2005; Yamada and Komagata 1972). Although the utilization of n-alkanes was described in the past for several undefined Corynebacterium species (Bouchez-Naitali et al. 1999; Cardini and Jurtshuk 1968; Ikeda et al. 1994; McGowan et al. 2004), the integration of these bacteria into the genus Curtobacterium remains unclear. Chase et al. (2016) concluded that Curtobacterium may be a cosmopolitan and dominant player in the functional breakdown of dead organic material in leaf litter microbial communities (with high representation on grasses and in soils), and has the ability to utilize different carbohydrates such as starch, cellulose, or xylan. However, there are various different hydrocarbons in the cuticula of plant leaves, so that our characterization of Curtobacterium species as hydrocarbon degraders may not be surprising.
Fungi of the genus Sarocladium include nearly 20 species belonging to the Ascomycota order Hypocreales. They have been reported as pathogenic plant fungi or as saprobes, and many of them have been transferred from the morphologically similar (but phylogenetically rather distant) genus Acremonium to Sarocladium (Giraldo et al. 2015; Summerbell et al. 2011). While Sarocladium species are not known as alkane degraders, several Acremonium species have been described as hydrocarbon-assimilating (Barnes et al. 2018; Chaineau et al. 1999; Ma et al. 2015; Oudot et al. 1993).
Purpureocillium lilacinus is a ubiquitous saprobic filamentous fungus distributed in soil, decaying vegetation. It is a cause of infection in nematodes, insects, and humans, and belongs also to the Ascomycota order Hypocreales. Alkane degradation has not been described for the species Purpureocillium lilacinus (Luangsa-Ard et al. 2011), which was previously known as Paecilomyces lilacinus. For Paecilomyces lilacinus, there are several reports concerning the degradation of aromatic compounds such as toluene (Vigueras et al. 2008), benzo(a)pyrene (Rafin et al. 2013), biphenyl (Gesell et al. 2001; Sietmann et al. 2006), or dibenzofuran (Gesell et al. 2004). To date, there is a single report concerning hexadecane utilization by Paecilomyces lilacinus (Vigueras et al. 2014). Apart from Paecilomyces lilacinus, some other Paecilomyces species such as P. variotii or P. brunneolus remained in the genus Paecilomyces. P. variotii was described as a petroleum hydrocarbon degrader (Ameen et al. 2016; Ezekoye et al. 2018). In view of this, our characterization of Purpureocillium lilacinus as a hydrocarbon degrader is not so surprising.
Our isolates of Curtobacterium, Sarocladium, and Purpureocillium degraded from 70 to 100% of tetradecane and produced several monocarboxylic acids using this substrate. This indicates a monoterminal alkane degradation by initial hydroxylation of tetradecane, oxidation to tetradecanoic acid and its further metabolism by ß-oxidation (Rojo 2009). The detection of 6-tetradecanone in the culture medium of Sarocladium sp. SBUG-M1728 indicated the possibility of an additional subterminal degradation pathway for this strain, and the identification of hexanedioic acid generated by Purpureocillium lilacinus SBUG-M 1730 indicated a diterminal oxidation pathway (Okuhara et al. 1971; Scheller et al. 1998).
Little is known about hydrocarbon degradation for the genera Bordetella, Lodderomyces, Pseudozyma, and Rhodotorula. Species of the bacterial genus Bordetella have been isolated as respiratory pathogens in humans and animals but they are frequently found as inhabitants of soils, water, sediments, or the plant rhizosphere (Soumana et al. 2017). Some of them (e.g., B. petrii and B. avium) have been suggested to be able to degrade benzenes (Wang et al. 2007) and PAHs (Abo-State et al. 2018; Obi et al. 2016; Yuan et al. 2009) whereas our isolate Bordetella sp. SBUG 2083 (similar to the cluster of Bordetella bronchialis/flabilis; Supplementary Material Table S13) is a tetradecane degrader. Several taxonomic analyses indicated a great similarity between the genera Bordetella, Achromobacter, and some Alcaligenes species partially reclassified to Achromobacter (Kronvall et al. 2000; Yabuuchi et al. 1998). For several Achromobacter species, the utilization of n-alkanes has been described (Deng et al. 2014; Kaczorek et al. 2013; Lal and Khanna 1996).
Our yeast isolate Lodderomyces elongisporus SBUG-Y 2196 is a moderate transformer of tetradecane, from which it produced monocarboxylic acids. L. elongisporus is known as a hexadecane utilizing yeast species (Bos and de Bruyn 1973; Hofmann and Schauer 1988; Kurtzman et al. 2011), but metabolic studies have not been performed until now.
Whereas other species of the genus Pseudozyma (P. antarctica and P. sp.) are known to metabolize n-alkanes (Kitamoto et al. 2001; Sajna et al. 2015), this has not previously been reported for Pseudozyma aphidis (Kurtzman et al. 2011). Here, we demonstrate that Pseudozyma aphidis SBUG-Y 2194 is able to almost completely degrade tetradecane and to generate thereby four monocarboxylic acids.
Rhodotorula mucilaginosa has previously been described as a degrader of aromatic hydrocarbons (Chandran and Das 2012; Lahav et al. 2002; Romero et al. 2002), whereas our isolate, Rhodotorula mucilaginosa SBUG-Y 2201, is a powerful n-alkane degrader. Several other Rhodotorula species are known as alkane degrading (Kurtzman et al. 2011; Schauer and Schauer 1986), but for R. mucilaginosa, the results are contradictory (Hofmann and Schauer 1988). In addition, our isolate of Rhodotorula mucilaginosa SBUG-Y 2202 metabolized cyclohexanone to a moderate extent. Current knowledge about fungal degradation of alicyclic hydrocarbons is scarce (Dallinger et al. 2016). Our isolate of Rhodotorula mucilaginosa SBUG-Y 2202 transformed cyclohexanone via the previously described microbial pathway to ε-caprolactone and hexanedioic acid (Anderson et al. 1980). The species Rhodotorula mucilaginosa, which has so far attracted less attention in the field of aliphatic and alicyclic hydrocarbon degradation, were found in two different soil samples (E3 and E4) separated by around 56 miles/90 km in our studies in Kazakhstan.
Sixty-two percent of the 32 isolated species of 14 different genera belong to well-described hydrocarbon degraders like Bacilli and Rhodococci as well as to Acinetobacter, Pseudomonas, Fusarium, and Candida species. Strains from these genera have been isolated from polluted areas and investigated for decades (Annweiler et al. 2000; Colombo et al. 1996; Hassanshahian et al. 2012; Kachholz and Rehm 1978; Kästner et al. 1994; Long et al. 2017; Mohammadian et al. 2017; Raju et al. 2017). We extend the identified sources to include contaminated soils in Kazakhstan. Furthermore, we confirm their importance for the degradative power of the microbiome in polluted soils by showing that they are able to grow on the isolation substrates used (Fig. 1a, b), and to degrade n-alkanes, cycloalkanes, branched chain alkanes, and aromatics (Table 4).
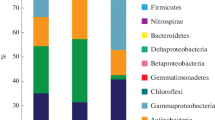
Recently, we isolated and examined a variety of bacterial strains from two oil-polluted deposits in Kazakhstan. These isolates belong mostly to the genera Gordonia and Rhodococcus (using a wide range of hydrocarbon substrates), and to Bacillus (using aromatic hydrocarbons, especially PAH) (Mikolasch et al. 2015, 2016). In the current study, 56% of the powerful isolates are fungi and only 44% of the species are bacteria. Furthermore, we found several bacteria and fungi species that are able to transform multiple hydrocarbons. The biodiversity of hydrocarbon degraders of the polluted soils from the north bank of the Lake Balkhash to the Talgar Pass as a whole (14 different genera) would seem to be higher than the diversity recovered from oil-polluted deposits (4 different genera) of the Mangystau and the Kyzylorda region (Fig. 2).
Comparative presentation of the “culturome” biodiversity of the hydrocarbon degraders of the polluted soils (E1–E8) from the north bank of the Lake Balkhash to the Talgar Pass and of the oil-polluted deposits of the Mangystau and the Kyzylorda region (around 994 miles/1600 km and 466 miles/750 km further to the west)
There are several recently published investigations characterizing the species diversity of microorganisms in petroleum-contaminated soils of oil fields (Mohammadian et al. 2017), crude oil sludge (Obi et al. 2016), or polluted soils (Long et al. 2017; Rajaei et al. 2013). In general, they focused either on bacteria or on fungi, while our studies include both bacteria and fungi and used multi-method analysis consisting of dry weight, HPLC-UV/Vis, and GC-MS measurements analyzing substrate consumption, biomass production, and metabolite formation.
Many authors, including ourselves, were able to isolate various promising bacteria and/or fungi for bioremediation purposes from contaminated samples. In contrast, the fungi from pristine and natural areas are less powerful in degradation of hydrocarbons (Martorell et al. 2017). For example, Martorell et al. (2017) reported that only one yeast strain from 105 was able to use n-alkanes as growth substrates. We, in contrast, isolated and characterized only 18 fungal strains from contaminated soil samples, but they are all potent hydrocarbon degraders, able to grow, and form detectable metabolites from hydrocarbon substrates. This indicates an adaptation of pristine areas to pollution. The samples collected on areas of petrol stations along the route from Lake Balkhash to the Talgar Pass in Kazakhstan suggest that these once-pristine areas adapted to oil pollution and hence permitted positive selection of species with the ability to degrade oil components. Such selection enables the removal of the pollutants by the local microorganisms and underlines the potential for self-regeneration of polluted areas.
Nevertheless, for acceleration of soil cleaning, heavily contaminated surfaces need to be restored either by feeding the native microbes with nutrients and/or other supplementary components (oxygen, biosurfactants, etc.) or—especially in case of scarcely degradable pollutants—by adding microorganisms with special degrading capabilities from strain collections or other habitats (Bento et al. 2005; Dua et al. 2002; Heipieper 2007; Juwarkar et al. 2010; Tyagi et al. 2011). This study illustrates the value of focused isolation efforts in times of increasing usage of cultivation-independent methods in applied and environmental microbiology.
References
Abo-State MAM, Riad BY, Bakr AA, Aziz MFA (2018) Biodegradation of naphthalene by Bordetella avium isolated from petroleum refinery wastewater in Egypt and its pathway. J Radiat Res Appl Sci 11(1):1–9. https://doi.org/10.1016/j.jrras.2017.10.001
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1006/jmbi.1990.9999
Ameen F, Moslem M, Hadi S, Al-Sabri AE (2016) Biodegradation of diesel fuel hydrocarbons by mangrove fungi from Red Sea Coast of Saudi Arabia. Saudi J Biol Sci 23(2):211–218. https://doi.org/10.1016/j.sjbs.2015.04.005
Anderson MS, Hall RA, Griffin M (1980) Microbial metabolism of alicyclic hydrocarbons: cyclohexane catabolism by a pure strain of Pseudomonas sp. J Gen Microbiol 120(SEP):89–94. https://doi.org/10.1099/00221287-120-1-89
Annweiler E, Richnow HH, Antranikian G, Hebenbrock S, Garms C, Franke S, Francke W, Michaelis W (2000) Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl Environ Microbiol 66(2):518–523. https://doi.org/10.1128/aem.66.2.518-523.2000
Awe S, Mikolasch A, Hammer E, Schauer F (2008) Degradation of phenylalkanes and characterization of aromatic intermediates acting as growth inhibiting substances in hydrocarbon utilizing yeast Candida maltosa. Int Biodeterior Biodegrad 62(4):408–414. https://doi.org/10.1016/j.ibiod.2008.03.007
Barnes NM, Khodse VB, Lotlikar NP, Meena RM, Damare SR (2018) Bioremediation potential of hydrocarbon-utilizing fungi from select marine niches of India. 3 Biotech 8(21):1–10. https://doi.org/10.1007/s13205-017-1043-8
Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96(9):1049–1055. https://doi.org/10.1016/j.biotech.2004.09.008
Bos P, de Bruyn JC (1973) The significance of hydrocarbon assimilation in yeast identification. Antonie Van Leeuwenhoek 39(1):99–107. https://doi.org/10.1007/bf02578845
Bouchez-Naitali M, Rakatozafy H, Marchal R, Leveau JY, Vandecasteele JP (1999) Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake. J Appl Microbiol 86(3):421–428. https://doi.org/10.1046/j.1365-2672.1999.00678.x
Cardini G, Jurtshuk P (1968) Cytochrome P-450 involvement in the oxidation of n-octane by cell-free extracts of Corynebacterium sp. strain 7E1C. J Biol Chem 243(22):6070–6072
Chaineau CH, Morel J, Dupont J, Bury E, Oudot J (1999) Comparison of the fuel oil biodegradation potential of hydrocarbon-assimilating microorganisms isolated from a temperate agricultural soil. Sci Total Environ 227(2–3):237–247. https://doi.org/10.1016/s0048-9697(99)00033-9
Chandran P, Das N (2012) Role of plasmid in diesel oil degradation by yeast species isolated from petroleum hydrocarbon-contaminated soil. Environ Technol 33(6):645–652. https://doi.org/10.1080/09593330.2011.587024
Chase AB, Arevalo P, Polz MF, Berlemont R, Martiny JBH (2016) Evidence for ecological flexibility in the cosmopolitan genus Curtobacterium. Front Microbiol 7:1–11. https://doi.org/10.3389/fmicb.2016.01874
Collins MD, Jones D (1983) Reclassification of Corynebacterium flaccumfaciens, Corynebacterium betae, Corynebacterium oortii and Corynebacterium poinsettiae in the genus Curtobacterium, as Curtobacterium flaccumfaciens comb. nov. J Gen Microbiol 129(NOV):3545–3548. https://doi.org/10.1099/00221287-129-11-3545
Colombo JC, Cabello M, Arambarri AM (1996) Biodegradation of aliphatic and aromatic hydrocarbons by natural soil microflora and pure cultures of imperfect and lignolitic fungi. Environ Pollut 94(3):355–362. https://doi.org/10.1016/s0269-7491(96)00044-9
Dallinger A, Duldhardt I, Kabisch J, Schluter R, Schauer F (2016) Biotransformation of cyclohexane and related alicyclic hydrocarbons by Candida maltosa and Trichosporon species. Int Biodeterior Biodegrad 107:132–139. https://doi.org/10.1016/j.ibiod.2015.11.015
Deng MC, Li J, Liang FR, Yi MS, Xu XM, Yuan JP, Peng J, Wu CF, Wang JH (2014) Isolation and characterization of a novel hydrocarbon-degrading bacterium Achromobacter sp HZ01 from the crude oil-contaminated seawater at the Daya Bay, southern China. Mar Pollut Bull 83(1):79–86. https://doi.org/10.1016/j.marpolbul.2014.04.018
Dua M, Singh A, Sethunathan N, Johri AK (2002) Biotechnology and bioremediation: successes and limitations. Appl Microbiol Biotechnol 59(2–3):143–152. https://doi.org/10.1007/s00253-002-1024-6
Ezekoye CC, Chikere CB, Okpokwasili GC (2018) Fungal diversity associated with crude oil-impacted soil undergoing in-situ bioremediation. Sustain Chem Pharm 10:148–152. https://doi.org/10.1016/j.scp.2018.11.003
Funke G, Aravena-Roman M, Frodl R (2005) First description of Curtobacterium spp. isolated from human clinical specimens. J Clin Microbiol 43(3):1032–1036. https://doi.org/10.1128/jcm.43.3.1032-1036.2005
Gesell M, Hammer E, Specht M, Francke W, Schauer F (2001) Biotransformation of biphenyl by Paecilomyces lilacinus and characterization of ring cleavage products. Appl Environ Microbiol 67(4):1551–1557. https://doi.org/10.1128/aem.67.4.1551-1557.2001
Gesell M, Hammer E, Mikolasch A, Schauer F (2004) Oxidation and ring cleavage of dibenzofuran by the filamentous fungus Paecilomyces lilacinus. Arch Microbiol 182(1):51–59. https://doi.org/10.1007/s00203-004-0695-z
Giraldo A, Gene J, Sutton DA, Madrid H, de Hoog GS, Cano J, Decock C, Crous PW, Guarro J (2015) Phylogeny of Sarocladium (Hypocreales). Persoonia 34:10–24. https://doi.org/10.3767/003158515x685364
Hassanshahian M, Emtiazi G, Cappello S (2012) Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull 64(1):7–12. https://doi.org/10.1016/j.marpolbul.2011.11.006
Heipieper HJ (2007) Bioremediation of soils contaminated with aromatic compounds: effects of rhizosphere, bioavailability, gene regulation and stress adaptation. In: Heipieper HJ (ed) Bioremediation of soils contaminated with aromatic compounds. NATO Science Series IV-Earth and Environmental Sciences, vol 76. Springer, Dordrecht, pp 1–4. https://doi.org/10.1007/978-1-4020-5693-2
Hofmann KH, Schauer F (1988) Utilization of phenol by hydrocarbon assimilating yeasts. Antonie Van Leeuwenhoek 54(2):179–188. https://doi.org/10.1007/bf00419204
Hugh R, Leifson E (1953) The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol 66(1):24–26
Hundt K, Wagner M, Becher D, Hammer E, Schauer F (1998) Effect of selected environmental factors on degradation and mineralization of biaryl compounds by the bacterium Ralstonia pickettii in soil and compost. Chemosphere 36(10):2321–2335. https://doi.org/10.1016/S0045-6535(97)10201-6
Ikeda K, Nakajima K, Yumoto I (1994) Isolation and characterization of a novel facultatively alkaliphilic bacterium, Corynebacterium sp., grown on n-alkanes. Arch Microbiol 162(6):381–386. https://doi.org/10.1007/bf00282101
Juretschko S, Timmermann G, Schmid M, Schleifer KH, Pommerening-Roser A, Koops HP, Wagner M (1998) Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol 64:3042–3051
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9(3):215–288. https://doi.org/10.1007/s11157-010-9215-6
Kachholz T, Rehm HJ (1978) Degradation of long chain alkanes by bacilli II. Metabolic pathways. Eur J Appl Microbiol Biotechnol 6(1):39–54. https://doi.org/10.1007/bf00500855
Kaczorek E, Salek K, Guzik U, Dudzinska-Bajorek B, Olszanowski A (2013) The impact of long-term contact of Achromobacter sp. 4(2010) with diesel oil—changes in biodegradation, surface properties and hexadecane monooxygenase activity. Int Biodeterior Biodegrad 78:7–16. https://doi.org/10.1016/j.ibiod.2012.12.003
Kane MD, Poulsen LK, Stahl DA (1993) Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol 59:682–686
Kästner M, Breuer-Jammali M, Mahro B (1994) Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH). Appl Microbiol Biotechnol 41(2):267–273. https://doi.org/10.1007/BF00186971
Kitamoto D, Ikegami T, Suzuki GT, Sasaki A, Takeyama Y, Idemoto Y, Koura N, Yanagishita H (2001) Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida) antarctica. Biotechnol Lett 23(20):1709–1714. https://doi.org/10.1023/a:1012464717259
Kronvall G, Hanson HS, von Stedingk LV, Tornqvist E, Falsen E (2000) Septic arthritis caused by a gram-negative bacterium representing a new species related to the Bordetella-Alcaligenes complex. Apmis 108(3):187–194. https://doi.org/10.1034/j.1600-0463.2000.d01-43.x
Krupa EG, Barinova SS, Tsoy VN, Lopareva TY, Sadyrbaeva NN (2017) Spatial analysis of hydro-chemical and toxicological variables of the Balkhash Lake, Kazakhstan. Res J Pharm Biol Chem Sci 8(3):1827–1839
Kurtzman C, Fell JW, Boekhout T (2011) The yeasts: a taxonomic study. Elsevier Science
Lahav R, Fareleira P, Nejidat A, Abeliovich A (2002) The identification and characterization of osmotolerant yeast isolates from chemical wastewater evaporation ponds. Microb Ecol 43(3):388–396. https://doi.org/10.1007/s00248-002-2001-4
Lal B, Khanna S (1996) Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenes odorans. J Appl Bacteriol 81(4):355–362. https://doi.org/10.1111/j.1365-2672.1996.tb03519.x
Long HZ, Wang YL, Chang SJ, Liu GX, Chen T, Huo GH, Zhang W, Wu XK, Tai XS, Sun LK, Zhang BG (2017) Diversity of crude oil-degrading bacteria and alkane hydroxylase (alkB) genes from the Qinghai-Tibet Plateau. Environ Monit Assess 189(3):1–14. https://doi.org/10.1007/s10661-017-5798-5
Luangsa-Ard J, Houbraken J, van Doorn T, Hong SB, Borman AM, Hywel-Jones NL, Samson RA (2011) Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett 321(2):141–149. https://doi.org/10.1111/j.1574-6968.2011.02322.x
Ma XK, Ding N, Peterson EC (2015) Bioaugmentation of soil contaminated with high-level crude oil through inoculation with mixed cultures including Acremonium sp. Biodegradation 26(3):259–269. https://doi.org/10.1007/s10532-015-9732-7
Martorell MM, Ruberto LAM, Fernandez PM, de Figueroa LIC, Mac Cormack WP (2017) Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J Basic Microbiol 57(6):504–516. https://doi.org/10.1002/jobm.201700021
McGowan L, Herbert R, Muyzer G (2004) A comparative study of hydrocarbon degradation by Marinobacter sp., Rhodococcus sp. and Corynebacterium sp. isolated from different mat systems. Ophelia 58(3):271–281. https://doi.org/10.1080/00785236.2004.10410235
Mikolasch A, Omirbekova A, Schumann P, Reinhard A, Sheikhany H, Berzhanova R, Mukasheva T, Schauer F (2015) Enrichment of aliphatic, alicyclic and aromatic acids by oil-degrading bacteria isolated from the rhizosphere of plants growing in oil-contaminated soil from Kazakhstan. Appl Microbiol Biotechnol 99(9):4071–4084. https://doi.org/10.1007/s00253-014-6320-4
Mikolasch A, Reinhard A, Alimbetova A, Omirbekova A, Pasler L, Schumann P, Kabisch J, Mukasheva T, Schauer F (2016) From oil spills to barley growth—oil-degrading soil bacteria and their promoting effects. J Basic Microbiol 56(11):1252–1273. https://doi.org/10.1002/jobm.201600300
Mohammadian E, Arzanlou M, Babai-Ahari A (2017) Diversity of culturable fungi inhabiting petroleum-contaminated soils in Southern Iran. Antonie Van Leeuwenhoek 110(7):903–923. https://doi.org/10.1007/s10482-017-0863-1
Nhi-Cong LT, Mikolasch A, Awe S, Sheikhany H, Klenk H-P, Schauer F (2010) Oxidation of aliphatic, branched chain, and aromatic hydrocarbons by Nocardia cyriacigeorgica isolated from oil-polluted sand samples collected in the Saudi Arabian Desert. J Basic Microbiol 50(3):241–253. https://doi.org/10.1002/jobm.200900358
Obi LU, Atagana HI, Adeleke RA (2016) Isolation and characterisation of crude oil sludge degrading bacteria. Springerplus 5:1–13. https://doi.org/10.1186/s40064-016-3617-z
Okuhara M, Kubochi Y, Harada T (1971) Formation of glutaric and adipic acids from n-alkanes with odd and even numbers of carbons by Candida tropicalis OH23. Agric Biol Chem 35(9):1376–1380. https://doi.org/10.1080/00021369.1971.10860083
Oudot J, Dupont J, Haloui S, Roquebert MF (1993) Biodegradation potential of hydrocarbon-assimilating tropical fungi. Soil Biol Biochem 25(9):1167–1173. https://doi.org/10.1016/0038-0717(93)90211-s
Rafin C, de Foucault B, Veignie E (2013) Exploring micromycetes biodiversity for screening benzo[a] pyrene degrading potential. Environ Sci Pollut Res 20(5):3280–3289. https://doi.org/10.1007/s11356-012-1255-8
Rajaei S, Seyedi SM, Raiesi F, Shiran B, Raheb J (2013) Characterization and potentials of indigenous oil-degrading bacteria inhabiting the rhizosphere of wild oat (Avena Fatua L.) in south west of Iran. Iran J Biotechnol 11(1):32–40. https://doi.org/10.5812/ijb.9334
Raju MN, Leo R, Herminia SS, Moran REB, Venkateswarlu K, Laura S (2017) Biodegradation of diesel, crude oil and spent lubricating oil by soil isolates of Bacillus spp. Bull Environ Contam Toxicol 98(5):698–705. https://doi.org/10.1007/s00128-017-2039-0
Rojo F (2009) Degradation of alkanes by bacteria. Environ Microbiol 11(10):2477–2490. https://doi.org/10.1111/j.1462-2920.2009.01948.x
Romero MC, Hammer E, Cazau MC, Arambarri AM (2002) Isolation and characterization of biarylic structure-degrading yeasts: hydroxylation potential of dibenzofuran. Environ Pollut 118(3):379–382. https://doi.org/10.1016/s0269-7491(01)00290-1
Sajna KV, Sukumaran RK, Gottumukkala LD, Pandey A (2015) Crude oil biodegradation aided by biosurfactants from Pseudozyma sp NII 08165 or its culture broth. Bioresour Technol 191:133–139. https://doi.org/10.1016/j.biortech.2015.04.126
Schauer F, Schauer M (1986) Alkanassimilierende Hefen. Systematische Stellung und Erfassung einiger Leistungsgrenzen (Alkane assimilating yeasts. Systematic position and determination of some physiological limitations). Wiss Z Ernst-Moritz-Arndt-Univ Greifswald, Math-natwiss Reihe 35:14–23
Scheller U, Zimmer T, Becher D, Schauer F, Schunck WH (1998) Oxygenation cascade in conversion of n-alkanes to alpha,omega-dioic acids catalyzed by cytochrome p450 52A3. J Biol Chem 273(49):32528–32534. https://doi.org/10.1074/jbc.273.49.32528
Seeliger HPR (1956) Use of a urease test for the screening and identification of cryptococci. J Bacteriol 72(2):127–131
Sietmann R, Hammer E, Moody J, Cerniglia CE, Schauer F (2000) Hydroxylation of biphenyl by the yeast Trichosporon mucoides. Arch Microbiol 174(5):353–361. https://doi.org/10.1007/s002030000219
Sietmann R, Gesell M, Hammer E, Schauer F (2006) Oxidative ring cleavage of low chlorinated biphenyl derivatives by fungi leads to the formation of chlorinated lactone derivatives. Chemosphere 64(4):672–685. https://doi.org/10.1016/j.chemosphere.2005.10.050
Soumana IH, Linz B, Harvill ET (2017) Environmental origin of the genus Bordetella. Front Microbiol 24(open access):1–10. https://doi.org/10.3389/fmicb.2017.00028
Statista (2019) Ranking der größten Erdölexporteure weltweit im Jahr 2017. https://de.statista.com/statistik/daten/studie/227060/umfrage/die-groessten-oelexporteure-weltweit. Accessed 10 May 2019
Summerbell RC, Gueidan C, Schroers HJ, de Hoog GS, Starink M, Rosete YA, Guarro J, Scott JA (2011) Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud Mycol 68:139–162. https://doi.org/10.3114/sim.2011.68.06
Suslow TV, Schroth MN, Isaka M (1982) Application of a rapid method for Gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathology 72(7):917–918. https://doi.org/10.1094/Phyto-72-917
Trading Economics (2019) Erdölförderung – Liste der Länder. https://de.tradingeconomics.com/country-list/crude-oil-production. Accessed 10 May 2019
Tyagi M, da Fonseca MMR, de Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22(2):231–241. https://doi.org/10.1007/s10532-010-9394-4
Varjani SJ, Gnansounou E, Pandey A (2017) Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 188:280–291. https://doi.org/10.1016/j.chemosphere.2017.09.005
Vigueras G, Shirai K, Martins D, Franco TT, Fleuri LF, Revah S (2008) Toluene gas phase biofiltration by Paecilomyces lilacinus and isolation and identification of a hydrophobin protein produced thereof. Appl Microbiol Biotechnol 80(1):147–154. https://doi.org/10.1007/s00253-008-1490-6
Vigueras G, Shirai K, Hernandez-Guerrero M, Morales M, Revah S (2014) Growth of the fungus Paecilomyces lilacinus with n-hexadecane in submerged and solid-state cultures and recovery of hydrophobin proteins. Process Biochem 49(10):1606–1611. https://doi.org/10.1016/j.procbio.2014.06.015
Wang F, Grundmann S, Schmid M, Dorfler U, Roherer S, Munch JC, Hartmann A, Jiang X, Schroll R (2007) Isolation and characterization of 1.2,4-trichlorobenzene mineralizing Bordetella sp. and its bioremediation potential in soil. Chemosphere 67(5):896–902. https://doi.org/10.1016/j.chemosphere.2006.11.019
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Yabuuchi E, Kawamura Y, Kosako Y, Ezaki T (1998) Emendation of genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and proposal of Achromobacter ruhlandii (Packer and Vishniac) comb. nov., Achromobacter piechaudii (Kiredjian at al.) comb. nov., and Achromobacter xylosoxidans subsp. denitrificans (Ruger and Tan) comb. nov. Microbiol Immunol 42(6):429–438. https://doi.org/10.1111/j.1348-0421.1998.tb02306.x
Yamada K, Komagata K (1972) Taxonomic studies on coryneform bacteria. V. Classification of coryneform bacteria. J Gen Appl Microbiol 18(6):417–431. https://doi.org/10.2323/jgam.18.417
Yuan SY, Su LM, Chang BV (2009) Biodegradation of phenanthrene and pyrene in compost-amended soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 44(7):648–653. https://doi.org/10.1080/10934520902847638
Acknowledgments
We thank R. Jack (Prof. emeritus, Institute of Immunology, University of Greifswald) for help in preparing the manuscript. We thank CABNET (Central Asian Biodiversity Network), in particular the project manager Michael Manthey (Institute of Botany and Landscape Ecology, University of Greifswald), for the opportunity to establish active contacts between scientists from the Al-Farabi Kazakh National University and the University of Greifswald.
Funding
This study was funded by DAAD (Deutscher Akademischer Austauschdienst) (project code 50754935, project title “CABNET-Central Asian Biodiversity Network”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 842 kb)
Rights and permissions
About this article
Cite this article
Mikolasch, A., Donath, M., Reinhard, A. et al. Diversity and degradative capabilities of bacteria and fungi isolated from oil-contaminated and hydrocarbon-polluted soils in Kazakhstan. Appl Microbiol Biotechnol 103, 7261–7274 (2019). https://doi.org/10.1007/s00253-019-10032-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10032-9