Abstract
A low production rate for calcium carbonate with microbial solidification technology at low temperatures often restricts its application. For this reason, adding urea to the medium and the domestication of Bacillus megaterium at low temperature were proposed to produce more calcium carbonate based on an analysis of growth characteristics, urease activity, and the production rates for calcium carbonate under different conditions. Sand solidification tests were conducted to demonstrate improvements caused by the methods. The results showed that the higher the temperature, the faster the growth of Bacillus megaterium and the stronger the urease activity. Growth was fastest and urease activity strongest at a pH of 8. Adding urea to the medium and the domestication of B. megaterium at low temperature can both improve the production rate, effectively increasing calcium carbonate precipitation at low temperature. Combining the two methods resulted in greater improvement of the production rate for calcium carbonate. The two methods were also found to improve the effect of sand solidification. Therefore, our study provides a solid foundation for the actual engineering application of bio-cementation technology at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, many researchers in the geotechnical and environmental engineering field have paid increasing attention to microbially induced carbonate precipitation (MICP) (Cuthbert et al. 2013; Montoya et al. 2013; Soon et al. 2014). The essence of the technology is that metal ions bind with acid radical ions to form minerals, such as calcium carbonate, which form a fundamental part of biogeochemical processes (DeJong et al. 2006). It is widely recognized that carbonate precipitation, as an important aspect of biomineralization, has significant implementation potential (Wijngaarden et al. 2011; Wu et al. 2011). Applications of carbonate mineralization induced by bacteria include the production of biomimetic materials, bioremediation, and plugging-cementation in porous media cracks (Montoya et al. 2013; Whiffin et al. 2007; Martinez et al. 2013). The hydrolysis of urea by urease-producing bacteria is one of the most common pathways used to induce carbonate precipitation (Mostafa et al. 2017; Mostafa and Aydin 2019).
A problem with the application of MICP is its use at low temperatures, which has not been well studied. Palin et al. (2017) presented a bacteria-based self-healing cementitious composite for application in low-temperature marine environments, while Ferris et al. (2004) investigated the kinetics of calcite precipitation induced in response to the hydrolysis of urea by Bacillus pasteurii at different temperatures in artificial groundwater. Jiang et al. (2016) focused on quantifying ureolytic efficiency and showed that the anoxic ureolytic performance of B. megaterium is greater than its oxic counterpart. Hence, B. megaterium was used in this paper to identify production rates for calcium carbonate at low temperature. According to Sun et al. (2018b), microbial activity is low at temperatures as low as 10 °C. However, B. megaterium has been shown to grow and produce calcium carbonate, even at temperatures as low as 3 °C (Vos et al. 2011). In addition, much of the world’s underground infrastructure is located in cool climatic zones (annual average temperature < 10 °C, and average summer temperature generally < 20 °C), but many studies have been shown to work exclusively at room temperature (Erşan et al. 2015; Tziviloglou et al. 2016; Seifan et al. 2017; Seifan et al. 2018). Urease may be impaired under low-temperature conditions, leading to insufficient activity (Sun et al. 2018b). Weak urease activity results in a lack of precipitation, making it difficult to bond sand particles firmly together. Therefore, increasing microbial activity at low temperatures to produce more calcium carbonate is of great significance. For this reason, the primary objective of this study was to improve low production rates for calcium carbonate at low temperature. Through controlling temperature and pH, the growth characteristics and urease activity of B. megaterium were analyzed, and the production rates for calcium carbonate at different temperatures were studied. Adding urea to the medium and the domestication of B. megaterium at low temperature were found to improve the low production rate. Finally, sand solidification tests were conducted to study the curing effect after adding urea to the medium or the domestication of B. megaterium at low temperature. Studies in this paper provide a foundation for the subsequent application of MICP technology at low temperature.
Materials and methods
Bacteria and culture media
In this paper, B. megaterium (ATCC 14581, from the Guangdong culture collection center in China), a Gram-positive, rod-shaped soil bacterium ranging in size from 2 to 5 μm (Lian et al. 2006), was used as a urease-producing microbe to determine production rates at low temperature. B. megaterium was cultivated in LB (Luria Bertani) medium (as listed in the ATCC database), which is comprised of yeast extract 15.0 g/L, polypeptone 10.0 g/L, NaCl 10.0 g/L, and distilled water.
Growth profile and enzyme activity
Measurement of optical density and enzyme activity
Absorbance (optical density) of a suspension of B. megaterium was measured with a spectrophotometer at 600-nm wavelength (OD600) to measure the growth phase, as for Fredrickson et al. (2001).
According to the method proposed by Whiffin (2004), 6 mL of bacterial suspension was mixed with 54 mL urea solution (1 mol/L) and then electrical conductivity was measured every 5 min. The average change in conductivity per minute (ms/cm ∙ min) was calculated and Whiffin (2004) proposed that it corresponded to 11 mM urea hydrolyzed/min, which was derived experimentally. Therefore, the change in conductivity per minute (ms/min) can be converted to the amount of urease hydrolysis in unit time and, eventually, the rate of hydrolysis of urea per minute (mM urea hydrolyzed ∙ min−1), representing enzyme activity, was obtained by multiplying by the dilution factor of 10. The method was used at room temperature and the conversion factor might not remain constant at different temperatures; however, in order to conveniently compare activity, this method was used at different temperatures.
Comparative tests of optical density and enzyme activity with various temperatures and pH
With the view to differentiating the effects of temperature or pH on the growth of B. megaterium, five different temperatures were tested: 10, 15, 20, 25, and 30 °C and the initial pH of the medium was adjusted to 7, 8, 9, or 10. Triplicate samples of each condition were prepared. Khan et al. (2015) found that growth was affected by the amount of bacterial culture added to the liquid culture medium. Therefore, the all cultures were started with the same inoculum of OD600 0.989. Optical densities and enzyme activity were monitored after 48-h cultivation.
Comparative tests of production rates for calcium carbonate
Production rates under various temperature
Comparative tests of production rates for calcium carbonate were conducted in transparent polypropylene (PP) tubes. Bacterial suspension with an OD600 of 1.09 was added to the gelling solution (mixture of 0.5 M calcium acetate and 0.5 M urea solution). The volumes of the bacterial suspension and the gelling solution were both 20 mL resulting in a total solution volume of 40 mL. Nutrients were added to the gelling solution (5 g/L yeast extract, 10 g/L peptone, and 10 g/L sodium chloride) to provide energy for the bacteria and to prevent the decrease of total urease activity in the process of the calcification reaction.
In a sterile environment, samples were prepared corresponding to different temperatures (10, 15, 20, 25, or 30 °C). Every group consisted of three parallel samples, all with a starting pH of 8.0. The evaluation criterion was production rate for calcium carbonate, i.e., the ratio of calcium carbonate produced to the theoretical total amount, which was obtained every 2 days during the 96-hour reaction process.
The method for measuring the actual amount of calcium carbonate produced is described below.
After the precipitation reaction, the precipitate and the filter paper were dried at 70 °C after filtration and the total mass of the filter paper and precipitate was obtained (M1). Then, diluted hydrochloric acid was used to dissolve the calcium precipitate, followed by washing with water. Finally, the filter paper and insoluble matter were dried to obtain the total mass (M2). The actual amount of calcium carbonate (△m) was obtained via M1 − M2.
The theoretical total mass of CaCO3 is determined by C × V × M, where C is the concentration of the solution in moles/liter, V is the volume of gelling solution, and M is the molar mass of CaCO3 (100.087 g/mol).
Comparison of production rates under various amounts of urea added
Urea was added to 100 mL of medium at various urea concentrations (0 g/L, 5 g/L, 10 g/L, 15 g/L, or 20 g/L), with the same 1% inoculum and then the culture was cultivated at 10 °C for 48 h. The OD600 of bacterial suspension was 0.99. Three samples per group were prepared.
After 48 h of culture, the mixed solution of calcium acetate and urea was added to the bacterial suspension to determine the effect of urea concentration on production rates for calcium carbonate. The concentrations of calcium acetate and urea in the mixture were both 0.5 mol/L. Comparative tests of production rates were conducted at low temperature of 10 °C, pH 8, and production rates for calcium carbonate were calculated after 96 h.
Comparison of production rates for calcium carbonate with domestication at low temperature
During the process of domestication, the pH was kept constant (8.0). Four different temperatures were used (30 °C, 25 °C, 20 °C, and 15 °C). The period time of each phase was 6 days and bacteria were inoculated with 5% v/v every 2 days, which meant three inoculations per phase. The percentage of inoculum (5%) used here was different from previous tests (1%), as increased number of bacteria improve the likelihood of domestication. After domestication, performance tests at low temperature between domesticated B. megaterium from each stage and an undomesticated strain (culturing at 30 °C) were performed. All strains were cultured for 48 h at 10 °C with an inoculum of 1% v/v and OD600 of about 0.8 following which, calcification tests were also carried out at 10 °C. A similar percentage of inoculum (1%) was used for calcification tests, in keeping with previous experiments. Absorbance values after 24 and 48 h were measured and the production rates for calcium carbonate at 2 and 4 days were obtained.
Final comparison of production rates
To further improve production rates for calcium carbonate, the two methods mentioned above, adding urea to medium and the domestication tests at low temperature, were combined. Undomesticated B. megaterium and domesticated strains from each stage were inoculated at 1% v/v with an OD600 of approximately 0.8 and the temperature was controlled at 10 °C. At the same time, the samples were divided in two, where urea was added to one sample (20 g/L) the other sample without urea. After 48 h of culture, the production rates at 2 days and 4 days were obtained via calcification tests.
MICP test in PVC cylinders
Materials
B. megaterium and sand from the Yangtze River were used for solidification tests in polyvinyl chloride (PVC) cylinders with an inner diameter of 4.6 cm and a height of 20 cm. The sand grain size was smaller than 0.25 mm. The sand had poor gradation and they were sterilized by high temperature and pressure before being placed in PVC cylinders. In order to control variables, all sand samples had the same initial dry density of approximately 1.59 g/cm3. Therefore, for a column with an approximate height of 8 cm, 210 g of sand was used.
Methods
Bacteria were cultured aerobically in LB medium at 10 °C and pH 8 for 48 h. Two layers of gauze were placed on the two ends of the samples to avoid sand leakage. A diagram of the solidification tests in the PVC cylinders is shown in Fig. 1. The bacterial suspension was injected with an electric pump into the PVC cylinders at a low speed (4 mL/min) until it reached saturation and the sand samples were then left for 2 h. The injection speed of the gelling solution (150 mL mixture of 0.5 mol/L of urea and calcium acetate) was also controlled at 4 mL/min. To perform the experiments efficiently, the abovementioned steps were repeated once each day. To determine the influence of different conditions on the physical and mechanical features of the specimens, samples were divided into four groups: (1) samples with undomesticated B. megaterium and no urea added to the medium; (2) samples with undomesticated B. megaterium and urea added to the medium (20 g/L); (3) samples with domesticated B. megaterium at 15 °C and no urea added to the medium; (4) samples with domesticated B. megaterium at 15 °C and no urea added to the medium (20 g/L). Curing tests were conducted at 10 °C and initial pH values of the bacterial suspension and gelling solution were both 8.0. After 14 days of curing, the unconfined compressive strength (UCS) values of the specimens were measured. The loading speed was constant at 1 mm/min for the duration of the UCS tests. Experiments were stopped once specimens reached brittle failure. Curing tests were conducted at 10 °C and less calcium carbonate was produced, so that uneven curing was unlikely. UCS was used to evaluate improvements due to the methods proposed in this paper. Sun et al. (2018b) have demonstrated the existence of calcium carbonate and its crystal morphology via XRD tests; therefore, SEM/EDS was not used.
Results
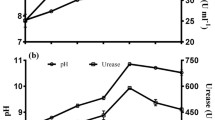
Varying temperatures and pH values were used to determine the effect of altering these parameters on the growth and urease activity of B. megaterium. Temperature had a significant impact on the eventual OD600 values of B. megaterium, as shown in Fig. 2. It can be observed that the number of bacterial cells varied with temperature. There was a significant decrease in absorbance from 15 to 10 °C. The OD600 at 10 °C was less than one half of that at 30 °C. At different temperatures, at a pH of 8, the growth of B. megaterium was the fastest, although there was no significant difference from the results at pH 7. Absorbance values decreased significantly with increasing pH to a value of 10.
Similar to the optical density curves, enzyme activity curves at various temperatures are shown in Fig. 3. An improvement of enzyme activity was seen with increasing temperature. Jiang et al. (2016) previously had similar results. At 10 °C, the enzyme activity of B. megaterium was significantly lower than at other temperatures. For different temperatures, the enzyme activity of B. megaterium was strongest at pH 8, which was consistent with the above absorbance results.
Different production rates for calcium carbonate at various temperatures were calculated on the 2nd and 4th day, as shown in Fig. 4. The amount of calcium precipitation by B. megaterium increased with time. The higher the temperature, the greater the calcium precipitation, which was consistent with the conclusions of Whiffin (2004) who used S. pasteurii for calcification. There were differences among the production rates at diverse temperatures, especially on the 4th day. In particular, the precipitation rate for calcium carbonate at 10 °C was less than 30%, much lower than that at 30 °C (almost 60%), which would restrict the application of MICP technology at low temperature. Therefore, it was reasonable to study how to increase production rates for calcium carbonate at low temperature.
The first method was to add urea to medium. In the process of MICP, urea is hydrolyzed by catalysis, leading to the production of carbonate and ammonium, which explains why the pH and carbonate concentration grow in the microbial environment and CaCO3 is generated afterwards (Stocks-Fischer et al. 1999). Therefore, the whole process was divided into two parts: hydrolysis of urea and formation of CaCO3. Here, we proposed a method to accelerate the MICP process. With the method used, production rates after 4 days are shown in Fig. 5. From the figure, the precipitation rate for calcium carbonate increased with urea added to the medium, in addition, production rates also increased with increasing urea concentration. When the concentration of urea was 20 g/L, the precipitation rate for calcium carbonate was almost 43%, growing by 11% compared to the no urea sample.
The second applied method was the domestication of B. megaterium at low temperature to study the improvement of precipitation rates for calcium carbonate at low temperature. The domestication tests were performed at temperatures from 15 to 30 °C. The time period of each phase was 6 days and bacteria were inoculated at 5% v/v every 2 days. The absorbance was measured after every inoculation, as shown in Fig. 6. When B. megaterium entered each new temperature stage, the OD600 after 48 h of culture decreased (Fig. 6). But as the domestication time increased, the absorbance on the 6th day after domestication was higher than that on the 2nd day. When the domestication temperature decreased to 15 °C, the absorbance value at day 2 was smaller than that for other temperatures on the 2nd day. However, with increasing domestication time, the absorbance value also grew, and after 6 days, it reached 1.1.
A comparison of the absorbance values and production rates for calcium carbonate is shown in Table 1. By using domesticated B. megaterium, absorbance values and production rates both rose, compared to when undomesticated B. megaterium was used. Furthermore, the lower the temperature of domestication, the faster the growth, and the larger the precipitation rate for calcium carbonate at 10 °C. The OD600 of B. megaterium domesticated at 15 °C was 0.2 higher than undomesticated B. megaterium, and the precipitation rate of the 4th day increased by 15%, reaching 46%. A significant increase of production rates for calcium carbonate was noted between 15 °C domesticated B. megaterium and 20 °C domesticated B. megaterium. OD600 at 24 and 48 h both grew by about 0.1, and precipitation rate of the 4th day went up by 7%.
The addition of urea to the medium and domestication of B. megaterium at low temperature both increased the precipitation rate for calcium carbonate at 10 °C. For example, at a concentration of 20 g/L urea, the precipitation rate on the 4th day was improved to almost 43%. With 15 °C domesticated B. megaterium, the precipitation rate on the 4th day was increased to 46%. However, the precipitation rate after improvement was still much smaller than precipitation rate with 30 °C and not using the two methods (60%). Therefore, the two methods were combined to determine the final increase in the precipitation rate for calcium carbonate. Production rates on the 2nd and 4th days for all samples were measured (Fig. 7). Only production rates for calcium carbonate on the 2nd and 4th day instead of longer time period (6 days) were measured here, since our aim was to demonstrate an improvement of the methods proposed in this paper compared to standard methods, an improvement that can be seen in Fig. 7. In addition, the nutrients in the LB medium were not sufficient, resulting in a small increase from the 4th to 6th day.
B. megaterium after domestication produced more calcium precipitate than undomesticated B. megaterium regardless of whether urea was added to medium, and with decreasing domestication temperature, production rates on the 2nd and 4th day gradually increased. Moreover, samples with added urea had higher production rates than those without added urea. It is worth noting that the precipitation rates of samples on the 2nd day with B. megaterium domesticated at 25 °C and without added urea were lower than those with undomesticated B. megaterium and added urea, whereas the former surpassed the latter on the 4th day. The same phenomenon was found for samples with B. megaterium that was domesticated at 20 °C and without added urea and samples with domesticated at 15 °C and added urea (20 g/L).
When the two methods were combined, the production rate for calcium carbonate was significantly higher than with undomesticated B. megaterium and without added urea, as shown in Fig. 7. For B. megaterium domesticated at 15 °C and added urea (20 g/L), the precipitation rate on the 4th day was 58%, increasing by about 30% compared with production rates for calcium carbonate at 30 °C, and almost reached 60%. Therefore, the combination of the two methods enabled B. megaterium to produce more calcium carbonate precipitation.
To study the effect of improving the MICP method on sand solidification, we considered different methods of improvement (adding urea to the medium and domestication of B. megaterium at low temperature), and the UCS values of the samples are shown in Table 2. All specimens were improved compared to the control. Samples with undomesticated B. megaterium and no added urea had the minimum strength. Compared with these, the strength of samples with domesticated B. megaterium and no added urea increased by 0.82 Mpa, suggesting that B. megaterium domesticated at 15 °C could improve the curing effect, which corresponded to previous research about the production rates of calcium carbonate. It was found through a comparison of the effect of adding urea to the medium that an increase in the strength of samples with undomesticated B. megaterium was 1 MPa and with 15 °C domesticated B. megaterium was nearly 0.8 MPa. Hence, adding urea to the medium could also improve the curing effect. Compared with NO. 4 and NO. 1 sand column, the strength increased nearly 1.7 MPa by combining the two methods. It was concluded that using the two methods together allowed the sand columns to have greater strength at low temperature, which was consistent with previous research about the production rates of calcium carbonate.
Discussion
The effect of temperature or pH on growth and enzyme activity of B. megaterium
B. megaterium can form endospores, which are highly resistant to extreme environmental conditions. More specifically, B. megaterium can grow at temperatures from 3 to 45 °C (Vos et al. 2011) and it can induce greater calcium precipitation than Sporosarcina pasteurii (Sun et al. 2018b); therefore, B. megaterium was used here.
Fredrickson et al. (2001) monitored the absorbance (optical density) of a bacterial suspension of S. pasteurii with a spectrophotometer at 600-nm wavelength (OD600). Jiang et al. (2016) also used spectrophotometry to measure the growth of B. megaterium, which is why we have used spectrophotometry here for comparison. Enzyme activity was measured as an increase in conductivity, because of the very strong positive linkage between conductivity increase and urea hydrolysis (Chin and Kroontje 1962; Whiffin 2004; Paassen 2009).
The effect of temperature on the growth of B. megaterium can be seen in Fig. 2. The higher the temperature, the greater the number of cells. The speed of growth and cell biomass at 30 °C trump the other conditions. At 10 °C, the number of bacteria was much lower than at other temperatures, as low temperature inhibits bacterial reproduction (Hattori 1973). Temperature greatly contributed to the reproduction of S. pasteurii, probably due to a higher sensitivity to temperature (Alexander 1961) and this phenomenon could also be seen in the growth of B. megaterium. Low temperature still impaired B. megaterium, causing slowed growth at 10 °C.
From Fig. 3, temperature had a huge impact on the enzyme activity of B. megaterium and enzyme activity was higher at high temperatures. Bachmeier et al. (2002) reached the same conclusion with S. pasteurii. Enzyme activity was greatly impaired at 10 °C and as the temperature rose from 15 to 30 °C, the urease activity of B. megaterium was not greatly altered. Strong adaptability to temperature of B. megaterium rested chiefly on the fact. Other studies have also shown that there was limited change in urease activity of B. megaterium despite variation in temperature (Vos et al. 2011; Whiffin 2004). However, when the temperature decreased to 10 °C, the urease activity of B. megaterium declined, meaning that B. megaterium was not active as the low temperature significantly impaired its urease activity. As for the effect of pH on OD600 and enzyme activity of B. megaterium, when pH was 8, the growth of B. megaterium was always the fastest and enzyme activity was highest; therefore, a pH of 8 is the optimum alkaline environment for B. megaterium and was used in subsequent tests. There is a linear relationship between OD600 and the ureolysis rate in S. pasteurii (Lauchnor et al. 2015) and by comparing Figs. 2 and 3, a linear relationship between OD600 and ureolysis rate in B. megaterium also existed.
Comparative tests of productive rates for calcium carbonate
MICP technology has been used extensively because of the production of calcium precipitate (Ivanov and Chu 2008). Only by analyzing the production rates for calcium carbonate can it demonstrated whether MICP technology can be used effectively at low temperatures. Many factors can have an impact on the type and amount of carbonate precipitation, for example, the functional attributes of a precipitating microorganism, the rate of urea hydrolysis, urea and calcium dosage, and amino acids such as glutamic acid (Rodriguez-Navarro et al. 2003; Whiffin 2004; De Muynck et al. 2013; Braissant et al. 2003; Li et al. 2010). Calcium carbonate produced from calcium chloride is called calcite, while the addition of calcium acetate leads to the formation of aragonite and the bonding effect of aragonite is better than calcite in solution (Muynck et al. 2008; Tittelboom et al. 2010). The reason may be that calcium carbonate produced from calcium acetate is heavy, easy to precipitate, and not dispersive. Consequently, calcium acetate was used as a calcium recourse.
Temperature affects carbonate precipitation by S. pasteurii by changing enzyme activity (Ferris et al. 2004). The effect of temperature on the production rate for calcium carbonate by B. megaterium is shown in Fig. 4. At 10 °C, the precipitation rate for calcium carbonate was lower than that at other temperatures, which was ascribed to a dual inhibition of low enzyme activity and low bacterial growth. Bacterial growth and enzyme activity of B. megaterium were impacted slightly by a change in temperature from 30 to 15 °C. However, when the temperature decreased to 10 °C, beyond the range of suitable environmental temperature, the precipitation rate was very low (less than 30%).
Urease is produced by bacteria to decompose urea into CO32−, binding free calcium ions to generate calcium carbonate precipitate (Mitchell et al. 2008). Generally, CO32− and Ca2+ are provided by the gelling solution. When bacteria and urea are added to the medium at the same time, urea would be decomposed into CO32− in advance during the growth of bacteria. Therefore, the process of calcium carbonate production consisted of two parts after the mixed solution was added. One was immediate calcification once the calcium source was added; i.e., calcium carbonate was produced as soon as the calcium source was added. The other was the common MICP process; i.e., urea was decomposed firstly and then bound to calcium ions to yield calcium carbonate. The curing reaction was divided into two parts: a quick chemical reaction and a biological reaction.
The precipitation rate for calcium carbonate at 10 °C was lower than at other temperatures. Hence, urea was added to the medium, which could accelerate the reaction at low temperature, producing more precipitate in the same time frame. Production rates rose with an increase in urea concentration, as more urea was hydrolyzed ahead of the addition of the mixed solution, as shown in Fig. 5. The method of adding urea to the medium was useful to induce greater precipitation by B. megaterium. Specifically, when the concentration of added urea was 20 g/L, the production rate of B. megaterium after 4 days increased from 32% to almost 43%.
The second method was domestication of B. megaterium at low temperature. Strain domestication is a great way to improve specific abilities by controlling certain factors. Cheng et al. (2015) studied the enhancement of fermentative hydrogen production from hydrolyzed water hyacinth with activated carbon detoxification and bacterial domestication. Lo et al. (2008) used xylose to domesticate a group of hydrogen-producing samples to identify the dominant bacterial strain. The energy conversion efficiency from hydrogen and methane cogeneration by Arthrospira maxima biomass by two-phase fermentation was improved by bacterial domestication (Cheng et al. 2011). Therefore, this paper adopted a similar method to domesticate B. megaterium at low temperature, and investigated the improvement in production rates.
As shown in Fig. 6, when B. megaterium entered each new temperature stage, the OD600 after a 48-h culture decreased. This was due to the lower temperature, where the speed of growth of B. megaterium was slower, but as the domestication time increased, the adaptability of B. megaterium to the low temperature was greater. This explains the increase in absorbance on the 6th day after domestication compared to the 2nd day. When the domestication temperature decreased to 15 °C, the absorbance values on the 2nd day were smaller than those at other temperature stages on the 2nd day, as the temperature was quite low for B. megaterium. However, with increasing domestication time, the absorbance value also increased and after 6 days it reached 1.1. By using domesticated B. megaterium, absorbance values and the production rates both increased and became larger with a decrease in domestication temperature, as listed in Table 1. The results demonstrated that this method improved the low-temperature resistance of B. megaterium. When it came to a comparison between 15 °C domesticated B. megaterium and 20 °C domesticated B. megaterium, it appears that B. megaterium domesticated at 15 °C had a stronger adaptability to 10 °C due to smaller difference between 15 and 10 °C compared with B. megaterium domesticated at 20 °C. Therefore, the domestication of B. megaterium at low temperature could improve the growth and production rates for calcium carbonate, but the precipitation rate was still much smaller than that with no urea addition at 30 °C, despite reaching 46%.
The two methods were used together to try to maximize precipitation rate so as to approach the result obtained at 30 °C. The precipitation rate of samples on the 2nd day with 25 °C domesticated B. megaterium and without added urea was lower than that with undomesticated B. megaterium and added urea, whereas the former surpassed the latter on day 4, as shown in Fig. 7. As calcium precipitation on the 2nd day was mainly produced by a chemical reaction for samples with urea added to the medium, this occurred faster than the normal MICP reaction. This reason also explained why the precipitation rate was higher under the same conditions. After that, most calcium carbonate precipitation was produced by a biological reaction. B. megaterium domesticated at low temperature grew better at low temperature, which allowed for more calcium carbonate precipitation.
In summary, the two methods, adding urea to the medium and domestication at low temperature, allowed greater precipitation, coping effectively with the problem of deficient calcium precipitation at low temperature. Moreover, this research laid the ground work for subsequent low-temperature sand solidification tests.
MICP test in PVC cylinders
The improvement of our method was demonstrated by an increase in production rates of calcium carbonate, but MICP technology needs to be utilized in a real engineering situation, which is why we performed sand solidification and crack repairing with MICP. Whiffin et al. (2007) suggested a two-phase injection method for bacterial retainment and this approach has been frequently used (Martinez et al. 2013; Sarmast et al. 2014). Cheng et al. (2014) compared the strength of samples improved with various densities in terms of calcite content. Our current study considered different environmental cases (adding urea to medium and domestication of B. megaterium at low temperature). Zhang et al. (2015) proposed, through mercury intrusion porosimetry analysis, that the pore size distribution in microbial mortar treated with Ca(CH3COO)2 was more uniform than when CaCl2 or Ca(NO3)2 was used, and Sun et al. (2018a) concluded that adding urea to the medium might result in poor uniform distribution of CaCO3 in sand columns due to a rapid chemical reaction; therefore, calcium acetate was utilized to alleviate this problem.
The sand-curing tests allowed the formation of a sand column by cementing loose sand grains due to the calcium carbonate produced. A chemical reaction took place immediately after the gelling solution was injected. The speed of the chemical reaction surpassed that of biological solidification reaction, which significantly accelerated the curing reaction and produced more calcium precipitation over the same time range. This explained why the UCS of the sand columns with added urea were greater than those with no added urea for the same curing time, as shown in Table 2. B. megaterium domesticated at a temperature of 15 °C had higher activity and stronger adaptability to incubation at 10 °C, which enabled them to grow and reproduce faster and produce more calcium precipitate during sand-curing tests at low temperature, resulting in a better sand-curing effect. When the two methods were used together for sand-curing tests, the sand column was dramatically improved, with the strength reaching 2.28 Mpa, as shown in Table 2. The results confirmed the validity of our improvements to the MICP method at low temperature.
In this study, different temperature and pH conditions were investigated to analyze some characteristics of B. megaterium, for instance, growth and enzyme activity. Production rates for calcium carbonate were also studied at different temperatures and then two methods were proposed to increase production rates for calcium carbonate and sand-curing effects. The conclusions are as follows:
-
(1)
The growth of B. megaterium was faster and urease activity was greater with increased temperature and low temperature significantly inhibits these factors. At various temperatures, B. megaterium grew the fastest and urease activity was greatest at a pH of 8.
-
(2)
Production rates for calcium carbonate increased with increasing temperature. Low production rates at low temperature were ascribed to both low enzyme activity and low bacterial cell numbers.
-
(3)
Adding urea to the medium for the duration of culture and domestication at low temperature can increase production rates for calcium carbonate, coping effectively with the problem of deficient calcium precipitation at low temperature and improving the effect of sand solidification. By combining the two methods, there was a significant enhancement of production rates for calcium carbonate and the curing effect of sand solidification was better.
-
(4)
Our study confirms the validity of our improvements to MICP at low temperature, laying a solid foundation for the practical engineering application of bio-cementation technology at low temperature.
References
Alexander M (1961) Introduction to soil microbiology. Wiley, New York, pp 472–428
Bachmeier KL, Williams AE, Warmington JR, Bang SS (2002) Urease activity in microbiologically-induced calcite precipitation. J Biotechnol 93(2):171–181
Braissant O, Cailleau G, Dupraz C, Verrecchia EP (2003) Bacterially induced mineralization of calcium carbonate in terrestrial environments: the role of exopolysaccharides and amino acids. J Sediment Res 73(3):485–490
Cheng J, Zhang M, Song W, Xia A, Zhou J, Cen K (2011) Cogeneration of hydrogen and methane from arthrospira maxima biomass with bacteria domestication and enzymatic hydrolysis. Int J Hydrog Energy 36(2):1474–1481
Cheng L, Shahin M, Cordruwisch R, Addis M, Hartanto T, Elms C (2014) Soil stabilisation by microbial-induced calcite precipitation (micp): investigation into some physical and environmental aspects. International Congress on Environmental Geotechnics
Cheng J, Lin R, Song W, Xia A, Zhou J, Cen K (2015) Enhancement of fermentative hydrogen production from hydrolyzed water hyacinth with activated carbon detoxification and bacteria domestication. Int J Hydrog Energy 40(6):2545–2551
Chin WT, Kroontje W (1962) Conductivity method for estimation of enzyme activity. Agric Food Chem 10:347–348
Cuthbert MO, Mcmillan LA, Handley-Sidhu S, Riley MS, Tobler DJ, Phoenix VR (2013) A field and modeling study of fractured rock permeability reduction using microbially induced calcite precipitation. Environ Sci Technol 47(23):13637–13643
De Muynck W, Verbeken K, De Belie N, Verstraete W (2013) Influence of temperature on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl Microbiol Biotechnol 97(3):1335–1347
DeJong JT, Fritzges MB, Nüsslein K (2006) Microbially induced cementation to control sand response to undrained shear. J Geotech Geoenviron Eng 132(11):1381–1392
Erşan YÇ, Gruyaert E, Louis G, Lors C, De Belie N, Boon N (2015) Self-protected nitrate reducing culture for intrinsic repair of concrete cracks. Front Microbiol 6:1228
Ferris FG, Phoenix V, Fujita Y, Smith RW (2004) Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20 C in artificial groundwater. Geochim Cosmochim Acta 68(8):1701–1710
Fredrickson J K, Fletcher M, Frederickson J K, Fletcher M (2001) Subsurface microbiology and biogeochemistry. John Wiley & Sons
Hattori T (1973) Microbial life in the soil: An introduction, vi. Dekker, New York, p 427
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7(2):139–153
Jiang NJ, Yoshioka H, Yamamoto K, Soga K (2016) Ureolytic activities of a urease-producing bacterium and purified urease enzyme in the anoxic condition: implication for subseafloor sand production control by microbially induced carbonate precipitation (micp). Ecol Eng 90:96–104
Khan MNH, Amarakoon GGNN, Shimazaki S, Kawasaki S (2015) Coral sand solidification test based on microbially induced carbonate precipitation using ureolytic bacteria. Mater Trans 56(10):1725–1732
Lauchnor EG, Topp DM, Parker AE, Gerlach R (2015) Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J Appl Microbiol 118(6):1321–1332
Li W, Liu L, Chen W, Yu L, Li W, Yu H (2010) Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Process Biochem 45(6):1017–1021
Lian B, Hu Q, Chen J, Ji J, Teng HH (2006) Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim Cosmochim Acta 70(22):5522–5535
Lo YC, Chen WM, Hung CH, Chen SD, Chang JS (2008) Dark h2 fermentation from sucrose and xylose using h2-producing indigenous bacteria: feasibility and kinetic studies. Water Res 42(4-5):827–0-842
Martinez B, DeJong J, Ginn T, Montoya BM, Barkouki TH, Hunt C, Tanyu B, Major D (2013) Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J Geotech Geoenviron Eng 139(4):587–598
Mitchell AC, Phillips AJ, Kaszuba JP, Hollis WK, Cunningham ALB, Gerlach R (2008) Microbially enhanced carbonate mineralization and the geologic containment of co2. Geochim Cosmochim Acta 72(12):A636–A636
Montoya B, DeJong J, Boulanger R (2013) Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. Géotechnique 63(4):302–312
Mostafa S, Aydin B (2019) Microbially induced calcium carbonate precipitation: a widespread phenomenon in the biological world. Appl Microbiol Biotechnol 3
Mostafa S, Khajeh SA, Shaun H, Aydin B (2017) The effect of cell immobilization by calcium alginate on bacterially induced calcium carbonate precipitation. Fermentation 3(4):57
Muynck WD, Cox K, Belie ND, Verstraete W (2008) Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build Mater 22(5):875–885
Paassen LAV (2009) Biogrout ground improvement by microbially induced carbonate precipitation. In: PhD thesis. Delft University of Technology, Netherlands
Palin D, Wiktor V, Jonkers HM (2017) A bacteria-based self-healing cementitious composite for application in low-temperature marine environments. Biomimetics 2(3):13
Rodriguez-Navarro C, Rodriguez-Gallego M, Ben Chekroun K, Gonzalez-Munoz MT (2003) Conservation of ornamental stone by myxococcus xanthus-induced carbonate biomineralization. Appl Environ Microbiol 69(4):2182–2193
Sarmast M, Farpoor MH, Sarcheshmehpoor M, Eghbal MK (2014) Micromorphological and biocalcification effects of sporosarcina pasteurii and sporosarcina ureae in sandy soil columns. J Agric Sci Technol 16(3):681–693
Seifan M, Samani AK, Berenjian A (2017) New insights into the role of ph and aeration in the bacterial production of calcium carbonate (caco3). Appl Microbiol Biotechnol 101(8):3131–3142
Seifan M, Ebrahiminezhad A, Ghasemi Y, Samani AK, Berenjian A (2018) The role of magnetic iron oxide nanoparticles in the bacterially induced calcium carbonate precipitation. Appl Microbiol Biotechnol 102:3595–3606
Soon NW, Lee LM, Khun TC, Ling HS (2014) Factors affecting improvement in engineering properties of residual soil through microbial-induced calcite precipitation. J Geotech Geoenviron Eng 140(5):04014006
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of caco3. Soil Biol Biochem 31(11):1563–1571
Sun XH, Miao LC, Tong TZ, WANG C C (2018a) Improvement of microbial-induced calcium carbonate precipitation technology for sand solidification. J Mater Civ Eng 30(11):04018301
Sun XH, Miao LC, Tong TZ, WANG C C (2018b) Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech:1–12
Tittelboom KV, Belie ND, Muynck WD, Verstraete W (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40(1):157–166
Tziviloglou E, Wiktor V, Jonkers HM, Schlangen E (2016) Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr Build Mater 122:118–125
Vos P, Garrity G, Jones D, Krieg N R, Ludwig W, Rainey F A, Schleifer K, Whitman W B (2011) Bergey’s manual of systematic bacteriology: volume 3: the firmicutes (Vol. 3). Springer Science and Business Media
Whiffin V S (2004) Microbial CaCO3 precipitation for the production of biocement. Perth: Murdoch University.
Whiffin VS, Paassen LAV, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24(5):417–423
Wijngaarden W, Vermolen F, Meurs G, Vuik C (2011) Modelling biogrout: a new ground improvement method based on microbial-induced carbonate precipitation. Transp Porous Media 87(2):397–420
Wu Y, Ajo-Franklin JB, Spycher N, Hubbard SS, Smith R (2011) Geophysical monitoring and reactive transport modeling of ureolytically-driven calcium carbonate precipitation. Geochem Trans 12(1):7
Zhang Y, Guo HX, Cheng XH (2015) Role of calcium sources in the strength and microstructure of microbial mortar. Constr Build Mater 77:160–167
Acknowledgments
The authors thank the valuable comments from the reviewers.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 51578147), and Scientific Research Foundation of Graduate School of Southeast University (grant number YBJJ1846). This work was also Supported by “the Fundamental Research Funds for the Central Universities” and “Postgraduate Research & Practice Innovation Program of Jiangsu Province” (grant number KYCX18_0107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, X., Miao, L., Wu, L. et al. Improvement of bio-cementation at low temperature based on Bacillus megaterium. Appl Microbiol Biotechnol 103, 7191–7202 (2019). https://doi.org/10.1007/s00253-019-09986-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09986-7