Abstract
The replacement of synthetic colors in food products by natural alternatives has been boosted by consumers willing to pay more for healthier products. However, the success of microbial colorants depends not only on its acceptability on the market but also its production costs. Talaromyces species can produce water-soluble red colorants induced by glucose and monosodium glutamate (MSG). In this study, the influence of several conditions was evaluated to produce natural red colorants by submerged culture of Talaromyces amestolkiae. Under optimal conditions (g/L: glucose 10, MSG 25, MgSO4 0.012, FeSO4 0.01, CaCl2 0.015; and initial pH of 5.0), a 30-fold increase in the production was achieved, reaching a red colorant production of 13.44 UA500nm. Depending on the initial pH, colorants with different hues and chroma values were obtained. Deep yellow colorants were derived from neutral and basic pH, while deep red colors were derived from acidic pH. The fluorescence spectrum of culture broth obtained before and after complexation with salts presented red colorants with yellow fluorescence spectra. The information generated in this study would be useful for the formulation of industrial media for large-scale cultivation of T. amestolkiae, which have the potential to produce Talaromyces fermented colorants for use in health foods and pharmaceutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most industrial products are colorful. Considering food and beverages, color is one of the most important product-intrinsic sensory cues of our expectations of taste and flavor (Vinha et al. 2018). The market for industrial colorants is primarily occupied by synthetic/artificial ones. However, there is a need for suitable coloring agents from natural sources because of serious safety problems with several artificial synthetic colorants (Lehto et al. 2017). Therefore, natural colorants have gained importance because some of them present pharmacological properties, such as antimicrobial activity against Escherichia coli (Kim et al. 2006) and Staphylococcus aureus (Zaccarim et al. 2018). The replacement of artificial colorants by their natural counterparts is a major challenge for the food, pharmaceutical, and cosmetics industries, both from a technical perspective and because of ingredient costs (Galaffu et al. 2015).
Currently, more than 50 patents concerning the use of microbial colorants for food have been issued in Japan, USA, France, and Germany. Nowadays, some fermentative food grade colorants are on the market: Monascus colorants, astaxanthin from Xanthophyllomyces dendrorhous, Arpink Red (or Natural Red) from Penicillium oxalicum, riboflavin from Ashbya gossypii, and β-carotene or lycopene from Blakeslea trispora (Dufossé 2017). However, new natural colorant producers are of great interest. In this sense, filamentous fungi as colorant producers are gaining ground in industrial production of biotechnological products because of the metabolic versatility of this group of microorganisms (Torres et al. 2016). The production of colorants from fungi is associated with high growth rates, high yields, and a large range of potential colorants. Efforts have been made and are still underway to reduce the production costs of colorants produced by microbial fermentation, since synthetic ones or those extracted from natural plant sources generally have lower production costs (Dufossé 2018).
The microbial production of colorants is dependent on several factors, such as the type of fermentation (submerged or state solid fermentation), the microorganism species, pH, carbon, nitrogen, and other nutrient sources, broth viscosity, dissolved oxygen, stirring frequency, and flow aeration (Orozco and Kilikian 2008; Vendruscolo et al. 2016; Terán-Hilares et al. 2018). An important feature about natural colorants is the range of new colors it adds to the available palette (Mapari et al. 2006). Natural colorants constitute a group of chemically heterogeneous and biosynthetically unrelated molecules that are grouped by a common feature: Their electronic structure contains a chromophore that is responsible for their characteristic colors (Torres et al. 2016). Among these colors, the red hues are of particular interest because red is the most popular food color, and true red natural colorants suitable for food use are difficult to obtain (Chen and Johns 1993; Ventura et al. 2013). Some red colorants have potential to replace nitrate and nitrite salts for color enhancement of meat and poultry products (Chen et al. 2017).
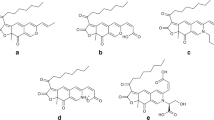
Among the filamentous fungi, species of Talaromyces generally produce yellow, orange, and red colorants in the mycelium or as diffusing colorants. Talaromyces amestolkiae (formerly Penicillium purpurogenum), evaluated in the present work, does not produce mycotoxins and presents absence of toxicity against Artemia salina (Santos-Ebinuma et al. 2013). In a former study, we used sequence data of the ITS, calmodulin, and β-tubulin gene regions to propose a sectional classification for Penicillium purpurogenum on the basis of molecular taxonomy and reclassified the fungus as Talaromyces (Zaccarim et al. 2018). Therefore, the strain DPUA1275 previously named Penicillium purpurogenum is now named Talaromyces amestolkiae. Moreover, the fermented broth containing the colorants produced by this microorganism present low cytotoxicity against fibroblasts cells and effective antimicrobial activity against S. aureus (Zaccarim et al. 2018). The colorants are azaphilone polyketide colorants similar to Monascus ones (Yilmaz et al. 2012; Venkatachalam et al. 2018). Polyketides are secondary metabolites formed by the condensation of an acetyl unit with malonyl units, with simultaneous decarboxylation (Mapari et al. 2010). From this process is formed a polyketide chromophore by polyketide synthase (Hajjaj et al. 2000). Some examples of polyketides colorants are anthraquinones, hydroxyanthraquinones, naphthoquinones, and azaphilone structures (Mapari et al. 2010). Azaphilone polyketide biosynthesis starts with a medium-chain fatty acid derived from the fatty acid biosynthetic pathway, which is then bound to the chromophore by trans-esterification generating the orange colorant. A reduction reaction turns the orange into the yellow colorant, while the red colorant is generated by amination of orange colorant with NH3 units (Hajjaj et al. 2000; Chen et al. 2017). Figure 1 shows the polyketide chromophore and some colorants produced by filamentous fungi reported in the literature.
Polyketide chromophore and chemical structure of yellow (Monascin and Ankaflavin produced by Monascus), orange (Rubropunctain and Monascorubrin produced by Monascus), and red colorants (Rubropunctamine, Monascorubramine) produced by Monascus and 6-[(Z)-2-carboxyvinyl]-NGABA-PP-V produced by Talaromyces. Chemical structures draw using ChemDraw professional adapted from Hajjaj et al. (2000), Chen et al. (2017), and Venkatachalam et al. (2018)
In this work, aiming to improve the knowledge about the colorant production by T. amestolkiae, a chemically defined medium for submerged fermentation with monosodium glutamate (MSG) as nitrogen source was investigated by submerged culture. The colorants produced were examined based on their CIELAB color characteristics, and 3D-fluorescence studies were also performed, which provided evidence of three fluorescent chromophores. These findings show that some characteristics of natural red colorants produced by T. amestolkiae could be used in food and pharmaceutical products.
Materials and methods
Chemicals
Glucose and MSG were purchased from LS Chemicals (Brazil) and Dinamica (Brazil), respectively. Potato dextrose agar and yeast extract were obtained from Acumedia (USA). All of the other reagents were of analytical grade and used as received. The PDA medium had the following composition (g/L in deionized water): agar potato dextrose (39.0) and yeast extract (5.0).
Microorganism and inoculum preparation
Talaromyces amestolkiae DPUA 1275 was generously provided by the Culture Collection of Federal University of Amazonas, DPUA, AM, Brazil. The microorganism preserved in distilled water was reactivated in potato dextrose agar supplemented with yeast extract (PDA) and maintained at 30 °C for 7 days. The PDA medium had the following composition (g/L in deionized water): agar potato dextrose (39.0) and yeast extract (5.0). Afterward, the cultures were kept in the refrigerator at 4 °C and defined as a stock culture for the whole work. For inoculum preparation, a loop of fungus from stock culture was inoculated on a PDA plate and maintained in the same reactivation conditions.
Colorant production by submerged culture
Initially, the production of natural colorants was studied varying the nitrogen source. For this study, five mycelial agar discs (8 mm diameter) of T. amestolkiae were punched out from the inoculum with a self-designed cutter and transferred to 50 mL of submerged culture medium in 250-mL Erlenmeyer flasks incubated at 30 °C and 150 rpm for 168 h. Ammonium sulfate, ammonium nitrate, sodium nitrate, and monosodium glutamate (MSG) were tested as nitrogen sources. The results achieved were compared with the best condition from previous work of our research group (Zaccarim et al. 2018) that used complex nitrogen sources, such as meat extract and meat peptone. Growth kinetics of colorants formed by T. amestolkiae grown in the best condition attained was monitored every 24 h in triplicate by 360 h. All media had the pH adjusted to 7.0 after sterilization at 121 °C for 15 min.
Then, a statistical design tool was used to improve the colorant production. The first two statistical designs were conducted using five mycelial agar discs per 50 mL of culture medium in 250-mL Erlenmeyer flasks. All of the assays were carried out in an orbital shaker incubator at 30 °C and 150 rpm for 168 h.
First, experiments were carried out employing a 23 full factorial design (8 experiments plus 3 central points) to investigate the effects of glucose, MSG, and pH on variable response red colorant production. From this factorial design, glucose was kept constant at 10.0 g/L, and the effects of pH and MSG on red colorant production were evaluated by a 22 central composite design. In this factorial design, a set of 12 experiments containing a central composite factorial matrix with center points and star points was performed. Table 1 shows the variables and factor levels for both experimental designs.
For the statistical analysis, the actual values of each independent variable (Xi) were coded to provide coded levels according to the Eq. (1):
where xi represents the corresponding coded values, Xo the actual values at the central point, and Xi the step change value.
Statistica software Version 7.0 (StatSoft, Tulsa, OK, USA) was used for the regression analysis of the data obtained and to estimate the coefficients of the regression equation. The quality of the fit of the polynomial model was expressed by the determination coefficient, R2, and its statistical significance was validated by an F-test at a significance level of (p) ≤ 0.05; the significance of the regression coefficients was tested by t tests.
Subsequently, a study to analyze the inoculum size was performed by varying the number of agar mycelial discs (5, 10, and 15) per 50 mL of culture medium (10 g/L glucose, 25 g/L MSG, and pH 5.0). Afterward, a Plackett–Burman design for 7 variables was used for macro- and micronutrient screening (Plackett and Burman 1946). In this set of experiments, the same medium aforementioned was employed by addition of 15 agar mycelial discs. Salt concentrations were combined based on the literature (Weinberg 1989; Lin and Demain 1991; Lee et al. 2001). Each variable was tested at two levels: high (+) and low (−) plus 3 central points. The range of variable levels for this experimental design is also depicted in Table 1. An additional experiment was performed varying the MgSO4 concentration (6, 12, and 24 mg/L), while the concentrations of FeSO4 and CaCl2 were kept at 10 and 15 mg/L. The other salts were not included in the culture medium of this study.
Analytical methods
The pH was measured using a pH meter (pH/Conductometer—Metrohm). The biomass concentration was calculated by dry weight. For the analysis of glucose consumption, aliquots of 20 μL of fermented broth filtrated through 0.45-μm filter (Millipore) were injected into the HPLC (Shimadzu - LC 20AD) using an Aminex HPX-87H column (300 × 7.8 mm) and eluted in a mobile phase of 0.005 mol/L H2SO4 in the following chromatographic conditions: 60 °C injection temperature and flow rate of 0.6 mL/min in an HPLC system equipped with a RID detector (Shimadzu model RID - 20A). Each analysis was performed in triplicate.
The concentration of extracellular colorants was estimated by spectrophotometric analysis by reading the absorbance of supernatant at 410, 470, and 500 nm after a spectrum scan of the fermented broth (Fig. S1 from Supplementary material), which corresponds to the maximum absorbance for yellow, orange, and red colorants, respectively. The measurement was performed using an EnSpire Alpha Plate Reader spectrophotometer (PerkinElmer®) and taking the dilution factor of each sample into consideration. The results were expressed in Units of Absorbance (UA). Although the main variable response was red colorant production, the production of yellow and orange colorants was also calculated. The results for yellow and orange colorants were expressed in terms of red/yellow colorant rate (R/Y) and red/orange colorant rate (R/O) according to Eqs. (2) and (3), respectively.
where AbsRed, AbsYellow, and AbsOrange represent, respectively, the red, yellow, and orange colorant absorbance.
Colorimetric analysis
The fermented broth containing the colorants was analyzed in terms of the quantitative description of the color. The L*, a*, and b* color coordinates were measured by a chromameter with the CIELAB color system (Hunterlab ColorQuest XE). These values were then used to calculate chroma (C*) and hue angle (hab) values according to Eqs. (4) and (5), respectively. L* indicates lightness from 0 (black) to 100 (white). Positives and negatives in a* represent red and green, respectively, whereas positives and negatives in b* represent yellow and blue, respectively. Chroma values denote the saturation or purity of the color. Values close to the center at the same L* value indicate dull or gray colors, whereas values near the circumference represent vivid or bright colors. Hue angle values represent 0 for redness, 90 for yellowness, 180 for greenness, and 270 for blueness.
Three-dimensional fluorescence analysis
To determine if the colorants present in the fermented broth emit fluorescence, a full 3D fluorescence spectrum was acquired at 25 °C with a variable wavelength range of excitation (λex) from 220 to 600 nm with a step width of 2 nm and emission wavelengths (λem) of 370–650 nm with a step width of 1 nm using the RF-6000 SHIMADZU spectrofluorophotometer. The 3D fluorescence spectroscopy patterns in the λex or λem maxima for each fluorophore found were assigned to a specific colorant based on comparison with standards found in the literature. Some excitation/emission wavelength patterns found for fermented broth could not be assigned to one specific colorant, as their fluorescence patterns had multiple possible identities.
Results
In the previous work of our research group, meat peptone and meat extract were found to be efficient nitrogen sources for microorganism growth and red colorant production by T. amestolkiae achieving 4.61 AU490nm of red natural colorants using glucose as carbon source in shake flask cultures (Zaccarim et al. 2018). However, complex nitrogen sources lean to major production of cell-bound colorant with low excretion rates (Lin and Demain 1991). Considering evidence found in the literature that cultures with glucose and other nitrogen sources, including MSG, produce water-soluble red colorant (Lin and Demain 1994), experiments were carried out employing MSG, ammonium, and nitrate sources as nitrogen source and glucose as carbon source. The effects of nitrogen source on microorganism growth, colorant production, and culture pH profiles are presented in Table 2.
All nitrogen sources studied promoted poor microorganism growth compared to biomass concentration obtained using meat peptone and meat extract. Ammonium sources limited both growth and colorant production. Sodium nitrate limited growth and promoted low colorants yield. MSG promoted poor microorganism growth associated with low colorant production, around 0.39 AU500nm. The association of low microorganism growth and colorants formation is quite interesting, considering that the main objective is to achieve high production of extracellular colorants. So, afterward, the red colorant production was evaluated over 360 h using MSG as nitrogen source. The results, depicted in Fig. S2 from Supplementary material, show that red colorant production reached a maximum value at 336 h (2.12 AU500nm) of cultivation and low rates of glucose consumption and biomass formation. Then, further studies were conducted analyzing lower glucose concentrations, different values of pH, and higher amounts of MSG through experimental design.
Factorial design experiments
To study the production of natural colorants by T. amestolkiae, two factorial designs were carried out as follows: (i) a 23 factorial design evaluating the independent variables glucose and MSG concentration and initial pH and (ii) a 22 central composite design evaluating the independent variables MSG concentration and initial pH. The results from the 23 factorial design, namely, red colorant production, final pH, biomass concentration, glucose consumption, and relation red/yellow (R/Y) and red/orange (R/O) are depicted in Table S1 in the Supplementary material. The general shape of pH profile was affected by changes in the initial glucose concentration. In the absence of glucose, the initial pH 5.0 tended to increase after 168 h of cultivation (Runs 1 and 5, Table S1 from Supplementary material). In the presence of glucose, there was a decrease in pH from 7.0 to 5.5 (Run 9(C), Table S1 from Supplementary material). As occurred in the aforementioned experiments, the microorganism growth was low (< 1.0 g/L). The relations between R/Y and R/O showed that orange and yellow colorants were the main components of almost all the conditions. The Pareto chart for this experimental design is presented at Fig. 2a. A standardized Pareto chart consists of bars with a length proportional to the absolute value of the estimated effects divided by the standard error. The bars are displayed in order of the size of the effects, with the largest effects on top. The chart includes a vertical line at the critical t-value for an α of 0.05 (Stowe and Mayer 1966). Effects for which the bars are smaller than the critical t-value are considered not significant and to not affect the response variables. For red colorant production, the pH had a confidence level above 95%. Hence, pH was considered the only significant variable for red colorant production, and the effect was negative, which means that lower levels of pH can promote the highest red colorant production. The fit of the regression was checked by the coefficient of determination, R2, which was 0.981, indicating that only 1.9% of the total variation could not be explained by the model. It was reasonable to use a regression model to analyze the trend in the response.
Pareto Chart of the 23 factorial design (a), 22 factorial design (b), and Plackett-Burman design (c) for studying the red colorant production by T. amestolkiae in orbital shaker. The line in the chart represents a reference line; any factor that extends past this line is of significant effect at p value < 0.05
The optimal initial pH for mycelial growth was 5.0, while the highest red colorant production reached 1.67 AU500nm (Run 7, Table S1 from Supplementary material) under maximum concentrations of glucose and MSG. Although glucose consumption was lower than 30%, it was decided to maintain the highest level of glucose (10.0 g/L) and study pH values lower than 5.0 by increasing the MSG concentration and employing a 22 central composite design. Figure 2 b presents the Pareto chart for this experimental design showing the estimated effects of the variables and their interactions on the responses in decreasing order of magnitude. The most relevant independent variables were those related to pH at both linear and quadratic levels. For linear pH, the factorial design showed that the highest level was better for obtaining maximum production. The results for red colorant production, final pH, biomass concentration, glucose consumption, and relations between red/yellow (R/Y) and red/orange (R/O) for the 22 central composite design are depicted in Table S2 from Supplementary material.
According to the results, extreme acidic pH conditions (pH values of 2.59 and 3) did not promote red colorant production. It was found that acidic values of pH promoted an increase in red colorant production until a limit (pH 4.0), for which there was a drastic reduction in production. The optimal pH recorded for mycelial growth and red colorant was 5.0. At pH 2.59, yellow colorant with maximum absorption at 410 nm was slightly detected (R/Y = 0.67). Higher amount of MSG (> 20 g/L) increased red colorant production to 2.11 AU500nm. Although red colorant production was more pronounced under the conditions of 22 factorial design, the glucose consumption remained lower than 30%, meaning that at least 7 g/L remained in the fermented broth. Since glucose supports microbial growth under these experimental conditions, biomass accumulation was very low reaching less than 2 g/L. So, the metabolic pathway of microorganism is mainly headed to produce natural colorants and other biocompounds than to growth. Moreover, the colorants produced in this set of experiments was extracellular which is quite interesting considering that under the conditions studied by Zaccarim et al. (2018) and other authors, the most part of colorants produced was intracellular.
Effects of inoculum size and micronutrients
Based on the results of factorial design experiments, the effect of inoculum size was evaluated on red colorant production with other parameters controlled (initial pH of 5.0, 25 g/L of MSG and 10 g/L of glucose). Table 3 shows the results of red colorant production varying inoculum size (5, 10, and 15 mycelial agar discs). The red colorant production and glucose consumption were almost the same either using 5 or 10 mycelial discs. However, 15 mycelial agar discs promoted an increase in the red colorant production around 39% and a glucose consumption higher than 50%. Moreover, the red/yellow and red/orange colorant rates were higher than 1 which means that red colorant was more produced than yellow and orange ones. As the red colorant is produced from the amination of orange colorant (Chen et al. 2017), more cells increased the production of orange colorant that was converted in red ones in the presence of MSG. From these results, it is clear that T. amestolkiae growth was intensively affected by the number of agar discs inoculated along with the increase in glucose consumption.
Afterward, it was evaluated the effect of seven different mineral supplements on microorganism growth and red colorant production. For this purpose, a Plackett-Burman design (PBD) for 8 trials with two levels of concentrations was performed using mineral elements (Zn2+, Mn2+, Mg2+, Fe2+, K+, Cu+, and Ca+). The results for red colorant production, final pH, biomass concentration, glucose consumption, and relation between red/yellow (R/Y) and red/orange (R/O) are shown in Table S3 from Supplementary material, while the Pareto chart is depicted in Fig. 2c. PBD is a powerful and useful tool for rapidly searching key factors from a multivariable system. PBD does not determine the exact quantity, but it can provide some valuable information about each factor with relatively few experiments (Plackett and Burman 1946). According to the experimental design, any independent variable was significant at the 95% level of confidence. However, the elements Mg2+, Fe2+, and Ca+ were beneficial for red colorant since its production increased 1.57-fold under 6, 10, and 15 mg/L of Mg2+, Fe2+, and Ca+, respectively. It is known that these elements are required in microorganism metabolism to produce colorants. Among these three metals, Mg2+ showed the strongest stimulatory effect on red colorant production. Therefore, an additional assay was performed varying Mg2+ concentration in the culture medium. The results are depicted at Table 4.
The red colorant production was more intensive and reached a maximum (13.44 AU500nm) at 12 mg/L of Mg2+ along with increase in glucose consumption. The addition of magnesium over 12 mg/L reduced the glucose consumption and red colorant production, which may be partly due to the strong negative regulating effect of high MgSO4 concentration on the action of the colorant synthases.
Figure 3 summarizes the improvement of red colorant production according to all experiments performed. An increment in red colorant production of, approximately, 30-fold was achieved, which shows that working with nutrients is an important tool to increase the production of biological products.
Color and three-dimensional fluorescence analysis
Color analysis of fermented broth containing the red colorant was performed using a colorimeter. There have been a couple of reports describing a color based on stimuli generated in the human eye by visible light of various wavelengths and intensities (Mapari et al. 2006). The so-called Commission International de l’Eclairage (CIE) system is based on the fact that light reflected from any colored surface can be visually matched by an additive mixture of the three primary colors: red, green, and blue (Zollinger 2003). The color characteristics of the samples largely depended on the color and the concentration of the dominant colorant in the mixture. For most conditions, the values of L*, a*, and b* were all positive, indicating yellowness and redness (Table S4 from Supplementary material). The lightness values were 13–87, and the hue angles ranged from 26 to 87, corresponding to a color ranging from deep red to deep yellow because of a scale of 0 for redness, 90 for yellowness, 180 for greenness, and 270 for blueness. Chroma value refers to how pure or “saturated” a color is, as high chroma is more vivid than low chroma. All chroma values varied in a range of black to gray colors. On the basis of hue angle and chroma values, the colorants derived from statistical improvement had various shades of orange and red.
Figure 4 depicts the qualitative color change in CIELAB color space of the best condition from each step of red colorant improvement. The hue angles of the deep yellow colorants were approximately 70. This range of colors was observed for the 23 factorial design employed to study neutral and basic pH. Red colorants exist in various shades of red color, and acidic pH tends to produce shades of red that varied from dull red to earth red, corresponding to vivid red colors. From the 22 central composite design, shades of red that varied from red-orange to middle orange corresponding to hue angles of approximately 40 to 50 were produced. Scarlet red was the red shade observed after we added 15 mycelial agar discs. With addition of salts, it was possible to reach lower hue angles and high chroma values that correspond to deep red colors.
Three-dimensional fluorescence (3D) studies provided additional evidence for structural changes in T. amestolkiae colorants after complexation with salts provided in the culture medium. This analysis lends useful insights into the characteristic conformational change of fluorophores. Figure 5 shows the representative 3D fluorescence spectra of fermented broth in the absence and presence of MgSO4, FeSO4, KCl, CuSO4, and CaCl2. Wavelengths of maximum absorption (≈ excitation) and emission are the typical terms used to refer to a given fluorophore, but the whole spectrum may be important to consider. The excitation wavelength spectrum may be a very narrow or a broad band, or it may be all beyond a cutoff level (Ismael et al. 2013).
Fluorescence excitation and emission 3D spectra of fermented broth in the absence (a) and presence of MgSO4, FeSO4, KCl, CuSO4, and CaCl2 (b). Three distinct fluorescence regions were identified as (a) I (λex 258 nm, λem 434 nm, intensity = 313,920), II (λex 316 nm, λem 432 nm, I = 150,000), and III (λex 476 nm, λem 520 nm, intensity = 133,400); and (b) I (λex 258 nm, λem 434 nm, I = 94,800), II (λex 314 nm, λem 426 nm, I = 54,600), and III (λex 480 nm, λem 520 nm, I = 258,360)
The fluorescence spectra of culture broths obtained before and after complexation with salts presented three distinct areas of fluorescence in the 3D spectrum. The three distinct areas presented a clear difference between the spectra obtained without salts and in the presence of them (Fig. 5a, b, respectively). Region I was the most intense peak at λex 258 nm and λem 434 nm before addition of salts (I = 313,920) and remained identical at the maximum wavelengths after complexation with them, but lost intensity (I = 94,800) (Fig. 5, regions I). The second region with lower intensity, λex 316 nm, λem 432 nm, and I = 150,000 (Fig. 5, regions II), not only lost intensity after addition of salts but also suffered a shift in the maximum wavelength peak. A slight shift in the peaks was also observed for the third region, but with an interesting increase in intensity of almost 2-fold.
Discussion
The results indicated that red colorant production can be orientated according to the nitrogen source employed. At low MSG concentration, the red colorant production by T. amestolkiae was low. However, improving the amount of MSG improved red colorant production, but the microorganism growth was low. The most interesting phenomenon was the poor biomass accumulation with MSG as nitrogen source. Low biomass yields are probably associated with the biosynthetic pathway of T. amestolkiae colorants. MSG seems to contribute to the high yield of colorants by suppressing central carbon metabolism and leading to carbon redirected to the colorant biosynthetic pathway. Moreover, the major part of the colorant produced in the medium with MSG was extracellular. Although the completely biochemical reactions and enzymes involved in azaphilone colorants formation are unknown, these lipophilic colorants appear to be polyketide-derived compounds (Jůzlová et al. 1996). In this sense, there is evidence that at least one and possibly two polyketide synthases must be involved in their biosynthesis, and fatty acids are a possible precursor of the azaphilone compounds (Hajjaj et al. 2000; Huang et al. 2017). The orange colorant will be formed by the esterification of a β-ketoacid (from the fatty acid pathway) to the polyketide chromophore (from polyketide pathway). The yellow colorant will be produced by a reduction of orange one while the red by an amination reaction (Chen et al. 2017). Therefore, the nitrogen source or the presence of amino acids will be mainly important in the production of red colorants. There are reports showing that a well-defined chemical medium with amino acids as nitrogen source promotes production and excretion of Monascus extracellular colorants (Lin and Demain 1994). Amino acids are side-chain precursors for soluble red colorants leading to formation of a glutamic acid–colorant complex by insertion of MSG into the chromophore, replacing the pyronoid oxygen. That arises from their affinity for the 4H-pyran nucleus to undergo substitution with primary amines to form the corresponding vinylogous γ-pyridones (Lin and Demain 1991; Hajjaj et al. 2000; Wei and Yao 2005). From the results achieved in this study, it seems that the microorganism metabolism had shifted the carbon metabolism from growth to production of secondary metabolites. This is facilitated once the insertion of MSG into the chromophore made the red colorant soluble in water and then excreted. These results support suggestions that solubilization of colorants and excretion to the medium enhances red colorant synthesis (Evans and Wang 1984).
Although some articles highlighted that ammonia assimilation is important for colorant production by filamentous fungi, including Penicillium and Monascus species (Arai et al. 2013; Shi et al. 2015), this nitrogen source had a negative effect on colorant production by T. amestolkiae. One possible reason is that ammonia is toxic for this strain. On the other hand, MSG promoted increased water-soluble red colorant production associated with poor microorganism growth. Considering that the colorant industry requires elevated concentration of natural colorants and the process cost is still a limitation for industrial production, it was established 27.05 g/L as the limit for MSG concentration in the culture media. A similar comprehension was used to perform the factorial design experiments until 168 h. Although the maximum colorant production was achieved at 336 h, the glucose consumption was only 3% higher than at 168 h, which shows that an improvement in other parameters could increase the glucose consumption and the red colorant production. Moreover, 336 h is a long period for producing a bioproduct industrially and has a high price considering energy consumption.
Considerable research has been done on colorant production in complex media (Arai et al. 2013; Kang et al. 2014; Terán-Hilares et al. 2018). However, complex media is known to produce cell-bound colorants. This study is presented the production of a glutamic acid-red colorant complex by T. amestolkiae in a chemically defined medium with glutamate as nitrogen source. The red colorant complex seems to be more water-soluble than those produced in complex media. Therefore, the production process developed here avoids the costly downstream process of colorant extraction needed for mycelium-associated compounds.
The factorial designs employed were able to improve the red colorant production and had shown which variable was more important in this process. The general shape of pH profiles was affected by changes in the initial glucose concentration. In the presence of glucose, there was a tendency of pH reduction when initial pH was settled at 7.0. This pattern was also observed for cultures with organic nitrogen sources using glucose as carbon source (Zaccarim et al. 2018). According to the results, acidic conditions promoted higher colorant production. Carels and Shepherd (1977) reported that reducing pH inhibits the formation of conidia and increases colorant production, suggesting that pH of the medium might affect the transport of certain media constituents, such as glucose and nitrogen sources, and could be a factor in low growth levels as well.
The growth profile indicates that there was no positive relationship between biomass and glucose since MSG can be used as both carbon and nitrogen source. However, the presence of glucose is quite important since in the conditions without this compound, there were no colorant production (Run 1, 2, 5, and 6 of 23 factorial design depicted at Table S1 from Supplementary material). One distinctive characteristic of secondary metabolism is its association with low growth levels. Therefore, secondary metabolism is often brought on by exhaustion of a nutrient or addition of an inducer and/or by a decrease in growth rate (Bibb 2005). These events generate signals that cause a cascade of regulatory events resulting in chemical (secondary metabolism) and morphological differentiation (morphogenesis) of the microbial secondary metabolite producers (Krairak et al. 2000). It seems that in T. amestolkiae, the activity of carbohydrate catabolic genes or operons is regulated in the hierarchy of carbohydrate utilization for colorant production metabolism. This finding suggests that T. amestolkiae colorants could serve as a carbon sink, since a low growth ratio was observed. This result is quite interesting since in a batch fermentation process, the mycelium morphology of filamentous fungi often induces high viscosity that can result in many problems to the process (poor mixing, insufficient oxygen mass transfer, high power requirement, and formation of nutrient concentration zones), affecting directly the productivity and excretion of different microbial metabolites (Blanch and Bhavaraju 1976). In this sense, many efforts have been made to alter fungal morphology in order to reduce broth viscosity (Lv et al. 2017). Therefore, a culture media that promotes bioproduct production at low microorganism growth is an advantage.
Although glucose consumption was not satisfactory in all conditions evaluated, it was maintained in the highest level (10 g/L) in the experiments that studied the presence of micronutrients. The osmotic stress caused by high levels of glucose along with MSG may act as a trigger for colorants metabolic pathway. Hajjaj et al. (1997) supported this finding by showing that Monascus can produce high levels of extracellular red colorant when grown in a chemically defined culture medium with excess glucose and MSG.
T. amestolkiae growth and glucose consumption were intensively affected by the number of agar mycelial discs inoculated. However, the same strain cultivated in different conditions presented the opposite pattern. Santos-Ebinuma et al. (2013) inoculated 5, 10, and 15 agar mycelial discs of T. amestolkiae (formerly known as P. purpurogenum DPUA 1275) on a Czapek Yeast Extract (CYA) medium at a pH of 6.5. The CYA medium was composed of complex nitrogen source such as yeast extract and sucrose as carbon source. These authors observed that a higher number of agar mycelia discs led to lower red colorant production. CYA medium is great for fungal growth; however, it can cause difficulties for the microorganism to develop metabolically when the inoculum is too dense. While on CYA medium, the biomass reached 17.54 g/L at 120 h of cultivation, in the present study, it reached only 2.24 g/L of growth on MSG/glucose medium with 15 mycelial disc inoculum and 168 h of cultivation.
By analyzing the effect of several minerals added at the culture media, it was found that some of them are not essential for T. amestolkiae colorant biosynthesis. Mg2+ showed the strongest stimulatory effect on red colorant production. However, high amounts of Mg2+ showed inhibitory effect for colorant biosynthesis. Lin and Demain (1991) also reported that high concentrations of MgSO4 had an inhibiting effect on the synthesis of Monascus colorants. Interestingly, higher concentrations of MgSO4 in the medium led to a remarkable increase in glucose consumption without an increase in cell proliferation, which is extremely important in scale-up processes.
The 3D fluorescence studies have become a useful tool to monitor the structural changes in fluorophores in recent years (Sá et al. 2017). Based on the literature, differences in the concentration of a colorant resulted in differences in the intensity of fluorescence, but without shifts in λex or λem maxima. However, higher concentrations of some colorants have been shown to result in shifts in emission maxima to longer wavelengths (Simonot et al. 2011). Beyond that, the fluorescence intensity in solution, as well as excitation and emission maxima, can be influenced by pH and complexing cations (Lakowicz 2006). Polyketide colorants with azaphilone skeleton are usually composed of a highly oxygenated pyranoquinone bicyclic core, known as isochromene, and a quaternary carbon center (Gao et al. 2013). Many studies have reported azaphilone metabolites from Monascus spp. with fluorescent properties (Gao et al. 2013; Hsu et al. 2011; Loret and Morel 2010). The red colorant produced by T. amestolkiae and the orange colorant reported by Huang et al. (2014) have similar yellow fluorescence spectra (λex 480 nm, λem 520 nm; Fig. 5b, fluorophore III) indicating the presence of an extended conjugated system. Fluorophores I and II found in culture broth could not be assigned to one specific colorant, as their fluorescence patterns had multiple possible identifications.
The results of colorimetry and fluorescence show that the colorants produced by T. amestolkiae present a wide range of application. For example, the fluorescence properties can be used to develop diagnostic kit. The variety of hue and chroma supports current industry trends in food production (Vinha et al. 2018) and in the cosmetics and pharmaceutical industries as natural fluorescent colorants. Red colorants are one of the most widely used in the food industry (Singh 2006).
As final remarks, the production of extracellular colorants by T. amestolkiae using MSG was confirmed. The fact that MSG supports low microorganism growth and high amounts of extracellular colorants can be used as new strategy to develop an efficient process to produce these biocompounds. Moreover, the fluorescence properties of colorants produced enlarge their application area.
References
Arai T, Koganei K, Umemura S, Kojima R, Kato J, Kasumi T, Ogihara J (2013) Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V, production. AMB Express 3:19
Bibb MJ (2005) Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol 8(2):208–215. https://doi.org/10.1016/j.mib.2005.02.016
Blanch HW, Bhavaraju SM (1976) Non-Newtonian fermentation broths: rheology and mass transfer. Biotechnol Bioeng 18(6):745–790. https://doi.org/10.1002/bit.260180602
Carels M, Shepherd D (1977) The effect of different nitrogen sources on pigment production and sporulation of Monascus species in submerged, shaken culture. Can J Microbiol 23(10):1360–1372. https://doi.org/10.1139/m77-205
Chen M-H, Johns MR (1993) Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl Microbiol Biotechnol 40(1):132–138
Chen W, Chen R, Liu Q, He Y, He K, Ding X, Kang L, Guo X, Xie N, Zhou Y, Lu Y, Cox RJ, Molnár I, Li M, Shao Y, Chen F (2017) Orange, red, yellow: biosynthesis of azaphilone pigments in Monascus fungi. Chem Sci 8:4917–4925. https://doi.org/10.1039/c7sc00475c
Dufossé L (2017) Pigments, microbial. In: Reference module in life sciences. Elsevier, Oxford, pp 1–16. https://doi.org/10.1016/B978-0-12-809633-8.13091-2
Dufossé L (2018) Red colourants from filamentous fungi: are they ready for the food industry? J Food Compos Anal 69:156–161. https://doi.org/10.1016/j.jfca.2017.11.002
Evans PJ, Wang HY (1984) Pigment production from immobilized Monascus spp utilizing polymeric resin adsorption. App Environ Microbiol 47(6):1323–1326
Galaffu N, Bortlik K, Michel M (2015) An industry perspective on natural food colour stability. In: Colour Additives for Foods and Beverages, Woodhead publishing, pp 91–130. https://doi.org/10.1016/C2013-0-16427-6
Gao JM, Yang SX, Qin JC (2013) Azaphilonoids: chemistry and biology. Chem Rev 113:4755–4811
Hajjaj H, Klaebe A, Loret MO, Tzedakis T, Goma G, Blanc PJ (1997) Production and identification of N-glucosylrubropunctamine and Nglucosylmonascorubramine from Monascus ruber and the occurrence of electron donor-acceptor complexes in these red pigments. Appl Environ Microbiol 63:2671–2678
Hajjaj H, Klaébé A, Goma G, Blanc PJ, Barbier E, François J (2000) Medium-chain fatty acids affect Citrinin production in the filamentous fungus Monascus ruber. Appl Environ Microbiol 66(3):1120–1125
Hsu YW, Hsu LC, Liang YH, Kuo YH, Pan TM (2011) New bioactive orange pigments with yellow fluorescence from Monascus-fermented dioscorea. J Agric Food Chem 59:4512–4518
Huang Z, Zhang S, Xu Y, Li L, Li Y (2014) Structural characterization of two new orange pigments with strong yellow fluorescence. Phytochem Lett 10:140–144. https://doi.org/10.1016/j.phytol.2014.08.020
Huang T, Tan H, Chen G, Wang L, Wu Z (2017) Rising temperature stimulates the biosynthesis of water-soluble fluorescent yellow pigments and gene expression in Monascus ruber CGMCC10910. AMB Express 7(134):1–10. https://doi.org/10.1186/s13568-017-0441-y
Ismael R, Schwander H, Hendrix P (2013) Fluorescent dyes and pigments. In: Ullmann’s encyclopedia of industrial chemistry. Wiley Online Library, pp 1–22
Jůzlová P, Martínková L, Křen V (1996) Secondary metabolites of the fungus Monascus: a review. J Ind Microbiol 16(3):163–170
Kang B, Zhang X, Wu Z, Wang Z, Park S (2014) Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzym Microbial Technol 55:50–57. https://doi.org/10.1016/j.enzmictec.2013.12.007
Kim C, Jung H, Kim JH, Shin CS (2006) Effect of Monascus pigment derivatives on the electrophoretic mobility of bacteria, and the cell adsorbtion and antibacterial activities of pigments. Colloid Surf B 47:153–159. https://doi.org/10.1016/j.colsurfb.2005.12.009
Krairak S, Yamamura K, Irie R, Nakajima M, Shimizu H, Chim-Anage P, Yongsmith B, Shioya S (2000) Maximizing yellow pigment production in fed-batch culture of Monascus sp. J Biosci Bioeng 90(4):363–367. https://doi.org/10.1016/S1389-1723(01)80002-5
Lakowicz JR (2006) Chapter sixteen – protein fluorescence. In: Principles of fluorescence spectroscopy, 3rd edn. Springer, New York, pp 529–569
Lee B-K, Park N-H, Piao HY, Chung W-J (2001) Production of red pigments by Monascus purpureus in submerged culture. Biotechnol Bioprocess Eng 6(5):341–346
Lehto S, Buchweitz M, Klimm A, Straßburger R, Bechtold C, Ulberth F (2017) Comparison of food colour regulations in the EU and the US: a review of current provisions. Food Addit Contam A 34(3):335–355. https://doi.org/10.1080/19440049.2016.1274431
Lin TF, Demain AL (1991) Effect of nutrition of Monascus sp. on formation of red pigments. Appl Microbiol Biotechnol 36(1):70–75
Lin TF, Demain AL (1994) Leucine interference in the production of water-soluble red Monascus pigments. Arch Microbiol 162(1–2):114–119
Loret MO, Morel S (2010) Isolation and structural characterization of two new metabolites from Monascus. J Agric Food Chem 58:1800–1803
Lv J, Zhang B-B, Liu X-D, Zhang C, Chen L, Xu G-R, Cheung PCK (2017) Enhanced production of natural yellow pigments from Monascus purpureus by liquid culture: the relationship between fermentation conditions and mycelial morphology. J Biosci Bioeng 124(4):452–458. https://doi.org/10.1016/j.jbiosc.2017.05.010
Mapari SAS, Meyer AS, Thrane U (2006) Colorimetric characterization for comparative analysis of fungal pigments and natural food colorants. J Agric Food Chem 54(19):7027–7035. https://doi.org/10.1021/jf062094n
Mapari SAS, Thrane U, Meyer AS (2010) Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol 28:300–307. https://doi.org/10.1016/j.tibtech.2010.03.004
Orozco SFB, Kilikian BV (2008) Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J Microb Biotechnol 24(2):263–268. https://doi.org/10.1007/s11274-007-9465-9
Plackett RL, Burman JP (1946) The Design of Optimum Multifactorial Experiments. Biometrika 33(4):305–325
Sá M, Monte J, Brazinha C, Galinha CF, Crespo JG (2017) 2D fluorescence spectroscopy for monitoring Dunaliella salina concentration and integrity during membrane harvesting. Algal Res 24:325–332. https://doi.org/10.1016/j.algal.2017.04.013
Santos-Ebinuma VC, Teixeira MFS, Pessoa A Jr (2013) Submerged culture conditions for the production of alternative natural colorants by a newly isolated Penicillium purpurogenum DPUA 1275. J Microbiol Biotechnol 23:802–810. https://doi.org/10.4014/jmb.1211.11057
Shi K, Song D, Chen G, Pistolozzi M, Wu Z, Quan L (2015) Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J Biosci Bioeng 120(2):145–154. https://doi.org/10.1016/j.jbiosc.2015.01.001
Simonot L, Thoury M, Delaney J (2011) Extension of the Kubelka–Munk theory for fluorescent turbid media to a nonopaque layer on a background. J Opt Soc Am A 28(7):1349–1357. https://doi.org/10.1364/JOSAA.28.001349
Singh S (2006) Impact of color on marketing. Manag Decis 44(6):783–789. https://doi.org/10.1108/00251740610673332
Stowe RA, Mayer RP (1966) Efficient screening of process variables. Ind Eng Chem 58(2):36–40
Terán-Hilares R, de Souza RA, Marcelino PF, da Silva SS, Dragone G, Mussatto SI, Santos JC (2018) Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem 245:786–791. https://doi.org/10.1016/j.foodchem.2017.11.111
Torres FAE, Zaccarim BR, Novaes LCL, Jozala AF, Santos CA, Teixeira MFS, Santos-Ebinuma VC (2016) Natural colorants from filamentous fungi. Appl Microbiol Biotechnol 100(6):2511–2521. https://doi.org/10.1007/s00253-015-7274-x
Vendruscolo F, Schmidell W, Moritz DE, Bühler RMM, de Oliveira D, Ninow JL (2016) Isoelectric point of amino acid: importance for Monascus pigment production. Biocatal Agric Biotechnol 5:179–185. https://doi.org/10.1016/j.bcab.2015.12.006
Venkatachalam M, Zelena M, Cacciola F, Ceslova L, Girard-Valenciennes E, Clerc P, Dugo P, Mondello L, Fouillaud M, Rotondo A, Giuffrida D, Dufossé L (2018) Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J Food Compos Anal 67:38–47. https://doi.org/10.1016/j.jfca.2017.12.036
Ventura SPM, Santos-Ebinuma VC, Pereira JFB, Teixeira MFS, Pessoa A Jr, Coutinho JAP (2013) Isolation of natural red colorants from fermented broth using ionic liquid-based aqueous two-phase systems. J Ind Microbiol Biotechnol 40:507–516. https://doi.org/10.1007/s10295-013-1237-y
Vinha AF, Rodrigues F, Nunes MA, Oliveira MBPP (2018) Natural pigments and colorants in foods and beverages. In: Polyphenols: properties, recovery, and applications. Woodhead Publishing, pp 363–391
Wei WG, Yao ZJ (2005) Synthesis studies toward chloroazaphilone and vinylogous γ -pyridones: two common natural product core structures. J Org Chem 70:4585–4590
Weinberg ED (1989) Chapter seven - roles of micronutrients in secondary metabolism of Actinomycetes. In: Shapiro S (ed) Regulation of Secondary Metabolism in Actinomycetes, 3rd edn. CRC Press, Boca Raton, pp 239–261
Yilmaz N, Houbraken J, Hoekstra ES, Frisvad JC, Visagie CM, Samson RA (2012) Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 29(1):39–54
Zaccarim BR, de Oliveira F, Passarini MRZ, Duarte AWF, Sette LD, Jozala AF, Santos Ebinuma VC (2018) Sequencing and phylogenetic analyses of Talaromyces amestolkiae from the Amazon, a producer of natural colorants. Biotechnol Prog. https://doi.org/10.1002/btpr.2684
Zollinger H (2003) Color of organic compounds. In: Color chemistry: syntheses, properties, and applications of organic dyes and pigments. Wiley-VHCA and Wiley-VCH, Zurich and Weinheim, p 51
Funding
This study was supported by the São Paulo Research Foundation (FAPESP) – Brazil [Grant no. 2014/01580-3], by the Conselho Nacional de Desenvolvimento Científico CNPq-Brazil [Grant no. 155317/2016-4], and partly by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 585 kb)
Rights and permissions
About this article
Cite this article
de Oliveira, F., Pedrolli, D.B., Teixeira, M.F.S. et al. Water-soluble fluorescent red colorant production by Talaromyces amestolkiae. Appl Microbiol Biotechnol 103, 6529–6541 (2019). https://doi.org/10.1007/s00253-019-09972-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09972-z