Abstract
Wheat straws (WS) were pretreated by a two-step pretreatment for enhanced enzymatic hydrolysis. The raw wheat straw (RWS) was firstly soaked in microwave-assisted NaOH solution at the presence of a mixed solution of H2O2 and hexadecyl trimethyl ammonium chloride (HTAC), and the residue was put into methyl orange (MO) solution to obtain the final substrate for enzymatic hydrolysis. It was interesting to find that the WS after the first step of the pretreatment could effectively remove MO in the second step of the pretreatment with the highest removal efficiency of 98.86% at the MO concentration of 25 mg/L and at the adsorbent-adsorbate ratio of 0.8%. Meanwhile, the enzymatic hydrolysis efficiency of the WS that was pretreated by the two steps was higher than that of the RWS as well as the first-step-pretreated WS. Enzymatic hydrolysis experiment showed that the glucose concentration after 48-h hydrolysis was 4.85 g/L at the solid loading of 5%, compared with the RWS of 0.90 g/L and the first-step-pretreated WS of 4.33 g/L. This suggested that the two-step pretreatment method could not only make WS as a good adsorbent but also a more biodegradable lignocellulosic material. Characterization analysis showed that the specific surface area and the cellulose content of the WS were both increased after the two-step pretreatment, indicating that the pretreatment method was significant to properly utilizing WS as biosorbent and biofuel. Besides, a possible mechanism for the effect of the pretreatment on the WS was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing consumption of fossil fuels in recent years has urged people to find other suitable substitutes which are greener, cheaper, and abundant. Biofuels such as bioethanol, biogas, and biodiesel are such promising new energy that can meet the standard of sustainable development of the environment (Pant et al. 2012). China as a great agricultural country in the world produces a large amount of crop residues every year, and approximately 135 million tons of wheat straws are annually generated, making wheat straw an abundant and available biomass resource to be utilized (Zeng et al. 2007). Studies show that agricultural wastes can be used as adsorbents to remove dyes from wastewater, because they contain many active functional groups such as hydroxyl and carboxyl. However, using raw agricultural residues as adsorbents directly obtains very low adsorption capacity, so proper pretreatment methods are rather necessary to improve their adsorption capacity (Wu et al. 2017). Besides, agricultural residues are especially abundant in lignocellulose, which makes them potential substrates for bioethanol production (Hassan and Shukry 2008). The production of bioethanol involves two steps, the first step is to hydrolyze lignocellulose substance into reducing sugars, and the second is to ferment reducing sugars into bioethanol. Nevertheless, some hydrolysis inhibitory factors like cellulose crystallinity, surface area, the protection by lignin and hemicellulose can all impede the process of biomass hydrolysis (Zhao et al. 2009). Besides, the compact heteromatrix structure of cellulose, hemicellulose, and lignin requires pretreatment to make them more biodegradable and therefore produce more fermentable sugars (Haruta et al. 2002). General pretreatment methods used to increase the biodegradability of biomass include physical (steam explosion, microwaving, ultrasonication, etc.), chemical (acid/alkaline pretreatment, ionic liquid pretreatment, diluted inorganic acids, etc.), and biological pretreatment (fermentation, degradation, etc.) and their combinations (Kim et al. 2016; Saha and Cotta 2011). However, those methods have some shortcomings such as high cost, low efficiency, and probable secondary pollution (He et al. 2018; Mosier et al. 2005). Therefore, proper pretreatment methods are needed to tackle the problem and enhance the yield of reducing sugar.
Among various agricultural wastes that are used for biofuels production, wheat straw (WS) has drawn increasing attention not only due to its wide availability but also because of its higher ash content and less degree of lignifications compared with woody biomass (Chen et al. 2015; Anwar et al. 2014; Saini et al. 2014). Apart from being used for biofuel production, WS is also a common biosorbent for dyes removal (Pan et al. 2010).
Given that using agricultural residues as effective biosorbent and biofuel both needs pretreatment, finding suitable pretreatment methods to simultaneously achieve both goals is of novelty and importance. In addition, studies on reusing agricultural waste biosorbents for producing reducing sugar are insufficient. In other words, pretreatments can improve the efficiency of enzymatic hydrolysis and the adsorption capacity of lignocellulosic materials. Therefore, if the modified WS could effectively remove dyes from wastewater and then produce more reducing sugar by enzymatic hydrolysis, the pretreatment methods would be significant.
In this study, WS samples were pretreated by a two-step pretreatment for higher reducing sugar yield. The objective of the present work is to find an appropriate pretreatment method which can achieve the dual use of wheat straw, namely as an efficient adsorbent for dye removal and a good enzymatic hydrolysis substrate. The effects of different pretreatment methods were evaluated on both adsorption behavior and enzymatic hydrolysis of wheat straw. In addition, characteristic analyses were performed to better understand the possible mechanism of those pretreatment methods.
Materials and methods
Materials
Raw wheat straw (RWS) was collected from a local farm in Zhengzhou (Henan Province, China). Before pretreatment, RWS was cut to 1–3 cm, washed with ultrapure water until the washings were clean, dried at 80 °C for 48 h, ground and passed through a 100-mesh sieve, and stored in plastic sealable bags for further use. Sodium hydrate (NaOH), hydrogen peroxide (H2O2, 30% w/v), methyl orange (MO, C14H14N3SO3Na), hexadecyl trimethyl ammonium chloride (HTAC, C19H42ClN), citric acid (CA, C6H8O7), trisodium citrate dehydrate (TCD, Na3C6H5O7·2H2O), and 3,5-dinitrosalicylic acid (DNS, C7H4N2O7) were purchased from Sinopharm Chemical Reagent Co., Ltd. and were all analytically pure grade. The cellulase (CAS # 9012-54-8) used for enzymatic hydrolysis was purchased from Aladdin Chemistry Co., Ltd., and the recommended BG activity and xylanase activity was 14 and 20 IU per gram of dry biomass, respectively. Ultrapure water was used in all the experiments and all the containers were soaked in dilute nitric acid overnight and thoroughly washed with ultrapure water before using. All the experiments were performed in triplicate.
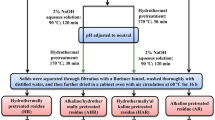
The first step of the pretreatment
The first step of the pretreatment could be seen as a preparation for WS adsorbents. Two kinds of WS adsorbent were synthesized by the following steps: (1) NHWS: adding 10 g of RWS and 250 mL of 1.5% NaOH (w/v) into a 500-mL Erlenmeyer flask, mixing the WS and NaOH solution evenly, and putting it at 25 °C without mechanical agitation. After 24 h, stirring the flask for 3 h at a speed of 200 rpm, using ultrapure water to wash the WS until its pH was neutral, oven-drying the alkali-treated WS at 80 °C for 48 h. Taking 4 g of the NaOH pretreated WS, mixed with 100 mL of 10% H2O2 and 300 mL 2.5 mmol/L HTAC solution, magnetically stirring with a speed of 1000 rpm at 80 °C for 3 h. Washing the WS to neutral pH, oven-drying it to constant weight, and labeling it as NHWS. (2) NMHWS: this adsorbent was obtained by the above pretreatment step with only one difference of using a 5-min microwaving to supplement the alkali pretreatment. Besides, adsorbent obtained by directly soaking RWS in the mixed solution of H2O2 and HTAC (labeled as CK) was used to rule out the dissolution effect of HTAC on removing MO. All the modified WS were stored in plastic sealable bags for later use.
The second step of the pretreatment
As mentioned before, step 1 could be regarded as a preparation of WS biosorbents, thus step 2 was actually an adsorption process. Three parameters including adsorbent-adsorbate ratios, initial MO concentrations, and contact time were investigated.
In order to study the effect of different adsorbent-adsorbate ratios on the adsorption behavior of the modified WS, the experiment was carried out by the following procedures: the initial concentration of the MO solution was about 30 mg/L. Adsorbents were weighed and MO solution was measured, the adsorbent-adsorbate ratios were set as 0.2, 0.4, 0.6, 0.8, 1.0% g/mL. When the WS was mixed with MO solution, put it under continuous stirring at 25 °C and 200 rpm for 12 h to reach the adsorption equilibrium. Whereafter, filter the MO solution by 0.45 μm syringe filters and analyzing at the wavelength of 464 nm by a Victor 722 Vis spectrophotometer. As the standard curve only covered a concentration range of 0–15 mg/L, appropriate dilution was carried out to make sure that the MO solution was within the range of the standard curve. The adsorption capacity was calculated by Eq. (1) and the removal efficiency of MO was measured by Eq. (2):
where C0 (mg/L) is the initial concentration of the MO solution and Ce (mg/L) is the final concentration of MO after adding adsorbents; V (L) is the volume of the MO and m (g) is the mass of the adsorbent; r (%) refers to the removal efficiency of the adsorbent.
To investigate the time of adsorption equilibrium, three MO concentrations were set: 20, 25, and 30 mg/L. After adding adsorbent into MO solution, continuously shaking the mixture at a speed of 200 rpm and the temperature of 25 °C, and taking 4 mL of mixed solution each time after 5, 10, 15, 30, 45, 60, 90, 120, 180, 240 min and filtering by 0.45 μm syringe filters. The concentration of MO was measured by ultraviolet spectrophotometer. Furthermore, the effect of initial MO concentration was tested at the MO concentrations ranging from 10 to 35 mg/L.
Enzymatic hydrolysis
After NHWS and NMHWS were used to absorb MO, the used adsorbents were collected and oven-dried at 80 °C, labeled them as NHWS-MO and NMHWS-MO, and then prepared for enzymatic hydrolysis experiment. To evaluate the effectiveness of the pretreatment, five samples were chosen to perform enzymatic hydrolysis experiment, namely, RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO. Of each sample, 0.1 g was added to a 50-mL centrifuge tube containing 10 mL of CA-TCD buffer solution (pH = 4.8) and 0.006 g of cellulase. Besides, 50 μL 250 mg/L tetracycline solution was added into the tube to inhibit the influence of microorganisms. The enzymatic hydrolysis reactions were respectively conducted for 0, 4, 6, 8, 12, 24, 36, 48, and 72 h, shaking at 150 rpm and at the temperature of 50 °C. Nine tubes of each type of WS were prepared, one tube was withdrawn when the reaction was terminated at each time point by taking 2 mL of hydrolysates using 0.22 μm syringe filters, and the filtrate was stored at − 20 °C for further reducing sugar measurement. The amount of total reducing sugars was determined by dinitrosalicylic acid method (DNS method). Enzymatic hydrolysis efficiency was evaluated by reducing sugar concentration and reducing sugar yield, and the latter was calculated by Eq. (3):
where mRS (mg) is the total mass of reducing sugar obtained by enzymatic hydrolysis, mD (mg) is dry weight of the samples, and YRS (%) is reducing sugar yield.
The effect of different solid loadings on enzymatic hydrolysis was examined by setting five solid loadings: 2, 4, 5, 10, and 15%. The final reducing sugar concentration obtained at each solid loading was compared to determine the optimal condition.
Characterization and compositional analysis
Three samples, RWS, NHWS, and NMHWS, were characterized by scanning electron microscope (SEM, JEOL, JEM-200CX, Japan) and Brunauer-Emmett-Teller surface area measurement (BET, BELSORP-miniII_Microtrac. BEL, Japan). The infrared spectra of RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO were determined by Fourier transform infrared spectroscopy (FTIR, IR Affinity-1, Japan). The main compositions of the WS including cellulose, hemicelluloses, lignin, and ash were measured according to NREL method (Sluiter et al. 2008). High performance liquid chromatography (HPLC, 1200, Agilent, Switzerland) was used to determine the quantities of acid hydrolysis products.
Results
Effect of adsorbent-adsorbate ratios on the removal efficiency of methyl orange
In order to investigate the optimum adsorption efficiency of the WS adsorbents and the effect of MO amount absorbed onto the adsorbents on further enzymatic hydrolysis, different adsorbent-adsorbate ratios were set to demonstrate this. Sufficient contact time was given to ensure a complete adsorption and enzymatic hydrolysis process. Figure 1a showed the removal efficiency of MO at five adsorbent-adsorbate ratios: 0.2, 0.4, 0.6, 0.8, 1.0% (g/mL). From Fig. 1a, it could be seen that the two adsorbents had much higher removal efficiency than that of the RWS. The removal efficiency of MO increased with the increase of adsorbent-adsorbate ratio, and at the ratios of 0.8 and 1.0%, the removal efficiencies were approximately the same. When the adsorbent-adsorbate ratio was 0.8%, the removal efficiency of RWS, NHWS, and NMHWS was 21.35, 97.72, and 99.32%, respectively. Figure 1b presented the enzymatic hydrolysis performance of those used adsorbents with different amount of MO absorbed. No obvious changes of reducing sugar yield of each sample at different adsorbent-adsorbate ratios were observed. The reducing sugar yield decreased in the series of NMHWS>NHWS>RWS, and for each sample its reducing sugar yield was on a slight rise with the increase of the adsorbent-adsorbate ratio.
Effect of contact time on the removal of methyl orange
The adsorption performance of NHWS and NMHWS as a function of contact time was presented in Fig. 2a and b, respectively. Three initial concentrations of MO solution (20, 25, and 30 mg/L) were set to study the adsorption equilibrium. As shown in Fig. 2, both adsorbents had a rapid adsorption stage during the first 15 min, and the adsorption capacity increased with the increase of the initial MO concentration. Another phenomenon that drew attention was that the time of adsorption equilibrium was a little bit different for the three initial MO concentrations. When the initial MO concentration was 20 mg/L, it only took 15 min to finish the rapid adsorption stage, while at concentrations of 25 and 30 mg/L, it took about 45 min to cover this stage. After the rapid adsorption stage, a relatively slow adsorption stage came. When the initial MO concentration was 20 mg/L, the slow adsorption stage was the period of 15–180 min while at the initial concentrations of 25 and 30 mg/L, the slow adsorption stage moved to the period of 45–180 min. Therefore, the time of adsorption equilibrium for both adsorbents was 180 min. Compared with the adsorption capacity of NHWS and NMHWS, it could be seen that the later was higher than the former. The maximum adsorption capacity of NMHWS was 2.57 mg/g when the initial MO concentration was 30 mg/L, while the adsorbent of NHWS obtained a maximum adsorption capacity of 2.24 mg/g.
Effect of initial methyl orange concentration on removal efficiency
Figure 3 demonstrated how the removal efficiency of the two adsorbents changed with the variation of the initial MO concentration. In general, the removal efficiency of NHWS and NMHWS was above 95% when the initial MO concentration was between 10 and 30 mg/L. As the MO concentration increased, the removal efficiency of both adsorbents was on the slight decline. For NMHWS, at the concentrations of 10, 15, and 20 mg/L, the removal efficiency of MO was over 99.00% and no obvious difference was observed. When the MO concentrations were 25 and 30 mg/L, the removal efficiency was 98.86 and 97.17%, respectively, and when the concentration was 35 mg/L, the removal efficiency was only 93.60%. For NHWS, the overall variation trend was similar to NMHWS but lower removal efficiency was obtained.
Effect of hydrolysis time on enzymatic hydrolysis
The enzymatic hydrolysis experiment was performed using five kinds of substrate, namely RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO. Referring to the above experiment results, NHWS-MO and NMHWS-MO were the used adsorbents with the maximum amount of MO absorbed. From Fig. 4, it could be seen that in the first 24 h, the producing speed of reducing sugar was very fast, and it gradually slowed down from 24 to 48 h. When the enzymatic hydrolysis time was over 48 h, the concentration of reducing sugar did not increase anymore, so it took 48 h for cellulose to be completely hydrolyzed. After 12-h enzymatic hydrolysis, the reducing sugars’ concentrations of NHWS, NMHWS, NHWS-MO, and NMHWS-MO were very close to each other, while the reducing sugars’ concentration produced by RWS was relatively low. Figure 4 also showed that the reducing sugar concentration of NMHWS-MO after 48 h of hydrolysis was the highest among the five samples.
Effect of pretreatment methods on compositional change of wheat straw
The structure of lignocellulose was so compact that it was rather difficult for lignocellulose to be hydrolyzed. Hemicellulose and lignin were linked together by covalent bonds, and cellulose was embedded between them (Xu et al. 2010). It formed a mesh-shaped framework that hindered the hydrolysis process. The change of the cellulose, hemicellulose, and lignin content in the WS could indirectly reflect its enzymatic hydrolysis efficiency, so the compositional analysis of the raw and pretreated WS was conducted. As presented in Table 1, the RWS contained 34.5% of cellulose, 23.1% of hemicellulose, and 20.6% of lignin. Apart from that, it also contained 5.8% of ash. After pretreatment and adsorption, the proportions of cellulose, hemicellulose, and lignin were changed. A general trend of the compositional change was that the cellulose content was increased while the hemicellulose and lignin contents were decreased. According to Table 1, the cellulose content of the NHMWS-MO increased the most, about 51.3% higher than that of the RWS. The contents of hemicellulose and lignin were slightly decreased. For instance, hemicellulose content of NMHWS-MO decreased from 23.1 to 14.7%, showing that the amount of hemicellulose was reduced by the first and second step of pretreatment. Moreover, the lignin content of NMHWS-MO was also reduced to 14.8%. It suggested that the amount of hemicellulose might be reduced by pretreatment and MO adsorption.
Effect of different pretreatment methods on enzymatic hydrolysis
Pretreatment methods such as dilute acid and alkaline pretreatment could remove lignin and hemicelluloses so as to increase not only the porosity and specific surface area but also the reducing sugar yield (Cheng et al. 2016a). Figure 5a, b showed the reducing sugar concentration and reducing sugar yield of RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO after 48 h of enzymatic hydrolysis. The final reducing sugar concentration of RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO was 0.90, 3.93, 4.07, 4.33, and 4.85 g/L, respectively, as presented in Fig. 5a, showing that the concentration of reducing sugar produced by the WS was greatly enhanced after pretreatment. The concentration of reducing sugar released by NMHWS-MO ranked the highest among the five samples, and it was nearly six times higher than that of the control group. Besides, the reducing sugar concentrations of NHWS, NMHWS, and NHWS-MO were all four to five times higher than that of the RWS after hydrolysis. As Fig. 5b suggested, the reducing sugar yield of NMHWS-MO was the highest of 87.33% among the five samples.
Effect of solid loadings on enzymatic hydrolysis
Five solid loadings were set to examine the influence of solid loadings on enzymatic hydrolysis. NMHWS-MO was chosen to investigate this effect as it had the highest reducing sugar concentration after enzymatic hydrolysis according to the aforementioned result. The experiment results showed that cellulose in the NMHWS-MO was completely hydrolyzed after 48 h at five solid loadings. Among these solid loadings, namely 2, 4, 5, 10, and 15%, the highest reducing sugar concentration was observed at solid loading of 5%, followed by 10, 4, 15, and 2%. The final reducing sugar concentrations of the NMHWS-MO at the solid loadings of 2, 4, 5, 10, and 15% were 2.54, 4.66, 4.85, 4.82, and 3.22 g/L, respectively. When the solid loading was lower than 10%, the final reducing sugar concentration was increased with the substrate concentration; while at solid loadings higher than 10%, the enzymatic hydrolysis efficiency was slightly declined.
Characteristics of pretreated wheat straw
SEM analysis was used to examine the morphological changes caused by the step 1 and step 2 pretreatment. Figure 6 showed the SEM images of RWS, NHWS, and NMHWS. From the image of RWS, a tube-like structure with a relatively dense, smooth, and continuous surface could be seen. SEM images of NHWS and NMHWS showed rugged surfaces, their structures seemed to be torn and broken, besides, split and fibrillation could be observed. In addition, the specific surface area data of the RWS, NHWS, and NMHWS was 0.616, 2.073, and 4.256 m2/g, respectively. It clearly showed that the pretreated WS had larger specific surface area than the RWS.
FTIR analysis was performed to examine the structural changes of WS after pretreatment. In Fig. 7, it suggested that the characteristic peaks of the RWS were mainly located at the wavenumber of 3500–3350 cm−1 (–OH stretching vibration region), 3000–2800 cm−1 (–CH3 and –CH2 stretching vibration region), 2400–2100 cm−1 (triple bond and cumulative double bond stretching vibration region), 1900–1630 cm−1 (C=O stretching vibration region), 1675–1500 cm−1 (double bond stretching vibration region), and 1475–1300 cm−1 (saturated –CH in-plane bending vibration region). The FTIR spectra of the unpretreated and pretreated WS were different from one another. For NHWS and NHWS-MO, the major characteristic peaks did not change so much. At the wavenumber of 3500–3300 cm−1 and 3000–2800 cm−1, the intensity of the adsorption peaks was weakened, indicating that the amount of –OH and –CH was decreased.
Discussion
Effective pretreatment methods could enhance the enzymatic hydrolysis efficiency of the biomass materials, and the key to realizing higher lignocellulosic conversion rate is to break the compact cross-linked polysaccharide network that was constructed by cellulose, hemicellulose, and lignin (Unrean et al. 2015; Wang et al. 2014). Alkali and microwave had long been used for the removal of hemicellulose and lignin, and NaOH solution was a common reagent for this purpose. Moreover, the use of surfactant to pretreat agricultural residues was also often reported (Zhou et al. 2010a). For example, Tween 80 could be used to pretreat WS with the enzymatic hydrolysis efficiency increased by 21.3% (Jalc 2002); Triton X-100 could enhance the enzymatic hydrolysis of WS by 18–25% (Qi et al. 2009). In this study, a two-step pretreatment method combining microwave-assisted dilute alkali at the presence of surfactant of HTAC and MO solution was employed to improve the enzymatic hydrolysis efficiency of WS. For the second step of the pretreatment was actually an adsorption process, variations concerning adsorption were investigated, and thus the whole experiment was divided into three parts, which included adsorption experiment, enzymatic hydrolysis, and characterization analysis.
The effect of different adsorbent-adsorbate ratios on MO removal was clearly demonstrated (Fig. 1). When the adsorbent-adsorbate ratio was lower than 0.8%, the removal rate of MO improved with the adsorbent-adsorbate ratio, and at the ratio of 0.8%, most MO would be removed thus there was no need to increase the adsorbent dosage (Fig. 1a). This also showed that the first step of the pretreatment was effective to make the WS a good biosorbent. In other words, the combined pretreatment of microwave-assisted NaOH solution together with a mixture of H2O2 and HTAC achieved the highest removal efficiency of MO. The results were consistent with literature about alkali, microwaving or surfactant pretreatment of crop straw that improved the removal efficiency of dyes was numerous (He et al. 2017; Wu et al. 2018). For instance, Pirbazari et al. (2015) used anionic surfactant to pretreat rice straw and obtained a high removal efficiency of methylene blue. The phenomenon could be explained by the reason that those pretreatment methods could help straws get much rougher surface and therefore increased their adsorption capacity (Cheng et al. 2016b). Besides, the enzymatic hydrolysis efficiency of the pretreated WS was slightly increased with the increase of the mass of MO that it had absorbed (Fig. 1b), which meant that before using as enzymatic hydrolysis substrate, the pretreated WS could be used an effective biosorbent and absorb as much MO as possible.
Contact time and the adsorption capacity were two important factors that could show some properties of the adsorbents. The equilibrium time decided how long the second step of pretreatment should take; therefore, an adsorption time of 180 min was appropriate for the second step of pretreatment (Fig. 2a, b). The adsorption capacity of both NHWS and NMHWS increased with the increase of the initial MO concentration. What lay behind this phenomenon might be that the adsorbents did not reach their maximum adsorption capacity at low MO concentration. When the initial concentration of MO was low, the MO only occupied some of the adsorption sites of the adsorbents and many other unoccupied sites remained to be occupied, so it took shorter time to finish the rapid adsorption stage than that of the higher initial MO concentration (Huang et al. 2015; Yu et al. 2018). Comparing the adsorption capacity of the two adsorbents, it was found that NMHWS was higher than NHWS, suggesting that the first step pretreatment with the assistance of microwave improved the adsorption capacity of the WS more obviously. The sample CK was used to investigate the effect of HTAC alone on the removal of MO, and the result was that the removal efficiency of the CK was about 37.56%, which was much lower than that of the NHWS and NMHWS, suggesting that the dissolution effect of HTAC was not remarkable.
Enzymatic hydrolysis as one of the common methods for degradation of cellulose into glucose can take place under relatively mild conditions, and it produces nontoxic chemicals when the reaction was over. In this study, the enzymatic hydrolysis of pretreated WS was conducted by cellulase. The performance of enzymatic hydrolysis was determined by reducing sugar production from the conversion of the biomass.
The enzymatic hydrolysis experiment mainly focused on four aspects, namely the hydrolysis time, the compositional changes, final reducing sugar concentration, and different solid loadings. For the hydrolysis time of the enzymatic hydrolysis, it was similar to the previous adsorption process. A fast enzymolysis stage was observed in the first 36 h, this might be due to the fact that at the beginning of the enzymatic hydrolysis the substrate fully contacted with cellulase so that the enzymatic hydrolysis rate was rapid. With time passing and the amount of reducing sugar increasing, inhibitory effect of reducing sugar producing was becoming seeable, which caused the slow enzymatic hydrolysis stage of the WS. However, slight difference could be observed regarding the enzymolysis time of the RWS. This suggested that the RWS itself did not have much cellulose to be hydrolyzed, and all the cellulose was totally hydrolyzed by the cellulase in the first 12 h.
The compositional change of WS was a significant parameter which could indirectly reflect the effectiveness of the pretreatment methods applied to the WS. Compositional change of RWS, NHWS, NMHWS, NHWS-MO, and NMHWS-MO was studied to understand the enzymatic hydrolysis from a molecular perspective. The increase of cellulose content after the two-step pretreatment proved that the pretreatment methods could increase the accessibility of the cellulose and thus guaranteed higher enzymatic hydrolysis (Table 1). A possible explanation was that as alkali could destroy the intricate structure of cellulose, hemicellulose, and lignin, the NaOH pretreatment could dissolve a part of hemicellulose and lignin, which could increase the content of cellulose and expand the cellulose to get larger porosity (Mcintosh and Vancov 2011). Previous researches proved that cellulose, hemicellulose, and lignin formed a compact entity by chemical bonds such as covalent bond and ionic bond, and Van der Waals force (Pant and Adholeya 2007; Zhou et al. 2010b; Zhu et al. 2018). The loss of hemicellulose and lignin indicated that those pretreatment methods destroyed the compact entity of cellulose, hemicellulose, and lignin so that the relative content of cellulose would increase. Sitepu et al. (2014) pointed out that NaOH and H2O2 could efficiently remove hemicellulose and lignin of agricultural residues. Satpathy et al. (2014) used different powers of microwave to pretreat WS and found that the carbon content of the WS increased with the increase of power and time, and it could also reduce the content of lipid materials existed on the surface of the WS. Thus, both alkali and microwaving could help to accelerate the “dewaxing” process of WS. This result was also in accordance with the result of FTIR, showing that NMHWS and NMHWS-MO were supposed to acquire the higher enzymatic hydrolysis efficiency and reducing sugar concentration than RWS, NHWS, and NHWS-MO.
In addition, it was interesting to notice that both the reducing sugar concentrations of NHWS-MO and NMHWS-MO were a little higher than the two unused adsorbents, namely NHWS and NMHWS (Fig. 3). A possible reason was that MO changed the structure of the WS and made cellulose more accessible (Inglesby and Zeronian 1996). The results showed that the pretreatment methods and adsorption process did change the structure of WS and enhance their enzymatic hydrolysis. Besides, WS pretreated by microwave-NaOH at the presence of HTAC together with adsorption was proved to be an effective pretreatment method to improve enzymolysis efficiency, which might be attributed to that the combined pretreatment method could effectively change the structure of WS. This provided a new insight into enzymolysis of agricultural residues.
High solid loading is beneficial to the production of reducing sugars; however, it is inevitable that the enzymatic hydrolysis efficiency will be inhibited by high solid loadings. In general, the reducing sugar concentration was higher at higher solid loading, but the end-product inhibition could hinder the enzymolysis of cellulose, the glucose as a final product might have a direct inhibitory effect on the activity of cellulase (Larsson et al. 1999). Moreover, the decrease of reducing sugar yield at higher solid loading could have something to do with the cellulase adsorption behavior (He et al. 2017; Wang and Chen 2006; Wang et al. 2011). For these reasons, only part of the substrate could be hydrolyzed by the enzyme. Meanwhile, the sampling process might also bring deviations. Considering the completeness of the enzymatic hydrolysis, the proper solid loading should be 5% in this experiment. Under this condition, the concentration of reducing sugar was 4.85 g/L.
SEM pictures were provided to further characterize the surface changes of RWS and the pretreated WS. The results proved that the pretreatment methods could damage the inner structure of the WS. A smooth surface of the RWS was presented, and it was the wax layer of the WS which mainly contained saturated hydrocarbon compounds and their derivates, and advanced fatty acid that made the surface smooth (Thomsen et al. 2009). The disrupted structure of NHWS and NMHWS indicated that the two pretreatment methods changed the structure of the WS to some extent, and components such as hemicellulose, lignin, and pectin might be removed after the pretreatment (Rossana et al. 2016). The less smooth surface of the pretreated WS suggested that the wax and siliceous layer that covered the surface of the WS was more or less removed. Such a loose structure would increase the binding sites of both MO and cellulase. As a result, the adsorption capacity of MO and enzymatic hydrolysis efficiency of cellulose might be both enhanced. Comparing the SEM images of NHWS with NMHWS, the later had more cracked and open structure than that of the former, demonstrating that microwaving resulted in more structural damages to the WS. It could make more microfibrils expose to the environment, which was expected to increase the specific surface area of the WS and thus enhance the adsorption capacity (Kabel et al. 2017). Some studies showed that microwaving pretreatment could change the crystal structure of cellulose (Yu et al. 2017). Moreover, microwaving was beneficial to the breakage of the hydrogen bond between cellulose molecules, making other solutions easily permeate through the inner area of the WS. The combination of microwaving and alkali would make the pretreatment of the WS more complete, which was embodied by the result that more lignin was removed. According to Zhang et al. (2011), alkali could basify WS and promote the etherification in follow-up pretreatment steps. NaOH damaged the structural unit of the lignin which was connected by ether linkage so that the lignin with high molecular weight would break down into smaller ones and be dissolved in solutions (Lin et al. 2018). At the same time, the ester linkage that linked lignin and hemicellulose was broken due to saponification, and the strength of hydrogen bond between hemicellulose and cellulose weakened (Abubackar et al. 2015; Chen et al. 2018). Because 10% H2O2 was added to assist the pretreatment, the content of lignin should be decreased, for H2O2 could also promote dissolution of other materials and therefore increase the content of cellulose. Furthermore, H2O2 could also have non-selective oxidation effect on cellulose. Therefore, the WS expanded and got bigger volume. This result was supported by the specific surface area data. The increase of specific surface area might be attributed to the change of the microstructure of the WS (Abubackar et al. 2016; Pant and Adholeya 2009). The increase of roughness and specific surface area could not only improve the adsorption capacity of the WS but also enhance its efficiency of enzymatic hydrolysis. This made the reuse of the adsorbents as suitable enzymatic hydrolysis substrates possible.
FTIR was often performed to reveal the changes of functional groups during different pretreatments. The FTIR data of the five samples demonstrated that the two-step pretreatment method changed the functional groups of the WS to some degree. It showed that NaOH did cause damage to the structure of cellulose and hemicellulose of the WS, and MO also had an impact on it. The missing of the region of 1850–1750 cm−1 showed that NaOH affected the –C=O and dissolved lignin and hemicellulose. This would increase the content of cellulose as well as the tensile strength of the WS, which would provide more cellulose to be hydrolyzed and increase the reducing sugar yield.
Possible mechanism that the pretreatments affected the structure of the WS was proposed. Generally speaking, the aims of pretreatment are to make the surface of WS much rougher or to change its inner structures so that both adsorption capacity and biodegradability will be enhanced. Different pretreatment methods have different effects on WS. For example, one of the prominent effects of microwaving is to remove the wax layer which is attached to the surface of the WS. In addition, microwaving could change the super-molecular structure of the plant fiber, which is embodied by the variation of the hydrogen bonds and the minifying of the size of crystalline regions (Wang and Chen 2009). Although the microwaving process alone could not remove the wax layer completely, it could assist the NaOH and H2O2 solutions to exert a better effect on WS pretreatment. As it was mentioned before, NaOH and H2O2 could decrease the content of lignin and increase the content of cellulose. Both microwaving and alkaline pretreatment could enhance the accessibility and reaction activity of cellulose because a larger specific surface area was obtained. Besides, NaOH solution broke the structural unit of lignin which was connected by ether bonds, which could greatly reduce the percentage of the lignin and increase the reducing sugar yield (Yang et al. 2018). In addition, it was worth noticing that when alkaline solution was used to pretreat WS, lignin would be dissolved rather than degraded (Zhou et al. 2018).
HTAC as a cationic surfactant as well as a kind of quaternary ammonium salts could also change the structure of WS. With previous alkaline pretreatment by NaOH solution, most lignin in WS was dissolved while hemicellulose was degraded into low molecular sugars. Meanwhile, insoluble cellulose was etherified by HTAC under alkaline condition, and cellulose cationic etherate was produced (Yang et al. 2010). From the FTIR figure, it could be seen that the characteristic peak of the quaternary ammonium salt was detected, which meant that the WS was quaternized successfully. A possible mechanism for the effects of NaOH and HTAC on cellulose was presented in Fig. 8. The H+ of the cellulose was replaced by Na+ when NaOH was added to pretreat WS, and then the basifying products combined with HTAC, quaternary ammonium cationic group was introduced to the WS. Although in this experiment those pretreated WS got slightly higher reducing sugar concentration after absorbing MO, studies on dyes’ effects on the enhancement of the enzymatic hydrolysis of WS was not enough. A reasonable guess was that MO made more cellulose exposed to the outside environment, and when cellulase was added it would be hydrolyzed more sufficiently. To further analyze the mechanism behind it, deeper researches should be carried on in future studies.
In conclusion, a biosorbent of NMHWS was successfully synthesized by the first step of pretreatment, and the highest removal efficiency was 98.86% at the initial MO concentration of 25 mg/L and adsorbent-adsorbate ratio of 0.8%. The adsorption process was regarded as the second step of pretreatment, and the following enzymatic hydrolysis experiment showed that the NMHWS-MO obtained the highest reducing sugar concentration of 4.85 g/L and reducing sugar yield of 87.33% among five samples within 48 h at the solid loading of 5%. The increased specific surface area and cellulose accessibility guaranteed both favorable adsorption capacity and reducing sugar yield of WS, indicating that the pretreatment methods were promising and effective for the reclamation of the WS.
References
Abubackar HN, Veiga MC, Kennes C (2015) Ethanol and acetic acid production from carbon monoxide in a Clostridium strain in batch and continuous gas-fed bioreactors. Int J Environ Res Public Health 12:1029–1043. https://doi.org/10.3390/ijerph120101029
Abubackar HN, Bengelsdorf FR, Dürre P, Veiga MC, Kennes C (2016) Improved operating strategy for continuous fermentation of carbon monoxide to fuel-ethanol by clostridia. Appl Energy 169:210–217. https://doi.org/10.1016/j.apenergy.2016.02.021
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Chen HM, Jia Z, Hu TH, Zhao XB, Liu DH (2015) A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: substrate digestibility, fermentability and structural features. Appl Energy 150:224–232. https://doi.org/10.1016/j.apenergy.2015.04.030
Chen YJ, He HJ, Liu HY, Zeng GM, Xia X, Yang CP (2018) Effect of salinity on removal performance and activated sludge characteristics in sequencing batch reactors. Bioresour Technol 249:890–899. https://doi.org/10.1016/j.biortech.2017.10.092
Cheng Y, He HJ, Yang CP, Zeng GM, Li X, Chen H, Yu GL (2016a) Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol Adv 34:1091–1102. https://doi.org/10.1016/j.biotechadv.2016.06.007
Cheng Y, Yang CP, He HJ, Zeng GM, Zhao K, Yan Y (2016b) Biosorption of Pb(II) ions from aqueous solution by waste biomass from biotrickling filters: kinetics, isotherms and thermodynamics. J Environ Eng 142:C4015001–C4015007. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000956
Haruta S, Cui Z, Huang Z, Li M, Ishii M, Igarashi Y (2002) Construction of a stable microbial community with high cellulose-degradation ability. Appl Microbiol Biotechnol 59:529–534. https://doi.org/10.1007/s00253-002-1026-4
Hassan EBM, Shukry N (2008) Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind Crop Prod 27:33–38. https://doi.org/10.1016/j.nbt.2014.12.007
He HJ, Chen YJ, Li X, Cheng Y, Yang CP, Zeng GM (2017) Influence of salinity on microorganisms in activated sludge processes: a review. Int Biodeterior Biodegrad 119:520–527. https://doi.org/10.1016/j.ibiod.2016.10.007
He HJ, Xiang ZH, Chen XJ, Chen H, Huang H, Wen M, Yang CP (2018) Biosorption of Cd(II) from synthetic wastewater using dry biofilms from biotrickling filters. Int J Environ Sci Technol 15:1491–1500. https://doi.org/10.1007/s13762-017-1507-8
Huang YQ, Yang CP, Sun ZC, Zeng GM, He HJ (2015) Removal of cadmium and lead from aqueous solutions using nitrilotriacetic acid anhydride modified ligno-cellulosic material. RSC Adv 5:11475–11484. https://doi.org/10.1039/C4RA14859B
Inglesby MK, Zeronian SH (1996) The accessibility of cellulose as determined by dye adsorption. Cellulose 3:165–181. https://doi.org/10.1007/BF02228799
Jalc D (2002) Effect of Tween 80 and monensin on ruminal fermentation of the diet containing 70% wheat straw treated by white-rot fungus in artificial rumen. Berl Munch Tierarztl Wochenschr 115:453–457
Kabel MA, Jurak E, Mäkelä MR, Vries RPD (2017) Occurrence and function of enzymes for lignocellulose degradation in commercial Agaricus bisporus cultivation. Appl Microbiol Biotechnol 101:4363–4369. https://doi.org/10.1007/s00253-017-8294-5
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant NO (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym Microb Technol 24:151–159. https://doi.org/10.1016/S0141-0229(98)00101-X
Lin Y, Wu SH, Li X, Wu X, Yang CP, Zeng GM, Peng YR, Zhou Q, Lu L (2018) Microstructure and performance of Z-scheme photocatalyst of silver phosphate modified by MWCNTs and Cr-doped SrTiO3 for malachite green degradation. Appl Catal B Environ 227:557–570. https://doi.org/10.1016/j.apcatb.2018.01.054
Mcintosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 35:3094–3103. https://doi.org/10.1016/j.biombioe.2011.04.018
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Pan R, Cao L, Huang H, Zhang R, Mo Y (2010) Biosorption of Cd, Cu, Pb, and Zn from aqueous solutions by the fruiting bodies of jelly fungi (Tremella fuciformis and Auricularia polytricha). Appl Microbiol Biotechnol 88:997–1005. https://doi.org/10.1007/s00253-010-2821-y
Pant D, Adholeya A (2007) Biological approaches for treatment of distillery wastewater: a review. Bioresour Technol 98:2321–2334. https://doi.org/10.1016/j.biortech.2006.09.027
Pant D, Adholeya A (2009) Nitrogen removal from biomethanated spentwash using hydroponic treatment followed by fungal decolorization. Environ Eng Sci 26:559–565. https://doi.org/10.1089/ees.2007.0328
Pant D, Singh A, Bogaert GV, Olsen SI, Nigam PS, Diels L, Vanbroekhoven K (2012) Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv 2:1248–1263. https://doi.org/10.1039/C1RA00839K
Pirbazari AE, Kisom BF, Khararoodi MG (2015) Anionic surfactant-modified rice straw for removal of methylene blue from aqueous solution. Desalin Water Treat 57:18202–18216. https://doi.org/10.1080/19443994.2015.1090919
Qi B, Chen X, Fei S, Yi S, Wan Y (2009) Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind Eng Chem Res 48(15):7346–7353. https://doi.org/10.1021/ie8016863
Rossana L, Valeria V, Olimpia P, Vincenza F (2016) Bioreactors for lignocellulose conversion into fermentable sugars for production of high added value products. Appl Microbiol Biotechnol 100:597–611. https://doi.org/10.1007/s00253-015-7125-9
Saha BC, Cotta MA (2011) Continuous ethanol production from wheat straw hydrolysate by recombinant ethanologenic Escherichia coli strain FBR5. Appl Microbiol Biotechnol 90:477–487. https://doi.org/10.1007/s00253-010-3082-5
Saini JK, Saini R, Tewari L (2014) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353. https://doi.org/10.1007/s13205-014-0246-5
Satpathy SK, Tabil LG, Meda V, Naik SN, Prasad R (2014) Torrefaction of wheat and barley straw after microwave heating. Fuel 124:269–278. https://doi.org/10.1016/j.fuel.2014.01.102
Sitepu IR, Jin MJ, Fernandez JE, Sousa LDC, Balan V, Boundymill KL (2014) Identification of oleaginous yeast strains able to accumulate high intracellular lipids when cultivated in alkaline pretreated corn stover. Appl Microbiol Biotechnol 98:7645–7657. https://doi.org/10.1007/s00253-014-5944-8
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templaton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory. Laboratory analytical procedure. Technical report NREL/TP-510-42618
Thomsen MH, Thygesen A, Thomsen AB (2009) Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl Microbiol Biotechnol 83:447–455. https://doi.org/10.1007/s00253-009-1867-1
Unrean P, Khajeeram S, Laoteng K (2015) Systematic optimization of fed-batch simultaneous saccharification and fermentation at high-solid loading based on enzymatic hydrolysis and dynamic metabolic modeling of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 100:2459–2470. https://doi.org/10.1007/s00253-015-7173-1
Wang JL, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Wang W, Kang L, Wei H, Arora R, Lee YY (2011) Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol 164:1139–1149. https://doi.org/10.1007/s12010-011-9200-8
Wang H, Yuan XZ, Wu ZB, Wang LL, Peng X, Leng LJ, Zeng GM (2014) Removal of basic dye from aqueous solution using Cinnamomum camphora sawdust: kinetics, isotherms, thermodynamics, and mass-transfer processes. Sep Sci Technol 49:2689–2699. https://doi.org/10.1080/01496395.2014.940590
Wu SH, Shen ZQ, Yang CP, Zhou YX, Li X, Zeng GM, Ai SJ, He HJ (2017) Effects of C/N ratio and bulking agent on speciation of Zn and Cu and enzymatic activity during pig manure composting. Int Biodeterior Biodegrad 119:429–436. https://doi.org/10.1016/j.ibiod.2016.09.016
Wu X, He HJ, Yang WL, Yu JP, Yang CP (2018) Efficient removal of atrazine from aqueous solutions using magnetic Saccharomyces cerevisiae bionanomaterial. Appl Microbiol Biotechnol 102:7597–7610. https://doi.org/10.1007/s00253-018-9143-x
Xu J, Thomsen MH, Thomsen AB (2010) Investigation of acetic acid-catalyzed hydrothermal pretreatment on corn stover. Appl Microbiol Biotechnol 86:509–516. https://doi.org/10.1007/s00253-009-2340-x
Yang CP, Wang JQ, Lei M, Xie GX, Zeng GM, Luo SL (2010) Biosorption of zinc(II) from aqueous solution by dried activated sludge. J Environ Sci 22:675–680. https://doi.org/10.1016/S1001-0742(09)60162-5
Yang CP, Qian H, Li X, Cheng Y, He HJ, Zeng GM, Xi JY (2018) Simultaneous removal of multi-component VOCs in biofilters. Trends Biotechnol 36:673–685. https://doi.org/10.1016/j.tibtech.2018.02.004
Yu T, Deng YH, Liu HY, Yang CP, Wu BW, Zeng GM, Lu L, Nishimura F (2017) Effect of alkaline microwaving pretreatment on anaerobic digestion and biogas production of swine manure. Sci Rep 7:1668. https://doi.org/10.1038/s41598-017-01706-3
Yu JP, He HJ, Yang WL, Yang CP, Zeng GM, Wu X (2018) Magnetic bionanoparticles of Penicillium sp. yz11-22N2 doped with Fe3O4 and encapsulated within PVA-SA gel beads for atrazine removal. Bioresour Technol 260:196–203. https://doi.org/10.1016/j.biortech.2018.03.103
Zeng XY, Ma YT, Ma LR (2007) Utilization of straw in biomass energy in China. Renew Sust Energ Rev 11:976–987. https://doi.org/10.1016/j.rser.2005.10.003
Zhang X, Xu J, Cheng JJ (2011) Pretreatment of corn stover for sugar production with combined alkaline reagents. Energy Fuel 25:4796–4802. https://doi.org/10.1021/ef201130d
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827. https://doi.org/10.1007/s00253-009-1883-1
Zhou BW, Seunggu S, Kwanghyun H, Johnghwa A, Seokhwan H (2010a) Effect of microwave irradiation on cellular disintegration of gram positive and negative cells. Appl Microbiol Biotechnol 87:765–770. https://doi.org/10.1007/s00253-010-2574-7
Zhou Y, Stuartwilliams H, Farquhar GD, Hocart CH (2010b) The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 71:982–993. https://doi.org/10.1016/j.phytochem.2010.03.001
Zhou Q, Lin Y, Li X, Yang CP, Han ZF, Zeng GM, Lu L, He SY (2018) Effect of zinc ions on nutrient removal and growth of Lemna aequinoctialis from anaerobically digested swine wastewater. Bioresour Technol 249:457–463. https://doi.org/10.1016/j.biortech.2017.10.044
Zhu CY, Yang WL, He HJ, Yang CP, Yu JP, Wu X, Zeng GM, Tarre S, Green M (2018) Preparation, performance and mechanisms of magnetic Saccharomyces cerevisiae bionanocomposites for atrazine removal. Chemosphere 200:380–387. https://doi.org/10.1016/j.chemosphere.2018.02.020
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51478172, 51278464, 51521006), the Natural Science Foundation of Zhejiang Province of China (Grant No. LY17E080002), and Department of Science and Technology of Hunan Province of China (Grant No. 2017JJ2029), and the International S&T Cooperation Program of China (Grant No. 2015DFG92750).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animal or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wu, M., Liu, H., Guo, J. et al. Enhanced enzymatic hydrolysis of wheat straw by two-step pretreatment combining alkalization and adsorption. Appl Microbiol Biotechnol 102, 9831–9842 (2018). https://doi.org/10.1007/s00253-018-9335-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9335-4