Abstract
The specific targeting of immunotoxins enables their wide application in cancer therapy. The A-chain of the ricin protein (RTA) is an N-glycosidase that catalyzes the removal of adenine from the 28S rRNA, preventing protein translation and leading to cell death. Ricin is highly toxic but can only exert its toxic effects from within the cytoplasm. In this study, we linked the anti-HER2 single-chain variable fragment 4D5 scFv and the endoplasmic reticulum-targeting peptide KDEL to the C-terminal of the RTA to construct immunotoxin RTA-4D5-KDEL. In vitro experiments showed that the anticancer effect of RTA-4D5-KDEL towards ovarian cancer cells SKOV-3 increased 440-fold and 28-fold relative to RTA and RTA-4D5, respectively. RTA-4D5-KDEL had a strong inhibitory effect on HER2-overexpressing SKOV-3 cells and caused little damage to normal HEK-293 cells and H460 lung cancer cells. Immunofluorescence experiments showed that the immunotoxin RTA-4D5 could specifically bind to SKOV-3 cells, but not to normal cells HEK-293. The immunotoxin RTA-4D5-KDEL could rapidly localize the recombinant protein to the endoplasmic reticulum. These results suggest that the recombinant immunotoxin RTA-4D5-KDEL has a strong inhibitory effect on ovarian cancer cells that overexpress HER2 but little harm to the normal cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ricin is a protein toxin extracted from the castor bean plant, Ricinus communis, containing two chains, A and B, that are connected through a disulfide bond (Fodstad et al. 1984). The A-chain (RTA) is the toxic chain consisting of 263 amino acids, and its molecular weight is 32 kD. It has N-glycoside activity, which can catalyze the 28S rRNA at the 4234-site to remove an adenine, leading to protein translation arrest and cell apoptosis (Endo et al. 1987). The B-chain (RTB) is one type of galactose-specific lectin, containing two galactose binding sites that could be combined with galactose residues on the surface of cell membrane glycoproteins or glycolipid molecules (Vitetta and Thorpe 1991). Ricin gets into cells through endocytosis by binding of the galactose-binding site of the RTB to the cell surface receptor containing galactose residue (Barbieri et al. 1993). Therefore, ricin passes through cell membrane vesicles via receptor-mediated endocytosis, translocates to the trans-Golgi network (TGN), and then undergoes retrograde translocation to the endoplasmic reticulum (ER) (Olsnes 2004). Next, RTA is released from RTB by protein disulfide isomerase (PDI) and transferred to the host cytoplasm by Sec61 in the ER (Spooner et al. 2008; Spooner and Lord 2012). Recent studies have shown that RTA can directly enter cells and plays a toxic role independent of RTB (Newton et al. 1992).
The treatment of diseases with ricin has a long history. Lin et al. (1970) confirmed that ricin has a preventive effect on the proliferation of Ehrlich ascites carcinoma. Since then, the antitumor effects of ricin have been demonstrated in many experimental models, such as human tumor cells transplanted into nude mice (Olsnes and Phil 1973). In recent years, ricin and RTA have been used to synthesize immunotoxins for targeted cancer therapy and demonstrate better specific anticancer activity (Lambert et al. 1991; Zhou et al. 2010).
The toxic of ricin is not selective and harmful to all cells. It could be fused with monoclonal antibodies to reduce damage to normal cells. Human epidermal growth factor 2 (HER2) is one of the antigen families that is overexpressed in many human cancers and has therefore become an important target for cancer therapy (Sommaruga et al. 2011). Herceptin is a type of fully humanized monoclonal antibody that treats tumors by binding to HER2 that is overexpressed at tumor cell surface. 4D5 scFv is the single-chain variable fragment of Herceptin which efficiently interacts with HER2 antigen with high thermodynamic stability and easy folding properties (Willuda et al. 1999).
The study of the reverse transport mechanism of protein toxins has shown that many toxins undergo endocytosis, intracellular trafficking, and reverse transmembrane translocation from the ER to the cytoplasm within the cell. Only 5% of ricin in the cell transport pathway arrives in the Golgi apparatus (Dyba et al. 2004). The traffic of ricin to the Golgi and ER is a key to its cytotoxicity (Van Deurs et al. 1988). The KDEL receptor captures the protein in the ER by recognizing the C-terminal tetrapeptide signal KDEL (Lys-Asp-Glu-Leu) (Lewis and Pelham 1990). KDEL is an endoplasmic reticulum-targeting peptide that can be used to target tumor cells using protein drugs.
In this study, 4D5 scFv was constructed as an antibody ligated at the C-terminal of RTA to construct the immunotoxin RTA-4D5. To further facilitate the transport of immunotoxins in cells, KDEL was attached to the C-terminal of RTA-4D5 to construct immunotoxin RTA-4D5-KDEL. The soluble expression of RTA-4D5-KDEL in the Escherichia coli system was achieved by adding small ubiquitin-related modifier (SUMO) fusion tag to the N-terminal of the immunotoxin RTA-4D5-KDEL. Then, the anticancer effect and targeting ability of immunotoxin RTA-4D5-KDEL were tested in vitro for SKOV-3 ovarian cancer cells.
Materials and methods
Materials
The Escherichia coli strains DH5α and BL21 (DE3), plasmid pET28a, were preserved in our laboratory. Restriction endonucleases and DNA polymerase were purchased from Takara (Shanghai, China). T4 DNA ligase was purchased from YEASEN (Shanghai, China). The PCR Purification Kit, Gel Extraction Kit, and Plasmid Mini Kit were obtained from Sigma (Shanghai, China). The Ni-NTA Sepharose FF was from GE (Piscataway, NJ, USA). SKOV-3, HEK-293, and H460 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Annexin V-FITC/PI Apoptosis Detection Kit and Cell Counting Kit (CCK-8) were obtained from YEASEN (Shanghai, China). Other cell detection reagents and biological reagents were purchased from Sigma (Shanghai, China).
Plasmid construction
The genes encoding the 4D5 scFv (GenBank No. KM016462) and SUMO (GenBank No. NM_001180818) were stored in our laboratory. The gene encoding RTA (GenBank No. X52908) (416–1216 bp) was synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China). The flexible linker (G4S)3 was used to fuse 4D5 scFv to the C-terminal of RTA to obtain target gene RTA-4D5. The gene encoding KDEL was connected at the C-terminal of RTA-4D5 to obtain target gene RTA-4D5-KDEL. To enhance the soluble expression level of the recombinant protein in the E. coli system, the SUMO fusion tag was attached at the N-terminal of RTA-4D5-KDEL. Overlapping PCR was used to construct the gene SUMO-RTA-4D5-KDEL. Next, the plasmid pET28a was digested using restriction enzymes NcoI and BamHI and then ligated with the target gene. The primers used in this study are shown in Table 1.
Expression and purification of RTA-4D5-KDEL
The recombinant plasmids were transformed into the E. coli BL21 (DE3) strains. The recombinant strains were cultured in LB medium containing 100 μg/mL kanamycin on a 37 °C shaker. When OD600 reached 0.6–0.8, 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was used to induce protein expression at 18 °C for 20 h. The induced cells were collected through centrifugation and washed in PBS (pH 7.4), then resuspended with PBS containing 10 mM imidazole and disrupted by ultrasonication. The supernatant was purified using the Ni-NTA Sepharose column. The His-tagged fusion protein was eluted by PBS containing different concentrations of imidazole. The eluate containing the SUMO-RTA-4D5-KDEL was dialyzed in PBS at 4 °C. Next, the target protein was digested by SUMO protease and purified again via a Ni-NTA Sepharose column to get rid of His-tagged SUMO protein, SUMO protease, and uncleaved fusion protein. The target protein RTA-4D5-KDEL was harvested in the flow-through section. The purity and concentration of RTA-4D5-KDEL were determined through SDS-PAGE and the Bradford method. RTA-4D5 and RTA were purified using the same method.
Cell proliferation assay
CCK-8 is a rapid and sensitive detection kit based on 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfobenzene)-2H-tetrazole salt (WST-8) and which is widely used in cytotoxicity assays. The cells were diluted with RPMI 1640 medium containing 10% fetal bovine serum and then added to 96-well plates at an amount of 1 × 104 per well, and incubated at 37 °C in 5% CO2 overnight. RTA, RTA-4D5, and RTA-4D5-KDEL in different concentrations were seeded at each well. After incubation for 3 days, 10 μL of CCK-8 was added and incubated at 37 °C for 4 h. The absorbance at 450 nm was detected using a microplate spectrophotometer.
Detection of apoptosis by flow cytometry
Flow cytometry was performed to observe the apoptotic effect of cancer cells that were treated with RTA, RTA-4D5, and RTA-4D5-KDEL. The cells were added to 6-well plates at 1 × 106 per well and incubated overnight at 37 °C in 5% CO2. Different concentrations of recombinant protein were incubated with cells for 2 days. The cells were digested by trypsin without EDTA and harvested by centrifugation. The collected cells were washed three times by pre-cooled PBS and resuspended with 100 μL binding buffer. Next, 5 μL of Annexin V-FITC and 10 μL PI staining solution were added and incubated at room temperature for 10 min. Cell apoptosis was quantified by flow cytometry (BD FACSCalibur™) and analyzed by Flow Jo 7.6.1 software (Zhang et al. 2016).
Recombinant protein localization
To determine the selectivity and targeting ability of the recombinant proteins RTA-4D5 and RTA-4D5-KDEL, immunofluorescence was used to monitor their intracellular localization. RTA-4D5 and RTA-4D5-KDEL were labeled with fluorescein isothiocyanate (FITC). First, the target protein was dialyzed against bicarbonate buffer (pH 9.0) three times, and FITC was added at a ratio of 1000:15 (protein: FITC), and then incubated at 4 °C for 8 h in darkness. 50 mM ammonium chloride was then added to stop the reaction for 2 h. The mixture was dialyzed with PBS (pH 7.4) to eliminate the unbonded FITC.
To observe the selectivity of RTA-4D5, the cells were seeded onto confocal dishes (20 mm) at 1 × 105 and cultured for 24 h. After 5 μg/mL FITC-labeled RTA-4D5 was added and incubated for 1 h, the cells were fixed with 4% paraformaldehyde for 15 min, and then 1 mL Hoechst 33258 was added to stain the nuclei for 15 min. Finally, the cells were observed using laser scanning confocal microscopy.
To determine whether RTA-4D5-KDEL could be localized in the ER, the cells inoculated in confocal dishes were incubated with 5 μg/mL FITC-labeled RTA-4D5-KDEL for 12 h. Cells were fixed and permeabilized by 0.2% Triton X-100 in PBS buffer at room temperature for 10 min. Then, cells were stained with Texas Red (TR)-conjugated anti-calreticulin antibody for 1.5 h at 37 °C. The cells were washed with TBST buffer and observed under a laser scanning confocal microscope.
Statistical analysis
Statistical analysis was performed by Student’s t test. Values were expressed as mean ± standard error (mean ± SE) from at least three experiments. A level of P < 0.05 was considered to be statistically significant.
Results
Construction and expression of recombinant immunotoxins
We constructed the recombinant plasmids containing the SUMO tag by overlapping PCR and one-step cloning. The primer design and construction of recombinant immunotoxin genes are shown in Fig. 1. The recombinant plasmids were checked for insertion of the target genes by sequencing.
Schematic representation of expression vectors. a Construction of SUMO-RTA-4D5-KDEL. b Construction of SUMO-RTA-4D5. c Construction of SUMO-RTA. Schematic diagram of target constructs containing His6 tag (His), SUMO fusion tag (SUMO), ricin A chain (RTA), single-chain variable fragment (4D5) scFv, and endoplasmic reticulum-targeting peptide (KDEL)
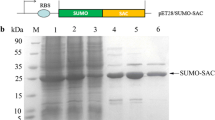
The recombinant expression strain containing the desired plasmid was induced with 0.1 mM IPTG at 18 °C, and protein expression level was detected by SDS-PAGE (Fig. 2). The results indicated that fusion proteins SUMO-RTA-4D5-KDEL, SUMO-RTA-4D5, and SUMO-RTA were mostly soluble expressed. Through Gel Pro Analyzer 4.0 analysis, SUMO-RTA and SUMO-RTA-4D5 accounted for 40.9 and 35.7% of soluble lysate fraction, respectively (Fig. 2a, b), and SUMO-RTA-4D5-KDEL in the supernatant was about 10% (Fig. 2c).
SDS-PAGE analysis of the expression of recombinant immunotoxin. a The expression of E. coli containing pET28a/SUMO-RTA induced at 18 °C for 20 h. Lane M, protein marker; lane 1, total cellular lysate of E. coli containing pET28a; lane 2, supernatant of lane 1; lane 3, total cellular lysate of E. coli containing pET28a/SUMO-RTA; lane 4, supernatant of lysate; lane 5, insoluble lysate. b The expression of E. coli containing pET28a/SUMO-RTA-4D5 induced at 18 °C for 20 h. Lane 1, total cellular lysate; lane 2, supernatant of lysate; lane 3, insoluble lysate; lane M, protein marker. c The expression of E. coli BL21 containing pET28a/SUMO-RTA-4D5-KDEL induced at 18 °C for 20 h. Lane M, protein marker; lane 1, total cellular lysate; lane 2, supernatant of lysate
Purification of RTA-4D5-KDEL
SUMO-RTA-4D5-KDEL was soluble expressed, thus the SUMO-tagged recombinant protein was purified using a Ni-NTA resin. The supernatant of the lysate was applied to the resin at a flow rate of 1 mL/min, and then the target protein containing the SUMO tag was eluted with 300 mM imidazole (Fig. 3c). The imidazole eluted was removed by dialysis against PBS, and then the fusion protein was digested by SUMO protease to remove the SUMO tag. The mixture was further purified using a Ni-NTA resin to obtain the target protein RTA-4D5-KDEL (Fig. 3e). The purification of RTA and RTA-4D5 was the same as that of RTA-4D5-KDEL (Fig. 3a, b, d). The purity of RTA-4D5-KDEL, RTA-4D5, and RTA was above 90%, and the yields were 10 mg/L, 15 mg/L, and 20 mg/L, respectively.
Purification of recombinant immunotoxin. a The purification of SUMO-RTA. Lanes 1–2, proteins eluted by 200 mM and 300 mM imidazole. b The purification of SUMO-RTA-4D5. Lanes 1–2, proteins eluted by 200 mM and 300 mM imidazole. c The purification of SUMO-RTA-4D5-KDEL. Lane 1, total cellular lysate; lane 2, supernatant lysate; lane 3, unbounded proteins; lanes 4–7, proteins eluted by 50 mM, 100 mM, 200 mM, and 300 mM imidazole, respectively. d The digestion and purification of RTA-4D5 and RTA. Lane 1, SUMO-RTA digested by SUMO protease; lane 2, purified RTA; lanes 3–4, eluted SUMO tag; lane 5, purified RTA-4D5; lane 6, SUMO-RTA-4D5 digested by SUMO protease. e The digestion and purification of RTA-4D5-KDEL. Lane 1, SUMO-RTA-4D5-KDEL digested by SUMO protease; lanes 2–3, purified RTA-4D5-KDEL; lane 4, eluted SUMO tag
RTA-4D5-KDEL significantly inhibits the proliferation of HER2-overexpressing cancer cells
The cell proliferation inhibition effect of recombinant proteins RTA-4D5-KDEL, RTA-4D5, and RTA was detected by CCK-8. The results showed that the inhibitory effects of RTA and RTA-4D5 on ovarian cells SKOV-3, lung cancer cells H460, and normal cells HEK-293 were concentration-dependent. The IC50 of RTA for SKOV-3 and HEK-293 cells was 11 μg/mL and 2.5 μg/mL, respectively (Fig. 4a). However, when the antibody 4D5 scFv was ligated, the toxicity of RTA-4D5 to SKOV-3 cells increased, while the damage to HEK-293 cells decreased. The IC50 of RTA-4D5 against SKOV-3 and HEK-293 cells was 0.7 μg/mL and 10 μg/mL, respectively (Fig. 4b). This indicated that RTA-4D5 had an enhanced inhibitory effect on HER2-overexpressing cancer cells and reduced the damage to normal cells. Furthermore, the IC50 of RTA-4D5-KDEL on SKOV-3 cells was 25 ng/mL (Fig. 4c), 440-fold and 28-fold lower compared to RTA and RTA-4D5, respectively. In addition, RTA-4D5-KDEL showed almost no damage to HER2-negative H460 cancer cells, even at 5 μg/mL, and the damage to normal cells was also greatly reduced. This indicated that RTA-4D5-KDEL had a higher specificity for HER2-overexpressing cancer cells.
RTA-4D5-KDEL induces apoptosis in cancer cells
Flow cytometry analysis was used to monitor the apoptosis inducing effect of recombinant immunotoxins. It was found that RTA-4D5-KDEL could remarkably induce the apoptosis of SKOV-3 cells (Fig. 5a). One microgram per milliliter of RTA-4D5-KDEL caused 53% early apoptosis and 10% late apoptosis in SKOV-3 cells. However, 1 μg/mL of RTA-4D5 caused a lower apoptosis rate in SKOV-3 than RTA-4D5-KDEL cells, and RTA at the same concentration did not induce apoptosis in SKOV-3 cells. Furthermore, RTA-4D5-KDEL and RTA-4D5 did not induce apoptosis in normal HEK-293 cells even at 1 μg/mL (Fig. 5b). Therefore, RTA-4D5-KDEL strongly induced the apoptosis of HER2-overexpressed SKOV-3 cells but caused almost no damage to normal HEK-293 cells, which was consensus with the results of the CCK-8 assay.
Effects of recombinant immunotoxin on cell apoptosis. The living cells were shown as Annexin V-/PI- (Q4), Annexin V-/PI+ represented mechanically damaged cells (Q1), Annexin V+/PI- represented early apoptotic cells (Q3), and Annexin V+/PI+ represented late apoptotic and necrotic cells (Q2). a The recombinant protein induces apoptosis of SKOV-3 cells. SKOV-3 cells were treated with RTA, RTA-4D5, and RTA-4D5-KDEL for 2 days and then labeled by Annexin V-FITC/PI for apoptosis analysis by flow cytometry. b The recombinant protein induces apoptosis of HEK-293 cells. HEK-293 cells were treated with RTA, RTA-4D5, and RTA-4D5-KDEL for 2 days and then labeled by Annexin V-FITC/PI for apoptosis analysis by flow cytometry
Specific selectivity and ER-targeting ability of RTA-4D5-KDEL
To detect the selectivity of immunotoxins, SKOV-3 and HEK-293 cells were incubated with FITC-labeled RTA-4D5 for 1 h. After the nuclei were stained with Hoechst 33258, protein localization was observed using confocal microscopy. As shown in Fig. 6, RTA-4D5 can specifically bind to the cell surface of SKOV-3, whereas no fluorescence was observed on HEK-293 cells (Fig. 6). However, RTA was barely observed on the surface of SKOV-3 and HEK-293 cells at the same concentration. Therefore, this suggests that RTA fused with 4D5 scFv can be specifically combined with HER2-overexpressed cancer cells.
Targeted selectivity analysis of RTA-4D5 in cancer cells. Cells were incubated with FITC-labeled RTA-4D5 or RTA at 37 °C for 1 h and then stained with Hoechst 33258 for 20 min. The blue color represents the nucleus stained with Hoechst 33258, and the green color represents the FITC-labeled target proteins
Immunofluorescence and confocal microscopy were used to observe the localization of RTA-4D5-KDEL in the ER. After incubation for 12 h, most of the RTA-4D5-KDEL was localized at the ER in SKOV-3 cells, whereas RTA-4D5 was dispersed in the cytoplasm (Fig. 7a). In addition, RTA-4D5 and RTA-4D5-KDEL were barely observed in HEK-293 cells on account of the lack of HER2 at cell surface (Fig. 7b). This suggests that RTA-4D5 fused with the ER-targeting tetrapeptide KDEL can be rapidly localized in the ER to induce apoptosis in cancer cells.
Laser confocal microscopy analysis of the localization of RTA-4D5-KDEL in cells. a Laser confocal microscopy detection of RTA-4D5-KDEL localized to ER of SKOV-3 cells. b Laser confocal microscopy detection of RTA-4D5-KDEL localized to ER of HEK-293 cells. The green color represents the FITC-labeled proteins, and the red color represents the endoplasmic reticulum (ER) stained with Texas Red (TR)-conjugated anti-calreticulin antibody. The yellow color represents the colocalization of the target protein and ER
Discussion
Immunotoxins have become a hot topic of research since they were successfully constructed (Pincus et al. 2001). Immunotoxins are fusion proteins of different target-specific antibodies or growth factors fused with toxins (Blythman et al. 1981). Therefore, immunotoxins have targeted selectivity and antitumor properties. Recently, bacterial toxins, phytotoxins, and ricin A-chains have been coupled as toxin moieties with monoclonal antibodies to form immunotoxins for cancer treatment (Schnell et al. 2002; Kreitman 2009).
RTA has N-glycosidase activity and can disrupt protein synthesis, leading to apoptosis. RTA is highly efficient; one molecule of RTA can inactivate thousands of ribosomes in 1 min (Olsnes et al. 1975). The cytotoxicity of ricin is only exerted after it is reversely transported to the cytoplasm by internalization. Therefore, RTA must be transported to the cytoplasm to kill tumor cells. Recently, some protein signal sequences have been found to mediate the introduction and retention of proteins in specific organelles during drug transportation. It has been demonstrated that the C-terminal of protein within the ER contains the tetrapeptide sequence KDEL, which localizes the protein to the ER mainly through binding to the KDEL receptor (Wales et al. 1992). Studies have confirmed that the fusion of ER-targeting tetrapeptide KDEL to the C-terminal of RTA can remarkably increase the cell-killing effect and translocation efficiency of RTA (Munro and Pelham 1987; Zhan et al. 2004). HER2 is overexpressed in many solid tumors, for example in 25–30% of breast cancers and in 20% of ovarian cancers, but is weakly expressed in normal epithelial tissues (Press et al. 1990). Therefore, HER2 is one of the main targets of cancer treatment (Sommaruga et al. 2011). Here, in order to enhance the targeting ability of RTA to cancer cells meanwhile to decrease the damage done towards normal cells, the anti-HER2 single-chain variable fragment 4D5 scFv and the ER positioning peptides KDEL were fused to the C-terminal of RTA to prepare a recombinant immunotoxin RTA-4D5-KDEL which could specifically target the tumor cells and rapidly localize to the ER. But RTA-4D5-KDEL exists as inclusion bodies in the E. coli system. Recent studies have shown that the SUMO tag linked at the N-terminal of the recombinant protein promotes the soluble expression of heterologous proteins in the E. coli system (Satakarni and Curtis 2011; Zhang et al. 2015; Lv et al. 2016). Furthermore, the SUMO tag was fused at the N-terminal of RTA-4D5-KDEL to facilitate its soluble expression.
Previous studies demonstrated that ricin has a preventive effect on the proliferation of cancer cells (Wales et al. 1993). The results of the CCK-8 assay indicated that the IC50 of RTA-4D5-KDEL in SKOV-3 cells was 25 ng/mL, while the IC50 of RTA-4D5 and RTA in SKOV-3 cells was 0.7 μg/mL and 11 μg/mL, respectively. Therefore, the anticancer effect of RTA-4D5 was increased 15-fold relative to RTA, while the anticancer effect of RTA-4D5-KDEL increased 28-fold and 440-fold compared to RTA-4D5 and RTA, respectively. However, RTA-4D5 still had a certain inhibitory effect towards normal cells HEK-293, but RTA-4D5-KDEL showed almost no damage towards normal cells HEK-293 and lung cancer cells H460 at the same concentration. It has been reported that the ER-located molecular chaperone GRP 78 contains an endoplasmic reticulum-locating tetrapeptide sequence KDEL that localized it in the ER. GRP 78 is preferentially positioned on the surface of cancer cells but not normal cells or organs (Munro and Pelham 1986; Tsai et al. 2015). Thus, we speculated that RTA-4D5-KDEL may bind not only to HER2 at the surface of SKOV-3 cells but also to GRP 78, further enhancing the binding of RTA-4D5-KDEL to SKOV-3 cells. This increased the amount of RTA-4D5-KDEL that enters SKOV-3 cells to exert a toxic effect. These results showed that RTA-4D5-KDEL exerted a strong proliferation inhibition effect on SKOV-3 cells that were overexpressing HER2 but had almost no apparent cytotoxicity to HER2-negative H460 and HEK-293 cells. In addition, flow cytometry analysis indicated that RTA-4D5-KDEL caused tumor cell death mainly through apoptosis and necrosis. RTA-4D5-KDEL induced more apoptosis in SKOV-3 cells than RTA and RTA-4D5.
Based on the above results, we used immunofluorescence experiments to demonstrate the targeting of RTA-4D5-KDEL to ovarian cancer cells SKOV-3. We determined that RTA-4D5 could effectively accumulate on the surface of SKOV-3 cells, but not HEK-293 cells. Furthermore, RTA-4D5-KDEL could rapidly localize to the ER of SKOV-3 cells, whereas the RTA-4D5 was dispersed at the cytoplasm. Therefore, the RTA that linked with the 4D5 scFv could specifically bind to HER2-overexpressed ovarian cancer cells SKOV-3 but not lung cancer cells H460 and normal cells HEK-293, and the fusion of ER-targeting tetrapeptide with the RTA-4D5 could rapidly localize the recombinant protein to the endoplasmic reticulum to increase the anticancer activity of the toxin toward cancer cells.
In summary, the recombinant immunotoxin RTA-4D5-KDEL is shown to have a targeted and potent proliferation inhibition effect on SKOV-3 cells that overexpress HER2 and can rapidly localize to the ER to enhance its anticancer activity, but almost no obvious cytotoxicity on HER2-negative cells. Therefore, RTA-4D5-KDEL could be used as a candidate for the therapy of HER2-positive tumor cells.
References
Barbieri L, Batteli MG, Stripe F (1993) Ribosome-inactivating proteins from plants. Biochim Biophys Acta 1154:237–282. https://doi.org/10.1016/0304-4157(93)90002-6
Blythman HE, Casellas P, Gros O, Gros P, Jansen FK, Paolucci F, Pau B, Vidal H (1981) Immunotoxins: hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 290:145–146. https://doi.org/10.1038/290145a0
Dyba M, Tarasova NI, Michejda CJ (2004) Small molecule toxins targeting tumor receptors. Curr Pharm Des 10:2311–2334. https://doi.org/10.2174/1381612043384024
Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 262:5908–5912
Fodstad O, Kvalheim G, Godal A, Lotsberg J, Aamdal S, Høst H, Pihl A (1984) Phase I study of the plant protein ricin. Cancer Res 44:862–865
Kreitman RJ (2009) Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies. BioDrugs 23:1–13. https://doi.org/10.2165/00063030-200923010-00001
Lambert JM, Goldmacher VS, Collinson AR, Nadler LM, Blättler WA (1991) An immunotoxin prepared with blocked ricin: a natural plant toxin adapted for therapeutic use. Cancer Res 51:6236–6242
Lewis MJ, Pelham HR (1990) A human homologue of the yeast HDEL receptor. Nature 348:162–163. https://doi.org/10.1038/348162a0
Lin JY, Tserng KY, Chen CC, Lin LT, Tung TC (1970) Abrin and ricin: new anti-tumor substances. Nature 227:292–293. https://doi.org/10.1038/227292a0
Lv XX, Zhang J, Xu R, Dong YG, Sun AY, Shen YL, Wei DZ (2016) Gigantoxin-4-4D5 scFv is a novel recombinant immunotoxin with specific toxicity against HER2/neu-positive ovarian carcinoma cells. Appl Microbiol Biotechnol 100:6403–6413. https://doi.org/10.1007/s00253-016-7487-7
Munro S, Pelham HR (1986) An Hsp70-like protein in the ER: identity with the 78 kD glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46:291–300. https://doi.org/10.1016/0092-8674(86)90746-4
Munro S, Pelham HR (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899–907. https://doi.org/10.1016/0092-8674(87)90086-9
Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, Ackerman EJ, Roberts LM, Lord JM, Youle RJ (1992) Cell surface and intracellular functions for ricin galactose binding. J Biol Chem 267:11917–11922
Olsnes S (2004) The history of ricin, abrin, and related toxins. Toxicon 44:361–370. https://doi.org/10.1016/j.toxicon.2004.05.003
Olsnes S, Phil A (1973) Different biological properties of the two constituent peptide chains of ricin a toxic protein inhibiting protein synthesis. Biochemistry 12:3121–3126. https://doi.org/10.1021/bi00740a028
Olsnes S, Fernandez-Puentes C, Carrasco L, Vazquez D (1975) Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem 60:281–288. https://doi.org/10.1111/j.1432-1033.1975.tb21001.x
Pincus SH, Marcotte TK, Forsyth BM, Fang H (2001) In vivo testing of anti-HIV immunotoxins. Methods Mol Biol 166:277–294
Press MF, Cordon-Cardo C, Slamon DJ (1990) Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5:953–962
Satakarni M, Curtis R (2011) Production of recombinant peptides as fusions with SUMO. Protein Expr Purif 78:113–119. https://doi.org/10.1016/j.pep.2011.04.015
Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, Schindler J, Ghetie V, Vitetta ES, Diehl V, Engert A (2002) A phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res 8:1779–1786
Sommaruga S, Lombardi A, Salvadè A, Mazzucchelli S, Corsi F, Galeffi P, Tortora P, Prosperi D (2011) Highly efficient production of anti-HER2 scFv antibody variant for targeting breast cancer cells. Appl Microbiol Biotechnol 91:613–621. https://doi.org/10.1007/s00253-011-3306-3
Spooner RA, Lord JM (2012) How ricin and Shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. Curr Top Microbiol Immunol 357:19–40. https://doi.org/10.1007/82_2011_154
Spooner RA, Hart PJ, Cook JP, Pietroni P, Rogon C, Höhfeld J, Roberts LM, Lord JM (2008) Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc Natl Acad Sci U S A 15:17408–17413. https://doi.org/10.1073/pnas.0809013105
Tsai YL, Zhang Y, Tseng CC, Stanciauskas R, Pinaud F, Lee AS (2015) Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose regulated protein (GRP78) to the cell surface. J Biol Chem 290:8049–8064. https://doi.org/10.1074/jbc.M114.618736
Van Deurs B, Sandvig K, Petersen OW, Olsnes S, Simons K, Griffiths G (1988) Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol 106:253–267. https://doi.org/10.1083/jcb.106.2.253
Vitetta ES, Thorpe PE (1991) Immunotoxins containing ricin or its A chain. Semin Cell Biol 2:47–58
Wales R, Chaddock JA, Roberts LM, Lord JM (1992) Addition of an ER retention signal to the ricin A chain increases the cytotoxicity of the holotoxin. Exp Cell Res 203:1–4. https://doi.org/10.1016/0014-4827(92)90032-4
Wales R, Roberts LM, Lord JM (1993) Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J Biol Chem 268:23986–23990
Willuda J, Honegger A, Waibel R, Schubiger PA, Stahel R, Zangemeister-Wittke U, Plückthun A (1999) High thermal stability is essential for tumor targeting of antibody fragments: engineering of a humanized anti-epithelial glycoprotein-2 (epithelial cell adhesion molecule) single-chain Fv fragment. Cancer Res 59:5758–5767
Zhan JB, Chen YD, Wang KY, Zheng S (2004) Expression of ricin A chain and ricin A chain-KDEL in Escherichia coli. Protein Expr Purif 34:197–201. https://doi.org/10.1016/j.pep.2003.11.009
Zhang J, Lv XX, Xu R, Tao XY, Dong YG, Sun AY, Wei DZ (2015) Soluble expression, rapid purification, and characterization of human interleukin-24 (IL-24) using a MBP-SUMO dual fusion system in Escherichia coli. Appl Microbiol Biotechnol 99:6705–6713. https://doi.org/10.1007/s00253-015-6441-4
Zhang J, Sun AY, Xu R, Tao XY, Dong YG, Lv XX, Wei DZ (2016) Cell-penetrating and endoplasmic reticulum-locating TAT-IL-24-KDEL fusion protein induces tumor apoptosis. J Cell Physiol 231:84–93. https://doi.org/10.1002/jcp.25054
Zhou XX, Ji F, Zhao JL, Cheng LF, Xu CF (2010) Anti-cancer activity of anti-p185HER-2 ricin A chain immunotoxin on gastric cancer cells. J Gastroenterol Hepatol 25:1266–1275. https://doi.org/10.1111/j.1440-1746.2010.06287.x
Funding
This work was funded by the National Natural Science Foundation of China (No. 21778018 and No. 21706072) and the National Special Fund for the State Key Laboratory of Bioreactor Engineering (2060204).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jiao, P., Zhang, J., Dong, Y. et al. Construction and characterization of the recombinant immunotoxin RTA-4D5-KDEL targeting HER2/neu-positive cancer cells and locating the endoplasmic reticulum. Appl Microbiol Biotechnol 102, 9585–9594 (2018). https://doi.org/10.1007/s00253-018-9291-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9291-z