Abstract
Glioblastoma, characterized by extensive microvascular proliferation and invasive tumor growth, is one of the most common and lethal malignancies in adults. Benefits of the conventional anti-angiogenic therapy were only observed in a subset of patients and limited by diverse relapse mechanism. Fortunately, recent advances in cancer immunotherapy have offered new hope for patients with glioblastoma. Herein, we reported a novel dual-targeting therapy for glioblastoma through simultaneous blockade of VEGF and CD47 signaling. Our results showed that VEGFR1D2-SIRPαD1, a VEGF and CD47 bispecific fusion protein, exerted potent anti-tumor effects via suppressing VEGF-induced angiogenesis and activating macrophage-mediated phagocytosis. Meanwhile, autophagy was activated by VEGFR1D2-SIRPαD1 through inactivating Akt/mTOR and Erk pathways in glioblastoma cells. Importantly, autophagy inhibitor or knockdown of autophagy-related protein 5 potentiated VEGFR1D2-SIRPαD1-induced macrophage phagocytosis and cytotoxicity against glioblastoma cells. Moreover, suppression of autophagy led to increased macrophage infiltration, angiogenesis inhibition, and tumor cell apoptosis triggered by VEGF and CD47 dual-targeting therapy, thus eliciting enhanced anti-tumor effects in glioblastoma. Our data revealed that VEGFR1D2-SIRPαD1 alone or in combination with autophagy inhibitor could effectively elicit potent anti-tumor effects, highlighting potential therapeutic strategies for glioblastoma through disrupting angiogenetic axis and CD47-SIRPα anti-phagocytic axis alone or in combination with autophagy inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is an incurable highly vascularized brain tumor characterized by aggressive proliferation of endothelial cells and abundance of disorganized microvessels causing progressive neurologic deterioration and death (Hu et al. 2016; Sareddy et al. 2017). Despite the existence of multimodality therapies, including surgical resection, chemotherapy, and radiotherapy, the prognosis of patients is still poor, with overall survival (OS) around 2 years (Kim et al. 2015). Thus, novel strategies for the therapy of glioblastoma patients are urgently required.
Vascular endothelial growth factor (VEGF), also named as vascular permeability factor, is a signal protein that promotes vasculogenesis and angiogenesis. For tumor cells, VEGF is secreted to promote tumor neo-angiogenesis and tumor progression (Boucher and Bautch 2014; Senger et al. 1983). Agents such as small molecular inhibitors, soluble VEGF receptor (VEGFR) Fc fusion, and monoclonal antibodies have shown clinical benefits for cancer patients through vessel regression in addition to preventing further VEGF-dependent outgrowth of new vessels (Bakas et al. 2017; Hagberg et al. 2012; Peng et al. 2017), while, in tumor-bearing hosts, high levels of VEGF facilitate tumor cells to evade immunological destruction by inhibiting the functions of leukocytes including macrophages, dendritic cells, and T cells (Allen et al. 2017). Therefore, activating immune responses would benefit the anti-tumor efficacy of anti-angiogenesis therapy. Emerging studies have identified CD47 as an immune checkpoint molecule that is overexpressed and correlated to poor prognosis in various human tumors, including glioma and glioblastoma (Chao et al. 2011; Vonderheide 2015; Willingham et al. 2012). CD47 binding to signal-regulatory protein alpha (SIRPα) initiates signaling cascade that transmits “don’t eat me” signal to macrophage and renders cancer cells resistant to immune surveillance (Willingham et al. 2012). Disruption of CD47-SIRPα axis by fusion protein SIRPαFc induced potent inhibitory effect against various solid tumors (Gholamin et al. 2017; Willingham et al. 2012). Based on the above mechanisms and the potent anti-tumor activities, SIRPαD1, the first extracellular domain of SIRPα, was fused to VEGFR1D2, the second extracellular domain of VEGFR1, to generate a novel fusion protein VEGFR1D2-SIRPαD1. And we hypothesized that simultaneously targeting VEGF and CD47 by VEGFR1D2-SIRPαD1 could elicit potent anti-tumor effects in glioblastoma.
Although clinical trials of anti-angiogenesis and CD47-based immunotherapy have shown their potential to control tumor, multiple mechanisms of therapy resistance exist in tumors (De Henau et al. 2016; Jung et al. 2017; Wen et al. 2015). Autophagy, a lysosome-dependent recycling process, could be activated in various stress conditions, including hypoxia, nutrient deprivation, and drug treatment (Rubinsztein et al. 2012). Investigation on the roles of autophagy in cancer therapy is increasing, and several data showed that many anti-tumor agents, including vismodegib, temozolomide, and asparaginase, activated cytoprotective autophagy in the treated cancer cells (Shen et al. 2017; Zeng et al. 2015; Zhang et al. 2016). Anti-angiogenesis agents could decrease and disrupt tumor vasculature and then reduce blood and nutrition supply, which subsequently induced cytoprotective autophagy in tumor cells against anti-angiogenesis therapy (Lee et al. 2014; Stanton et al. 2013). Previous studies reported that hypoxia-activated autophagy promoted glioblastoma cell and colon cancer cell survival and adaptation to anti-angiogenic treatment, and inhibition of autophagy by chloroquine could sensitize solid tumors to anti-angiogenic therapy, indicating that autophagy inhibitors might help prevent resistance to anti-angiogenic treatment in the clinic (Hu et al. 2012; Selvakumaran et al. 2013). Meanwhile, CD47 blockade triggered cytoprotective autophagy in non-small cell lung cancer (NSCLC) and glioblastoma (Zhang et al. 2018; 2017). Depleting autophagy significantly enhanced the anti-tumor efficacy of the above targeted agents. Thus, it is conceivable that autophagy participates in VEGF and CD47 bispecific therapy and targeting autophagy could increase the anti-tumor effects of VEGFR1D2-SIRPαD1 in glioblastoma.
In this study, we assessed the anti-tumor effects of dual-targeting therapy through simultaneously disrupting VEGF/VEGFR angiogenetic axis and CD47-SIRPα anti-phagocytic axis, and investigated the role of autophagy in VEGF/CD47 dual-specific therapy. Our data for the first time showed that VEGFR1D2-SIRPαD1 had significant anti-tumor effects in glioblastoma via anti-angiogenesis and activation of innate immune responses. Cytoprotective autophagy was activated during the treatment and inhibiting autophagy strengthened the anti-tumor effects by increased recruitment of macrophages, and apoptosis activation. The data elucidated the significant anti-tumor effects of VEGFR1D2-SIRPαD1, highlighting the promising therapeutics for glioblastoma by targeting VEGF and CD47 alone or in combination with autophagy suppression.
Materials and methods
Reagents and antibodies
Autophagy regulators and detectors were obtained as follows: rapamycin (Sangon Biotech, China), chloroquine (Sigma-Aldrich, USA), nonsilencing control (SCR) and ATG5 (siG10726164423) siRNA (Guangzhou RiboBio Co., Ltd., China), Cyto-ID and Hoechst 33342 (ENZO Life Science, Farmingdale, USA), LysoTracker® (Invitrogen, San Diego, USA). The primary antibodies against β-actin, SQSTM1, LC3, PARP, Caspase 9/3, phospho-Akt (Ser473), phospho-Erk (1/2) (Thr202/Tyr204), phospho-mTOR (Ser2448), and phospho-4E-BP1/2/3 (Thr45) were purchased from CST (Cell Signaling Technology, Danvers, USA). HRP-labelled goat anti-mouse/rabbit IgG were obtained from MR Biotech (Shanghai, China).
Preparation of VEGFR1D2-SIRPαD1
VEGFR1D2-SIRPαD1 is a novel fusion protein that consists of the second extracellular domain of VEGFR1 (VEGFR1D2) and the first extracellular domain of SIRPα (SIRPαD1) (Figure S1). The molecular weight of VEGFR1D2-SIRPαD1 is 100 KDa. The protein expression vector was constructed through three steps: (1) artificially designing: the coding sequence of SIRPαD1 was linked to VEGFR1D2 through the Fc coding sequence of human IgG1; (2) VEGFR1D2-Fc-SIRPαD1 expression sequence was synthesized (GenBank accession number MG920788); (3) the synthesized sequence was subcloned into pMac-Fc vector. Then, stable Chinese hamster ovary (CHO) clone cells with the highest expression capacity were inoculated into serum-free BalanCD® CHO growth A medium (Irvine Scientific, CA, USA) in a T-125 flask. After incubation for 5 days, cells were split into five T-175 flasks and were further cultured for 7 days at the same condition before harvesting cell culture supernatant. For protein purification, the cell culture supernatant was centrifuged at 10,000 rpm for 10 min and filtered through a 0.22-μm filter before applying to purification in a Protein A column. After washing the column with 25 mM Tris-HCl, the protein was eluted with eluting buffer (50 mM acetic acid, 100 mM NaCl), neutralized with 1 M Tris-HCl. The resulting protein solution was adjusted with 1 M NaOH and then clean-in-place overnight against phosphate-buffered saline. The purity was then analyzed by Bis-Tris Gel followed by Simply Blue staining (Invitrogen, San Diego, USA). The purity of VEGFR1D2-SIRPαD1 was above 95%, and the content of endotoxin was below 0.5 U/g.
Cell lines and cell culture
Glioblastoma cells U87 and U251 were obtained from the Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM, containing 10% fetal bovine serum (Invitrogen, San Diego, USA). Bone marrow-derived macrophage (BMM) was obtained from male C57BL/6 mice and cultured in DMEM medium with 100 ng/mL M-CSF. The purity was identified by flow cytometry with the stain of APC-labelled anti-F4/80 antibodies and Alexa Fluor 488-conjugated anti-CD11b.
Macrophage-mediated phagocytosis and cytotoxicity assay
As described previously (Zhang et al. 2017), carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) was used to label U87 cells. BMMs were pre-incubated in medium for 2 h before adding carboxyfluorescein succinimidyl ester (CFSE)-labelled glioblastoma cells. Then, the cells were co-incubated with VEGFR1D2-SIRPαD1 (10 μg/mL) for 2 h. Phagocytic index was presented as the number of phagocytosed CFSE-labelled glioblastoma cells in 100 BMMs. LDH release was used to measure the macrophage-mediated cytotoxicity. After VEGFR1D2-SIRPαD1 treatment, the cell culture supernatant was collected to determine LDH release by CytoTox 96® Non-Radio. Cytotoxicity Assay (Promega, USA).
Confocal microscopy
Planted on glass bottom cell culture dishes, glioblastoma cells were treated with VEGFR1D2-SIRPαD1. Then, glioblastoma cells were stained with Hoechst 33342, Cyto-ID, and LysoTracker following the manufacturer’s instruction. Subsequently, the images were got by confocal microscopy (Carl Zeiss LSM710, Germany) and relative fluoresent intensity was quantified by Image J.
Western blot analysis
After treated with VEGFR1D2-SIRPαD1 for the indicated time periods, U87 cells and U251 cells were harvested, and the protein was analyzed by SDS-PAGE. Following blockage with 5% BSA, the membranes were incubated with primary antibodies and HRP-labelled secondary antibodies. Immobilon™ Western Chemiluminescent substrate (Millipore, Billerica, USA) was purchased to examine the targeted bands.
In vivo experiment
Nude mice (BALB/c,6 weeks old) were purchased to construct glioblastoma U87 subcutaneous xenograft model. Well-established mice were randomly assigned into indicated cohorts. Chloroquine (50 mg/kg, once a day), VEGFR1D2-SIRPαD1 (10 mg/kg, twice a week), and temozolomide (20 mg/kg, once a day) were intraperitoneally injected. Tumor volume was detected as previously described (Zhang et al. 2017). After 22 days’ administration, tumors were weighed with an electronic balance. The established glioblastoma intracranial xenograft model was used to evaluate the effects of VEGFR1D2-SIRPαD1 and/or chloroquine on the median survival.
Statistical analysis
The results in this work was analyzed by GraphPad Prism 5 (GraphPad Software Inc., USA) and the data were presented as mean ± SD. Comparisons were calculated with Student’s t test and P value below 0.05 was considered statistically significant.
Results
VEGFR1D2-SIRPαD1 induced potent anti-tumor effects
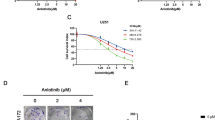
We first detect the cytotoxicity of VEGFR1D2-SIRPαD1 against glioblastoma U87 and U251 cells and found that VEGFR1D2-SIRPαD1 showed a negligible effect on the cell viability at the tested concentration (Figure S2). But VEGFR1D2-SIRPαD1 significantly increased macrophage cytotoxicity against glioblastoma cells (Fig. 1a). Compared with IgG1-Fc, the isotype control, VEGFR1D2-SIRPαD1 increased the phagocytic index from 10.2 to 24.6 in U87 cells and from 9.0 to 23.4 in U251 cells (Fig. 1b). Subcutaneous glioblastoma models were constructed to evaluate the anti-tumor effects of VEGFR1D2-SIRPαD1 in vivo. Well-established mice were randomly assigned into four cohorts: vehicle control, isotype control, VEGFR1D2-SIRPαD1, temozolomide. VEGFR1D2-SIRPαD1 decreased the mean tumor volume from day 4 and persisted until the end of the experiment (Fig. 1c). After the administration, mean tumor weight of VEGFR1D2-SIRPαD1 group was 178 ± 50.70 versus 1198 ± 237.64 mg of the isotype control (Fig. 1d). Besides, CD31, a microvessel-specific marker, was employed to detect microvessel density in the tumor tissues. We found that treatment with VEGFR1D2-SIRPαD1 decreased microvessel density of xenograft tumors (Fig. 1e). These data demonstrated that blocking CD47 and VEGF by VEGFR1D2-SIRPαD1 elicited potent anti-tumor effects in glioblastoma.
VEGFR1D2-SIRPαD1 induced valid anti-tumor effect in glioblastoma. a, b Phagocytic index and LDH release represented indicated the cytotoxicity and phagocytosis of macrophage against glioblastoma cells mediated by macrophages. (n = 5). c Xenograft tumor volume during the treatment. d After VEGFR1D2-SIRPαD1 treatment, the tumors were resected and tumor weight was calculated as mean ± SD (each point indicated an independent value). e The present images of glioblastoma xonograft tumor tissues under the stain of anti-CD31 antibody (× 200, magnification × 400). Statistical analysis of the vessel density was analyzed and normalized to the control and these data were shown as means ± SD (n = 3, Student’s t test, **P < 0.01)
VEGFR1D2-SIRPαD1 activated autophagy and autophagic flux
To detect whether VEGFR1D2-SIRPαD1 triggered autophagy in glioblastoma cells, ultrastructural analysis was used to observe the formulation of double-membrane-like vesicles, autophagosomes. After treatment with VEGFR1D2-SIRPαD1, more autophagosomes could be observed in U87 and U251 cells (Fig. 2a). To further confirm autophagy induction after VEGF and CD47 dual-targeting therapy, LC3 and SQSTM1, two autophagy activation-related proteins, were detected. Figure 2c and Figure S3 show the time-dependent increase of LC3-II while decrease of SQSTM1 in VEGFR1D2-SIRPαD1-treated glioblastoma cells. Moreover, Cyto-ID, one autophagosome specific green dye, was used to further confirm the induction of autophagy. VEGFR1D2-SIRPαD1 increased the fluorescent puncta localized in the cytoplasm of cells, which is similar to rapamycin (Fig. 2d). In fact, autophagosome accumulation only represented the autophagy induction, not the entire process of autophagy. LysoTracker, one lysosome specific red dye, and Cyto-ID were used to detect autophagic flux. Three stages of autophagy, formation of autophagosome at 12 h, autophagosome fused with lysosome at 24 h, and clearance of autophagosome in lysosome at 48 h in VEGFR1D2-SIRPαD1-treated tumor cells, were observed (Fig. 2e, f and Figure S4). In addition, ultrastructural analysis of xenograft tumors showed that VEGFR1D2-SIRPαD1 increased autophagosome accumulation in vivo (Figure S5).
Autophagy and autophagic flux were activated by VEGFR1D2-SIRPαD1 in glioblastoma cells. a, b TEM was applied to analyze autophagosomes in glioblastoma cells after being exposed to VEGFR1D2-SIRPαD1 (10 μg/mL) for 24 h. Red arrows point to autophagosomes. c SQSTM1 and LC3-II expression in tumor cells after VEGFR1D2-SIRPαD1 treatment. (β-actin, a loading control. Densitometric value was quantified by ImageJ. These data were presented from three independent experiments). d Representative images of glioblastoma cells stained with Cyto-ID after exposed to VEGFR1D2-SIRPαD1 (10 μg/mL) for the indicated time. e, f Representative images of glioblastoma cells stained with LysoTracker and Cyto-ID after being exposed to VEGFR1D2-SIRPαD1 for the indicated time (**P < 0.01)
In brief, VEGF and CD47 bispecific fusion protein VEGFR1D2-SIRPαD1 activated autophagy both in vitro and in vivo.
Inhibiting autophagy enhanced VEGFR1D2-SIRPαD1-induced macrophage phagocytosis and cytotoxicity
To elucidate the role of autophagy in CD47- and VEGF-targeting therapy, inhibitor and small interfering RNA (siRNA) were used to suppress VEGFR1D2-SIRPαD1-triggered autophagy. VEGFR1D2-SIRPαD1-activated autophagy was successfully blocked by chloroquine, a lysosome inhibitor (Fig. 3a, b). Chloroquine alone did not affect macrophage phagocytosis and cytotoxicity, while VEGFR1D2-SIRPαD1 combined with chloroquine markedly potentiated macrophage phagocytosis and cytotoxicity when compared to VEGFR1D2-SIRPαD1 (Fig. 3a, b). To further identify the critical role of autophagy, we knocked down ATG5, a key molecule in autophagy activation. As shown in Fig. 3c, d, compared with the nonsilencing scrambled control, ATG5-siRNA selectively reduced ATG5 expression (Fig. 3c, d). Knockdown of ATG5 also potentiated VEGFR1D2-SIRPαD1-triggered macrophage cytotoxicity and phagocytosis against glioblastoma cells.
Inhibiting autophagy enhanced the anti-tumor effects of VEGFR1D2-SIRPαD1 in vitro. a, b The expression of LC3-II in glioblastoma U87 and U251 cells after being exposed to VEGFR1D2-SIRPαD1 or combinated with chloroquine. c, d Glioblastoma cells were transfected with si-ATG5 siRNA. Phagocytic index and LDH release were presented as means ± SD (n = 5, *P < 0.05, **P < 0.01)
In summary, these data demonstrated that autophagy depletion reinforced VEGFR1D2-SIRPαD1-induced cytotoxicity and phagocytosis of macrophage against glioblastoma cells in vitro.
VEGFR1D2-SIRPαD1 inactivated Akt/mTOR and Erk signaling
To reveal the mechanisms of autophagy induced by VEGFR1D2-SIRPαD1 in glioblastoma cells, Akt/mTOR signaling pathway, one key regulator of autophagy, was explored. VEGFR1D2-SIRPαD1 downregulated phosphorylated mTOR in a time-dependent manner (Fig. 4 and Figure S6). Meanwhile, phosphorylated Akt, one upstream activator of mTOR, was also efficiently inhibited by VEGFR1D2-SIRPαD1. Furthermore, phosphorylation of 4E-BP1, one downstream substrate of mTOR, was also dramatically reduced after VEGFR1D2-SIRPαD1 administration. In addition, the phosphorylation of Erk was also assessed and the data showed that phosphorylated Erk was decreased in VEGFR1D2-SIRPαD1-treated glioblastoma cells. Collectively, these data showed that inactivation of Akt/mTOR and Erk pathways were most likely participated in VEGFR1D2-SIRPαD1-activated autophagy in glioblastoma U87 and U251 cells.
Akt/mTOR and Erk signal pathways were involved in VEGFR1D2-SIRPαD1-activated autophagy. a, b Glioblastoma U87 cells were treated with VEGFR1D2-SIRPαD1 for the indicated time. c, d Glioblastoma U251 cells were treated with VEGFR1D2-SIRPαD1 for the indicated time. The expression of p-Akt, p-mTOR, p-4E-BP1, and p-Erk was analyzed by Western blot. Densitometric value of control was set to 1.0 and the results were shown as means ± SD (n = 3, **P < 0.01)
Autophagy inhibition potentiated the anti-tumor effects of VEGFR1D2-SIRPαD1
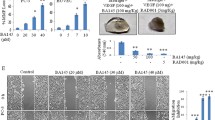
Next, we focused on evaluating whether combinational use of VEGFR1D2-SIRPαD1 and autophagy inhibitor could be a promising therapeutic for the therapy of glioblastoma therapy. In subcutaneous xenograft model, analysis of xenograft tumor volume revealed that there was no remarkable difference between the vehicle control and chloroquine-treated groups. However, the volume was significantly reduced from day 4 to the termination in VEGFR1D2-SIRPαD1 and chloroquine co-treatment group (P < 0.01) (Fig. 5a). After the administration, tumor weight of the group treated with VEGFR1D2-SIRPαD1 and chloroquine was 22 ± 22.80 versus 188 ± 78.23 mg of VEGFR1D2-SIRPαD1 alone (P < 0.01). Importantly, two mice were tumor free after 22-day co-treatment of VEGFR1D2-SIRPαD1 and chloroquine (Fig. 5b, c). Furthermore, in intracranial glioblastoma model, we assessed the effect of co-treatment of VEGFR1D2-SIRPαD1 and chloroquine on the survival. We found that chloroquine alone had no significant effect on the median survival when compared with the vehicle control. While median survival of mice treated with VEGFR1D2-SIRPαD1 was 45 days, mice co-treated with VEGFR1D2-SIRPαD1 and chloroquine had an extended median survival of 62 days (Fig. 5d).
Inhibiting autophagy enhanced the anti-tumor effects of VEGFR1D2-SIRPαD1 in glioblastoma. a Tumor volume was evaluated twice a week and showed as means ± SD. b, c After 21-day treatment with VEGFR1D2-SIRPαD1 and chloroquine, tumor weight was presented as mean ± SD and each point indicated an independent one. d The effect of VEGFR1D2-SIRPαD1 and chloroquine on the median survival of mice bearing intracranial glioblastoma (NS, no significance; *P < 0.05, **P < 0.01)
These data indicated that combinational use of VEGFR1D2-SIRPαD1 and autophagy inhibitor induced intensive anti-tumor effects in glioblastoma.
Co-treatment with VEGFR1D2-SIRPαD1 and chloroquine enhanced the effects of macrophages infiltration, anti-angiogenesis, and glioblastoma cell apoptosis
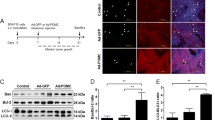
Meanwhile, H&E staining showed that combinational use of VEGFR1D2-SIRPαD1 and autophagy inhibitor elicited increased necrosis of tumor cells (Fig. 6a). CD31, a microvessel-specific marker, was employed to detect vessel density in the tumor tissues. Figure S7 showed that treatment with chloroquine further potentiated the anti-angiogenic effects of VEGFR1D2-SIRPαD1 in the xenograft tumors (P < 0.01). CD68 (a macrophage marker) was used to examine macrophage infiltration and the data presented that chloroquine increased VEGFR1D2-SIRPαD1-induced macrophage infiltration into the tumor site (Fig. 6b and Figure S8). To assess whether apoptosis was participated in the anti-tumor effect of simultaneously targeting CD47/VEGF and autophagy, PARP and Caspase 3/9 were detected. Western blot presented that blocking autophagy by chloroquine increased VEGFR1D2-SIRPαD1-induced cleavage of PARP and Caspase 3/9 (Fig. 6c, d).
Mechanisms of VEGFR1D2-SIRPαD1 and chloroquine-induced potent anti-tumor effects. H&E staining (a) and CD68 staining (b) of intracranial xenograft tumors (× 200, magnification ×400). Statistical analysis of the vessel density was quantified and presented as means ± SD (n = 3). c, d After treatment with VEGFR1D2-SIRPαD1 and/or chloroquine, intracranial xenograft tumor samples were analyzed to measure the expression of cleaved-PARP and cleaved-Caspase 3/9 (*P < 0.05, **P < 0.01)
In brief, inhibiting autophagy further enhanced VEGFR1D2-SIRPαD1-induced macrophage infiltration, anti-angiogenic effects, and tumor cell apoptosis, eliciting enhanced anti-tumor effects in glioblastoma.
Discussion
The life expectancy of patients with glioblastoma, the most aggressive and deadly brain tumor, is less than 2 years under the current standard treatment with surgery, radiation, and chemotherapies, leading to the current investigation and testing of novel treatment options against glioblastoma (Van Meir et al. 2010). Targeted therapeutics based on the immunology and biology of the lesion are finding their ways into clinical application, as evidenced by anti-angiogenic therapy with anti-VEGF antibodies and immune checkpoint-based immunotherapies (Allen et al. 2017; Ellingson et al. 2017; Gholamin et al. 2017). In the current context, VEGFR1D2-SIRPαD1, a two-in-one fusion protein against VEGF and CD47, was generated and employed to determine the anti-tumor effects in glioblastoma for the first time. And the results demonstrated that simultaneously targeting VEGF and CD47 elicited potent anti-tumor effects. Another principal finding of our work was that a cytoprotecitve autophagy was triggered in VEGFR1D2-SIRPαD1-treated glioblastoma cells. Autophagy inhibition further potentiated the anti-tumor effect of the VEGF and CD47 bispecific therapy in glioblastoma.
High tumor vascularity resulting from elevated production of pro-angiogenic growth factors has led to the development of therapies targeting pro-angiogenic signaling pathways (Ellingson et al. 2017; Van Meir et al. 2010). VEGF- and VEGFR-targeting antibodies or fusion protein was the general category of anti-angiogenic therapies. Bevacizumab, a humanized monoclonal antibody that neutralizes the effect of VEGF, has been approved for the therapy of recurrent glioblastoma in 2009 and improved progression-free survival (Diaz et al. 2017). After that, series of VEGF- or VEGFR-targeted agents such as aflibercept, cediranib, and cabozantinib have been generated for the therapy of glioblastoma (Ellingson et al. 2017; Jalali et al. 2014; Lombardi et al. 2017). Based on the promising initial data, VEGFR1D2 was employed to disrupt pro-angiogenic signaling pathways in malignant glioblastoma in the present study. Notwithstanding anti-VEGF therapies improved the progression-free survival, the randomized clinical trials have not demonstrated a significant benefit on the overall survival for glioblastoma patients (Diaz et al. 2017; Ellingson et al. 2017). Recently, immunotherapies, including the immune checkpoint inhibitors that aimed to stimulate the hosts’ immune systems to clear cancer cells, showed potent effect on treating hematologic malignancies and solid tumors (Jeanbart and Swartz 2015; Ramagopal et al. 2017; Sagiv-Barfi et al. 2015; Sockolosky et al. 2016). Emerging studies have reported that disruption of CD47/SIRPα resulted in potent anti-tumor efficacy in melanoma, lung cancer, and pancreatic ductal adenocarcinoma (Cioffi et al. 2015; Willingham et al. 2012; Zhang et al. 2017). Importantly, studies have demonstrated that targeting CD47 by humanized antibody and fusion protein could be effective therapeutic agents for central nervous system malignancies (Gholamin et al. 2017). Therefore, VEGFR1D2-SIRPαD1 was generated to evaluate the anti-tumor effect of simultaneously blocking VEGF and CD47 in glioblastoma. The present data indicated that simultaneously targeting VEGF and CD47 elicited notable anti-tumor effects through increasing anti-angiogenesis, macrophages infiltration, and glioblastoma cell apoptosis in glioblastoma.
Although enthusiasm for the progress of the above mentioned therapeutics is valuable, it must be tempered because glioblastoma is reliably becoming resistant during the therapies (Guo et al. 2017; Mondal et al. 2017). Mechanisms of resistance to molecular-targeted therapies depend on the tumor microenvironment (De Henau et al. 2016). Based on these, efforts have been done to increase the anti-tumor effect. Studies have reported that anti-angiogenic treatment activated autophagy in glioblastoma cells and colon cancer cells and inhibition of autophagy sensitized solid tumors to anti-angiogenic therapy (Hu et al. 2012; Selvakumaran et al. 2013). Another study has reported that CD47-targeted therapy activated a cytoprotective autophagy in NSCLC cells, inhibiting autophagy enhanced blocking CD47-induced efficacy (Zhang et al. 2017). In this study, we first found that simultaneous blocking VEGF and CD47 by VEGFR1D2-SIRPαD1 triggered autophagy via inactivation of Akt/mTOR signaling. Depletion of autophagy either by pharmacological agent or by siRNA could enhance VEGFR1D2-SIRPαD1-activated cytotoxicity and phagocytosis of macrophages against glioblastoma cells. Autophagy played a key role in cellular homeostasis. Depletion of autophagy by pharmaceutical inhibitors resulted in a series of cellular responses, including loss of stem cell characteristic and suppression of cell proliferation, which might disrupt the relative balance of pro-phagocytic and anti-phagocytic signals, and further promote macrophage phagocytosis (Boya et al. 2018; Zeng et al. 2015; Zhu et al. 2018). In vivo depleting autophagy significantly potentiated the anti-tumor effects of VEGFR1D2-SIRPαD1 and further extended the median survival, indicating that disruption of VEGF and CD47 signaling in combination with autophagy inhibition could be a prospective therapy for glioblastoma.
In conclusion, the present study provided novel therapeutic strategy for glioblastoma by simultaneously targeting VEGF and CD47. During VEGF and CD47 bispecific therapy, autophagy and the completed autophagic flux were activated as a cytoprotective mechanism in glioblastoma cells via inactivation of Akt/mTOR and Erk pathways. Blockade of autophagy induced by dual-targeting fusion protein elicited enhanced anti-tumor effects in glioblastoma (Scheme 1). Our data revealed the cytoprotective autophagy in VEGF and CD47 bispecific therapy, indicating that the novel treatment for glioblastoma: VEGF and CD47 bispecific therapy in combination with autophagy blockade.
References
Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G (2017) Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 9. https://doi.org/10.1126/scitranslmed.aak9679
Bakas S, Akbari H, Pisapia J, Martinez-Lage M, Rozycki M, Rathore S, Dahmane N, O’Rourke DM, Davatzikos C (2017) In vivo detection of EGFRvIII in glioblastoma via perfusion magnetic resonance imaging signature consistent with deep peritumoral infiltration: the phi-index. Clin Cancer Res 23:4724–4734. https://doi.org/10.1158/1078-0432.CCR-16-1871
Boucher JM, Bautch VL (2014) Antiangiogenic VEGF-A in peripheral artery disease. Nat Med 20:1383–1385. https://doi.org/10.1038/nm.3767
Boya P, Codogno P, Rodriguez-Muela N (2018) Autophagy in stem cells: repair, remodelling and metabolic reprogramming. Development 145. https://doi.org/10.1242/dev.146506
Chao MP, Majeti R, Weissman IL (2011) Programmed cell removal: a new obstacle in the road to developing cancer. Nat Rev. Cancer 12:58–67. https://doi.org/10.1038/nrc3171
Cioffi M, Trabulo S, Hidalgo M, Costello E, Greenhalf W, Erkan M, Kleeff J, Sainz B Jr, Heeschen C (2015) Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res 21:2325–2337. https://doi.org/10.1158/1078-0432.CCR-14-1399
De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T (2016) Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 539:443–447. https://doi.org/10.1038/nature20554
Diaz RJ, Ali S, Qadir MG, De La Fuente MI, Ivan ME, Komotar RJ (2017) The role of bevacizumab in the treatment of glioblastoma. J Neurooncol 133:455–467. https://doi.org/10.1007/s11060-017-2477-x
Ellingson BM, Gerstner E, Smits M, Huang RY, Colen RR, Abrey LE, Aftab DT, Schwab GM, Hessel C, Harris RJ, Chakhoyan A, Gahrmann R, Pope WB, Leu K, Raymond C, Woodworth DC, de Groot JF, Wen PY, Batchelor T, van den Bent MJ, Cloughesy TF (2017) Diffusion MRI phenotypes predict overall survival benefitfrom anti-VEGF monotherapy in recurrent glioblastoma:Converging evidence from phase II trials. Clin Cancer Res 23:5745–5756. https://doi.org/10.1158/1078-0432.CCR-16-2844
Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, Volkmer AK, Willingham S, Ponnuswami A, McCarty A, Lovelace P, Storm TA, Schubert S, Hutter G, Narayanan C, Chu P, Raabe EH, Harsh Gt, Taylor MD, Monje M, Cho YJ, Majeti R, Volkmer JP, Fisher PG, Grant G, Steinberg GK, Vogel H, Edwards M, Weissman IL, Cheshier SH (2017) Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 9. https://doi.org/10.1126/scitranslmed.aaf2968
Guo G, Gong K, Ali S, Ali N, Shallwani S, Hatanpaa KJ, Pan E, Mickey B, Burma S, Wang DH, Kesari S, Sarkaria JN, Zhao D, Habib AA (2017) A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat Neurosci 20:1074–1084. https://doi.org/10.1038/nn.4584
Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, Scotney P, Nyqvist D, Samen E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjoholm A, Nash A, Eriksson U (2012) Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490:426–430. https://doi.org/10.1038/nature11464
Hu B, Wang Q, Wang YA, Hua S, Sauve CG, Ong D, Lan ZD, Chang Q, Ho YW, Monasterio MM, Lu X, Zhong Y, Zhang J, Deng P, Tan Z, Wang G, Liao WT, Corley LJ, Yan H, Zhang J, You Y, Liu N, Cai L, Finocchiaro G, Phillips JJ, Berger MS, Spring DJ, Hu J, Sulman EP, Fuller GN, Chin L, Verhaak RG, DePinho RA (2016) Epigenetic activation of WNT5A drives glioblastoma stem cell differentiation and invasive growth. Cell 167:1281–1295. https://doi.org/10.1016/j.cell.2016.10.039
Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, Aghi MK (2012) Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res 72:1773–1783. https://doi.org/10.1158/0008-5472.CAN-11-3831
Jalali S, Chung C, Foltz W, Burrell K, Singh S, Hill R, Zadeh G (2014) MRI biomarkers identify the differential response of glioblastoma multiforme to anti-angiogenic therapy. Neuro Onco 16:868–879. https://doi.org/10.1093/neuonc/nou040
Jeanbart L, Swartz MA (2015) Engineering opportunities in cancer immunotherapy. Proc Natl Acad Sci U S A 112:14467–14,472. https://doi.org/10.1073/pnas.1508516112
Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, Rahbari NN, Chung E, Clark JW, Willett CG, Luster AD, Yun SH, Langer R, Anderson DG, Padera TP, Jain RK, Fukumura D (2017) Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J Clin Invest 127:3039–3051. https://doi.org/10.1172/JCI93182
Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM (2015) SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520:363–367. https://doi.org/10.1038/nature14363
Lee H, Lee JK, Park MH, Hong YR, Marti HH, Kim H, Okada Y, Otsu M, Seo EJ, Park JH, Bae JH, Okino N, He X, Schuchman EH, Bae JS, Jin HK (2014) Pathological roles of the VEGF/SphK pathway in Niemann-Pick type C neurons. Nat Commun 5:5514. https://doi.org/10.1038/ncomms6514
Lombardi G, Pambuku A, Bellu L, Farina M, Della Puppa A, Denaro L, Zagonel V (2017) Effectiveness of antiangiogenic drugs in glioblastoma patients: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 111:94–102. https://doi.org/10.1016/j.critrevonc.2017.01.018
Mondal A, Kumari Singh D, Panda S, Shiras A (2017) Extracellular vesicles as modulators of tumor microenvironment and disease progression in glioma. Front Oncol 7:144. https://doi.org/10.3389/fonc.2017.00144
Peng QX, Han YW, Zhang YL, Hu J, Fan J, Fu SZ, Xu S, Wan Q (2017) Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 8:52813–52,822. https://doi.org/10.18632/oncotarget.17264
Ramagopal UA, Liu W, Garrett-Thomson SC, Bonanno JB, Yan Q, Srinivasan M, Wong SC, Bell A, Mankikar S, Rangan VS, Deshpande S, Korman AJ, Almo SC (2017) Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci U S A 114:E4223–E4232. https://doi.org/10.1073/pnas.1617941114
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730. https://doi.org/10.1038/nrd3802
Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R (2015) Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 112:E966–E972. https://doi.org/10.1073/pnas.1500712112
Sareddy GR, Viswanadhapalli S, Surapaneni P, Suzuki T, Brenner A, Vadlamudi RK (2017) Novel KDM1A inhibitors induce differentiation and apoptosis of glioma stem cells via unfolded protein response pathway. Oncogene 36:2423–2434. https://doi.org/10.1038/onc.2016.395
Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O’Dwyer PJ (2013) Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res 19:2995–3007. https://doi.org/10.1158/1078-0432.CCR-12-1542
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985. https://doi.org/10.1126/science.6823562
Shen W, Zhang X, Fu X, Fan J, Luan J, Cao Z, Yang P, Xu Z, Ju D (2017) A novel and promising therapeutic approach for NSCLC: recombinant human arginase alone or combined with autophagy inhibitor. Cell Death Dis 8:e2720. https://doi.org/10.1038/cddis.2017.137
Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC (2016) Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A 113:E2646–E2654. https://doi.org/10.1073/pnas.1604268113
Stanton MJ, Dutta S, Zhang H, Polavaram NS, Leontovich AA, Honscheid P, Sinicrope FA, Tindall DJ, Muders MH, Datta K (2013) Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Res 73:160–171. https://doi.org/10.1158/0008-5472.CAN-11-3635
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60:166–193. https://doi.org/10.3322/caac.20069
Vonderheide RH (2015) CD47 blockade as another immune checkpoint therapy for cancer. Nat Med 21:1122–1123. https://doi.org/10.1038/nm.3965
Wen Y, Graybill WS, Previs RA, Hu W, Ivan C, Mangala LS, Zand B, Nick AM, Jennings NB, Dalton HJ, Sehgal V, Ram P, Lee JS, Vivas-Mejia PE, Coleman RL, Sood AK (2015) Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res 21:448–459. https://doi.org/10.1158/1078-0432.CCR-14-1578
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 109:6662–6667. https://doi.org/10.1073/pnas.1121623109
Zeng X, Zhao H, Li Y, Fan J, Sun Y, Wang S, Wang Z, Song P, Ju D (2015) Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy 11:355–372. https://doi.org/10.4161/15548627.2014.994368
Zhang B, Fan J, Zhang X, Shen W, Cao Z, Yang P, Xu Z, Ju D (2016) Targeting asparagine and autophagy for pulmonary adenocarcinoma therapy. Appl Microbiol Biotechnol 100:9145–9161. https://doi.org/10.1007/s00253-016-7640-3
Zhang X, Chen W, Fan J, Wang S, Xian Z, Luan J, Li Y, Wang Y, Nan Y, Luo M, Li S, Tian W, Ju D (2018) Disrupting CD47-SIRPα axis alone or combined with autophagy depletion for the therapy of glioblastoma. Carcinogenesis. https://doi.org/10.1093/carcin/bgy041
Zhang X, Fan J, Wang S, Li Y, Wang Y, Li S, Luan J, Wang Z, Song P, Chen Q, Tian W, Ju D (2017) Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res 5:363–375. https://doi.org/10.1158/2326-6066.CIR-16-0398
Zhu Y, Li H, Ding S, Wang Y (2018) Autophagy inhibition promotes phagocytosis of macrophage and protects mice from methicillin-resistant staphylococcus aureus pneumonia. J Cell Biochem. https://doi.org/10.1002/jcb.26677
Funding
The work was funded by the National Key Basic Research Program of China (grant number 2015CB931800), the National Natural Science Foundation of China (grant number 81573332 and 81773620), and the Special Research Foundation of State Key Laboratory of Medical Genomics and Collaborative Innovation Center of Systems Biomedicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Wenzhi Tian is the founder and Song Li is the employee of ImmuneOnco Biopharma (Shanghai) Co., Ltd. Others declared no conflict of interest.
Ethical approval
All experimental procedures involving animals were conducted in accordance with the standards approved by Animal Ethical Committee of School of Pharmacy at Fudan University.
Electronic supplementary material
ESM 1
(PDF 1507 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, S., Nan, Y. et al. Inhibition of autophagy potentiated the anti-tumor effects of VEGF and CD47 bispecific therapy in glioblastoma. Appl Microbiol Biotechnol 102, 6503–6513 (2018). https://doi.org/10.1007/s00253-018-9069-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9069-3