Abstract
Cordyceps militaris is a highly valued edible and medicinal fungus due to its production of various metabolites, including adenosine, cordycepin, N6-(2-hydroxyethyl)-adenosine, and carotenoids. The contents of these metabolites are indicative of the quality of commercially available fruit body of this fungus. In this work, the effects of environmental abiotic factors, including heat and light stresses, on the fruit body growth and metabolite production in C. militaris were evaluated during the late growth stage. The optimal growth temperature of C. militaris was 20 °C. It was found that a heat stress of 25 °C for 5–20 days during the late growth stage significantly promoted cordycepin and carotenoid production without affecting the biological efficiency. Light stress at 6000 lx for 5–20 days during the late growth stage significantly promoted cordycepin production but decreased the carotenoid content. Both heat and light stresses promoted N6-(2-hydroxyethyl)-adenosine production. In addition, gene expression analysis showed that there were simultaneous increases in the expression of genes encoding a metal-dependent phosphohydrolase (CCM_04437) and ATP phosphoribosyltransferase (CCM_04438) that are involved in the cordycepin biosynthesis pathway, which was consistent with the accumulation of cordycepin during heat stress for 5–20 days. A positive weak correlation between the cordycepin and adenosine contents was observed with a Pearson correlation coefficient of 0.338 (P < 0.05). The results presented herein provide a new strategy for the production of a superior quality fruit body of C. militaris and contribute to further elucidation of the effects of abiotic stress on metabolite accumulation in fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cordyceps militaris, a well-known edible and medicinal fungus, is the type species of Cordyceps that generally parasitizes the larvae or pupae of lepidopteran insects. It has been widely used as a tonic food in East Asia as well as a substitute for Ophiocordyceps sinensis (syn. Cordyceps sinensis) in traditional Chinese medicine. C. militaris is beneficial due to its anti-aging, anti-tumor, anti-fatigue, immunomodulatory, neuroprotective, liver-protective, and reno-protective activities (Das et al. 2010). These benefits have made this fungus marketable in Western countries as an over-the-counter medicine.
A series of active ingredients, including cordycepin, N6-(2-hydroxyethyl)-adenosine (HEA), adenosine, and carotenoids, have been isolated from the fruit body of C. militaris. Cordycepin (3′-deoxyadenosine), a nucleoside analogue, is an antibiotic that was first isolated from C. militaris (Cunningham et al. 1950). It is a broad-spectrum antimicrobial compound (Ahn et al. 2000) as well as a polyadenylation inhibitor that has undergone clinical trials against cancers (OncoVista Inc. 2008). It is also reported to have anti-metastatic, anti-platelet aggregation, and anti-inflammatory activities (Tuli et al. 2013).
Many studies have focused on improving cordycepin production during fermentation, including by high-energy ion beam irradiation (Das et al. 2008), UV-B irradiation (Huang et al. 2015), dissolved oxygen control (Mao and Zhong 2004), and medium optimization (Xie et al. 2009). However, the C. militaris products on the market are primarily the fruit body; thus, improving the cordycepin content in the fruit body is a more valuable pursuit. Until now, only laborious approaches, such as strain breeding (Kang et al. 2017; Lee et al. 2017) and culture medium optimization (Lim et al. 2012; Wu et al. 2016), have been performed to produce a larger amount of cordycepin in the fruit body.
Carotenoids are natural isoprenoid pigments that provide the natural yellow, orange, and red colors. It has been demonstrated that carotenoids showed anticancer, antioxidation, and eyesight enhancing activities (Milani et al. 2017). Carotenoids, including lutein, zeaxanthin, and four cordyxanthins, have been isolated from the fruit body of C. militaris (Yan et al. 2010; Chen et al. 2013; Dong et al. 2013). Our previous study has confirmed that light is necessary for carotenoid production for C. militaris and suggested that the carotenoid content should be considered as the quality standard of commercial products of this valued mushroom (Yang et al. 2014). However, there is little study on carotenoid production in C. militaris. Searching for a practical method to obtain high metabolite content, especially for cordycepin and carotenoid, without inhibiting fruit body growth in C. militaris is meaningful and challenging.
Fungi thrive in diverse niches around the world and are challenged by ever-changing variables in their environment. Heat and light stresses, two important environmental abiotic factors, have a significant impact on the physiology and secondary metabolism of many mushrooms. Heat stress is known to inhibit mycelial growth and impair fruit body formation, ultimately affecting the quality of the mushroom (Chang and Miles 2004). Fruit body growth of Agaricus bisporus is severely reduced concerning both the pileus diameter and biomass when exposed to high temperatures of 33 °C (Lu et al. 2014). Heat stress at 28 or 34 °C affects the strain growth and mycelial morphology in Tuber borchii (Leonardi et al. 2017). Heat stress at 42 °C inhibits the mycelium growth and reduces the hyphal branching of Ganoderma lucidum (Zhang et al. 2016). Song et al. (2014) reported that apoptosis-like cell death occurred in Pleurotus species when exposed to heat stress at 42 °C. Heat stress also has a significant effect on the secondary metabolism of fungi. For example, heat stress at 42 °C induces accumulation of ganoderic acid in the mycelium of G. lucidum (Zhang et al. 2016).
Light is also an important environmental factor for regulating the production of metabolites in addition to the growth and morphogenesis of mushrooms. Ganoderic acid and ganoderma polysaccharides are efficiently produced in submerged fermentation of G. lucidum using a three-stage light irradiation strategy (Zhang and Tang 2008). Shikimic acid, a key intermediate in the aromatic amino acid pathway, accumulates in oyster mushroom (Pleurotus ostreatus) mycelia under blue light by more than 200-fold compared to mycelia that are kept in the dark (Kojima et al. 2015). Light is more a beneficial condition than dark regarding antioxidant activity, linoleic acid content, and mycelial growth during cultivation of C. pruinosa mycelia on Sabouraud dextrose agar with yeast extract media (Oh et al. 2014). C. militaris was shown to accumulate carotenoids after light irradiation in our previous study (Yang et al. 2014). Few studies have focused on the effect of heat and light stresses on metabolite production in the fruit body of mushrooms.
C. militaris can complete its life cycle when cultured in vitro (Shrestha et al. 2012b). Furthermore, genome sequencing (Zheng et al. 2011), genetic transformation, and gene disruption (Yang et al. 2016) have been successful in this species, which has made it possible to conduct additional basic biology studies. Therefore, as an ascomycete with a few bioactive secondary metabolites, C. militaris has been used as a model organism for the study of Cordyceps spp. (Shrestha et al. 2012b), in which more than 400 species have been described. In this study, the effects of heat and light stresses on fruit body growth and metabolite production in C. militaris during the late growth stage were evaluated. It was found that during the late growth stage, heat stress significantly promoted cordycepin and carotenoid production without affecting the biological efficiency. Additionally, the expression levels of genes involved in the cordycepin biosynthesis pathway were analyzed. The results will provide a practical strategy for the production of high-quality fruit body in C. militaris.
Materials and methods
Fungal strain
The C. militaris strain CGMCC 3.16321 (Lian et al. 2014) used in this study was maintained on potato dextrose agar (PDA) at 4 °C as a stock. The strain was incubated on the same medium in a Petri dish at 20 °C for 2 weeks before being used.
For strain identification, the internal transcribed spacer (ITS1-5.8S-ITS2) of nuclear rDNA was amplified and sequenced. The nucleotide sequence has been deposited in GenBank under accession number MG753991. The BLAST searches of the NCBI nucleotide database indicated that ITS sequence of strain CGMCC 3.16321 exhibited a 100% similarity with C. militaris strain NBRC 30377 (JN943300.1). The morphology of conidia and conidiophores was observed under an optical microscope (Nikon Eclipse 80i, Nikon Instruments Inc., Tokyo, Japan) and was consistent with the previous description of this species (Shrestha et al. 2005b).
The optimal growth temperature
A 5-mm disc was punched with a sterilized cutter from the inoculum preparation and transferred to a fresh Petri dish containing the same medium. Dishes with inoculum were sealed with parafilm and incubated at 4, 10, 15, 20, 25, 30, 35, and 40 °C. The growth of the colony was measured every 3 days for 2 weeks. Cultures at higher temperatures with no growth, 35 and 40 °C, were moved to 20 °C after 2 weeks of incubation to determine whether they resumed growth at 20 °C. The growth rate was determined by measuring the colony diameter after 2 weeks of incubation, and six replicates were performed for each experiment.
Cultivation of the fruit body of C. militaris under heat and light stresses
The fungus was cultivated in wheat medium as previously described (Guo et al. 2016). Briefly, the strain was incubated on PDA dishes at 20 °C for 10 days and then transferred to the seed culture medium (PD broth) by punching out 6 discs of 9-mm in diameter from the agar plate with a sterilized cutter. The seed cultures were grown in a 250-ml flask containing 80 ml of medium at 20 °C on a rotary shaking incubator at 150 rpm for 3 days. The cultivation was performed in 500-ml glass bottles containing wheat medium at 20 °C, with 12 h of light and 12 h of dark, white fluorescent light at an intensity of 1700 lx and relative humidity greater than 80%.
When the nascent fruit body was approximately 4.50 cm in length after being cultured for 45 days, heat and light stresses were applied for another 20 days. The heat stress group was cultured at 25 °C and a white light intensity of 1700 lx, while the light stress group was cultured at 20 °C and a white light intensity of 6000 lx. The double stress group was cultured at 25 °C with a white light intensity of 6000 lx. Cultures at 20 °C and a white light intensity of 1700 lx were used as control. Six replicate bottles for each condition were applied.
Fruit bodies were harvested every 5 days after being subjected to heat and light stresses. The morphology of fruit body was observed, and the color was recorded based on the handbook of Kornerup and Wanscher (1978). The lengths and diameters of fruit bodies were calculated from six replicate bottles and 20 counts for each bottle under each condition. Fruit bodies were subsequently air-dried at 45 °C for 48 h to constant weight at the drying oven (Yiheng Scientific Instruments Co., Ltd., Shanghai, China). Biological efficiency was defined as the percentage of dry weight of harvested fruit body over dry weight of substrate. The dried fruit bodies were ground into a fine powder in a laboratory mill (HR2027, Philips, Hong Kong) and kept in glass jars that were wrapped with an aluminum foil at 4 °C.
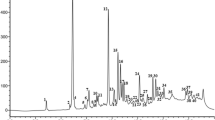
Determination of adenosine, cordycepin, and HEA by HPLC
Adenosine, cordycepin, and HEA were quantified using calibration curves of standard reference compounds. Specified amounts of adenosine (Sigma, Munich, Germany), cordycepin (Sigma, Munich, Germany), and HEA (Shanghai PureOne Biotechnology, Pudong, Shanghai) were dissolved in deionized water at 100 μg/ml as stock solutions. The appropriate amount of the stock solution was transferred to a 10-ml volumetric flask and brought up to a volume of 10 ml with the same solvent to obtain the desired concentrations (1, 2, 5, 10, 20, and 50 μg/ml).
Samples were extracted with deionized water using ultrasonication for 30 min and filtered through a 0.45-μm filter membrane prior to injection into the HPLC system. The production of adenosine, cordycepin, and HEA were analyzed by reversed-phase HPLC using a Waters 2695 Separations Module (Waters Corporation, Milford, USA) equipped with a built-in quaternary pump, 120 autosampler, Waters 2998 Photodiode Array Detector, and Empower program for data analysis. A pre-packed Puritex C18 column (4.6 × 250 mm, 5-μm particle size; Beijing Greenherbs Science and Technology Development Co. Ltd., Beijing, China) was used. Samples were eluted in the mobile phase, which consisted of acetonitrile to double-distilled H2O (0.1% acetic acid) (5:95), for 20 min at 25 °C. The flow rate was 1 mL/min, and the injection volume was 10 μL. Nucleosides were monitored and quantified at 260 nm.
Determination of carotenoids
Carotenoids were extracted from the fruit bodies using the acid-heating method (Yang et al. 2014). One gram of C. militaris fruit body powder was soaked in 15 mL of 1 M hydrochloric acid (HCl) at 30 °C for 30 min and centrifuged at 5000 rpm for 10 min. The supernatant was removed, and then, the residue was washed twice with distilled water. After the cell wall was broken, 15 ml of a 4:1 mixture of acetone to petroleum ether was added to the broken cells. Next, the carotenoid extraction was performed for 10 min at 30 °C while shaking at 100 rpm. The samples were subjected to centrifugation (5000 rpm, 10 min), and the supernatant was later used for determination of the carotenoids. The absorbance value was determined after dilution by colorimetry at 445 nm with a UV-2100 spectrophotometer (Unico Instrument Co. Ltd., Shanghai, China). The total carotenoid yield (μg/g dried biomass) was calculated according to the following equation:
where A is the absorbance value of the diluted extraction at λmax, V is the volume of the extraction reagent, D is the dilution ratio, 0.16 is the extinction coefficient of carotenoids, and W (g) is the weight of the dry fruit body of C. militaris.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from 100 mg frozen fruit body using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The extracted RNA was then treated with RQ1 RNase-Free DNase (Promega, Madison, WI, USA). We synthesized cDNA using the ReverTra Ace qPCR RT Master Mix (Toyobo Co., Ltd., Osaka, Japan). Quantitative real-time PCR (qPCR) was conducted using a Mastercycler ep realplex instrument (Eppendorf, Hamburg, Germany). The 25-μl qPCR solutions contained 5 ng cDNA, 0.1 μM primers, and 12.5 μl qPCR SYBR Green Mix (Toyobo Co., Ltd., Osaka, Japan). Relative gene expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). The obtained data represented six biological replicates, with two technical replicates each.
Statistical analysis
The data were expressed as the mean ± SD and were analyzed by one-way analysis of variance (ANOVA). Significant differences were determined by Duncan’s multiple range tests (P < 0.05). Data analyses were completed with SPSS 19.0 (SPSS, Inc., Chicago, USA) and OriginPro 8.5 (OriginLab Corporation, Massachusetts, USA). The graph was constructed using Graphpad Prism 6.0 (Graphpad Software, Inc., California, USA).
Results
The optimal growth temperature of C. militaris
After 2 weeks of culture, mycelia overspread the plates at 20 °C (Fig. 1a) and the average of colony diameters were 5.55, 7.40, and 6.35 cm at temperature 15, 20, and 25 °C, respectively. A range of 15–25 °C is favorable for the mycelial growth of C. militaris, with the highest growth rate at 20 °C followed by 25 °C (Fig. 1b). The growth rate at 20 °C was significantly higher than that at 25 °C (P < 0.05, Fig. 1b). The growth decreased rapidly outside that range, with very low growth at 10 and 30 °C while no growth occurred at 35 °C and beyond. The least sign of growth was observed at 4 °C (Fig. 1a). Cultures originally grown at 35 and 40 °C failed to regrow after being transferred to 20 °C for another 30 days of incubation and were apparently dead.
Colony and growth rate of Cordyceps militaris on agar medium under different temperatures. a Colony of C. militaris on agar medium for 2 weeks under different temperatures. b Growth rate of C. militaris on agar medium under different temperatures. Different letters above the bars indicated significant differences (ANOVA followed by Duncan’s multiple range tests, P < 0.05)
Fruit body growth of C. militaris under heat and light stresses
The fruit bodies under different stresses for 20 days had distinctly different appearances (Fig. 2), especially the color and spinules. The color of the fruit body under light stress was the palest, with a pale yellow color (4A3, LS in Fig. 2), while the deepest colored fruit body was that grown under heat stress with a deep yellow (4A8, HS in Fig. 2). The color of the control (C in Fig. 2) and double stress groups (HL in Fig. 2) were intermediate and appeared yellow (4A5). Additionally, some reddish spinules appeared on the top of the fruit body grown under heat stress (HS in Fig. 2).
Appearance of the Cordyceps militaris fruit body under different stresses. C is the control, which was kept at 20 °C and 1700 lx during the whole growth period; LS indicates the light stress group under a light intensity of 6000 lx and a temperature of 20 °C for 20 days; HS indicates the heat stress treatment at 25 °C and 1700 lx for 20 days; HL indicates heat and light stresses at 25 °C and 6000 lx for 20 days
The fresh, dry weights and biological efficiency of the fruit bodies under different stresses are shown in Table 1. The fresh weight exhibited almost the same decreasing trend after being treated for 5 to 20 days in each group. However, a significant decrease was only detected in the heat stress group after being treated for 20 days (P < 0.5). Comparing between different groups, the fresh weight of the double stress group was significantly lower than that of the other groups after being treated for 5 to 20 days. When the stress duration was 15 and 20 days, the fresh weight of the control group was significantly higher than that of the other groups, suggesting that both heat and light stresses may affect the fresh weight of the fruit body.
A different result was obtained for the dry weight of the fruit body (Table 1). There was no significant difference between each group after being treated for 5 to 20 days. Moreover, there was no significant difference between groups when the treatment lasted for 20 days. The same trend was found in biological efficiency (Table 1).
The fruit body length increased in the control and light stress groups with the extension of the growth period (Table 2). It was significantly longer than those of the other two groups when the treatment lasted for 20 days. The maximum length of the fruit body was observed in the control group for 20 days.
The diameter of the fruit bodies of the control and double stress group increased significantly with the extension of the growth period (Table 2). However, there was no significant difference when comparing between different groups after being treated for the same number of days.
Adenosine, cordycepin, and HEA contents of the fruit body of C. militaris under different stresses
The cordycepin content of fruit body increased significantly every 5 days in all groups, and the maximum content was achieved in each group after being treated for 20 days (Fig. 3a). The cordycepin content in the experimental groups was significantly higher than in the control group after being treated for 10 days and longer. The cordycepin content in the light stress group was significantly higher than that of the other groups after being treated for 5 and 10 days. However, after being treated for 15 or 20 days, the cordycepin content in the temperature stress group was significantly higher than that of the other groups. Among all of the groups, the maximum content (5.56 ± 0.05 mg/g) was achieved in the heat stress group after being treated for 20 days and was almost two times that of the control group over the same growth period.
Cordycepin, adenosine, and HEA contents of the fruit body of Cordyceps militaris under different stresses. a Cordycepin. b Adenosine. c HEA. d Correlation analysis of cordycepin and adenosine contents. C is the control, which was kept at 20 °C and 1700 lx during the entire growth period; LS indicates light stress treatment under a light intensity of 6000 lx and a temperature of 20 °C; HS indicates the heat stress treatment at 25 °C and 1700 lx; HL indicates heat and light stresses at 25 °C and 6000 lx. Error bars indicate the standard deviation of three replicate bottles, with two technical replicates each. Different letters above the bars for the same day indicated significant differences (ANOVA followed by Duncan’s multiple range tests, P < 0.05)
In the control, temperature stress, and light stress groups, the adenosine content increased with the extension of the growth period, whereas in the double stress group, the adenosine content decreased after being treated for 10 days (Fig. 3b). Compared to the other groups, the adenosine content of the double stress group was significantly higher when treated for 5 or 10 days. The opposite result was obtained when the groups were treated for 15 or 20 days.
The HEA content of fruit body varied from 1.34 ± 0.02 to 2.05 ± 0.05 mg/g during the growth period in all groups. The maximal HEA content (2.05 ± 0.05 mg/g) was observed in the double stress group after being treated for 20 days. In general, the HEA content of the control group was lower than that of the other groups (Fig. 3c).
Correlation analysis of the adenosine, cordycepin, and HEA contents
Nucleosides, including adenosine, cordycepin, and HEA, represent the major active components in Cordyceps spp. (Li et al. 2006). Correlation analysis of these three nucleosides was performed using SPSS. A positive weak correlation between the cordycepin and adenosine contents was observed with a Pearson correlation coefficient of 0.338 (P < 0.05, Fig. 3d); however, there was no correlation between the adenosine and HEA contents.
Carotenoid content of the fruit body of C. militaris under different stresses
The carotenoid content of C. militaris was consistent with the color of the fruit body. During the growth period, the carotenoid content of the heat stress group was significantly higher than that of the other groups, except for groups treated for 5 days (Fig. 4). In the control and heat stress groups, the carotenoid content increased with the growth period. The maximal content (4204.688 ± 19.887 μg/g) was achieved in the heat stress group after being treated for 20 days, and it was over two times that of the light and double stress group over the same growth period.
Carotenoid content of the fruit body of Cordyceps militaris under different stresses. C is the control, which was kept at 20 °C and 1700 lx during the entire growth period; LS indicates light stress treatment under a light intensity of 6000 lx and a temperature of 20 °C; HS indicates the heat stress treatment at 25 °C and 1700 lx; HL indicates heat and light stresses at 25 °C and 6000 lx. Error bars indicate the standard deviation of three replicate bottles, with two technical replicates each. Different letters above the bars for the same day indicated significant differences (ANOVA followed by Duncan’s multiple range tests, P < 0.05).
Expression of genes involved in the cordycepin biosynthesis pathway
Cordycepin is the most important metabolite in C. militaris, and the cordycepin content significantly increased after heat stress. We analyzed the expression of the genes involved in the cordycepin biosynthesis pathway under heat stress (Fig. 5). The three key genes encoding an oxidoreductase (CCM_04436), metal-dependent phosphohydrolase (CCM_04437), and ATP phosphoribosyltransferase (CCM_04438), which are responsible for cordycepin biosynthesis from adenosine, were analyzed. The result showed that no change was observed regarding expression of the gene encoding CCM_04436 between the control and heat stress groups that were treated for 5–20 days. However, the expression levels of the genes encoding CCM_04437 and CCM_04438 significantly increased under heat stress compared to the control and remained increased by two to three times of the control during the heat stress period.
Expression of genes involved in cordycepin biosynthesis under heat stress for different days
Control is the sample before heat stress treatment; HS-5, HS-10, HS-15, and HS-20 indicate heat stress treatment under temperature of 25 °C for 5, 10, 15, and 20 days, respectively. Error bars indicate the standard deviation of three replicate bottles, with two technical replicates each.
Discussion
Production of secondary metabolites that respond to environmental changes in the fungi has received widespread attention due to the economic and medicinal value of the fungi. Cordycepin, as the most important bioactive metabolite in C. militaris, has a significant therapeutic potential (Tuli et al. 2013). C. militaris is also a potential source of natural carotenoids, an antioxidant, and a pro-vitamin A. Cordycepin and carotenoid have been used or suggested as quality standard for commercial products of C. militaris (Yu et al. 2006; Yang et al. 2014). In this study, we focused on the effects of heat and light stresses on both fruit body growth and metabolite production, especially cordycepin and carotenoids, in C. militaris during the late growth stage. Our study proved that heat and light stresses during the late growth stage of fruit body led to a significant increase in cordycepin biosynthesis without affecting biological efficiency and heat stress also significantly promoted carotenoid production. This study provides a new strategy for producing superior quality fruit body of C. militaris and serves as a good example of the effect of abiotic stress on the regulation of metabolite production in fungi.
The optimal growing temperature for C. militaris was observed to be 20 °C on agar medium, and the growth at 25 °C was significantly lower than at 20 °C (P < 0.05, Fig. 1a, b). The optimal temperature for mycelial growth of C. militaris is apparently lower than that for other species in the same or related genera, such as 26 °C for Ophiocordyceps jiangxiensis JXPJ0109 (Xiao et al. 2004) and 28 °C for Paecilomyces tenuipes (current name Cordyceps tenuipes) (Xu et al. 2003). It was also different from O. sinensis, whose optimal growth temperature was 15 and 18 °C and no growth was observed at 25 °C (Dong and Yao 2011). Strains of C. militaris can withstand relatively high temperatures, e.g., 30 °C, but die if being kept at higher temperatures of 35 °C or 40 °C for 2 weeks.
Different temperatures were used in other reported heat stress experiments, such as 42 °C in G. lucidum (Zhang et al. 2016), 33 °C in A. bisporus (Lu et al. 2014), and 28 °C and 34 °C in T. borchii (Leonardi et al. 2017). The fruit body of C. militaris is most often cultivated at 20 °C (Shrestha et al. 2005a; Sung et al. 2006) or 23 °C (Chen et al. 2017). In the present study, 25 °C was used as the heat stress because the dry weight of the fruit body and the biological efficiency were not affected at this temperature. Actually, heat stress under different temperatures were also used, e.g., 30 °C and 35 °C for 5 days, and then transfer to 20 °C for another 20 days at a light intensity of 1700 lx. It was found that the fruit bodies grown at 30 and 35 °C for 5 days were not only vulnerable to be contaminated, but also showed a morphological abnormality (Fig. S1) and cannot be used as a commodity.
When the treatment lasted for 20 days, the fresh weight of the fruit body of the control group was significantly higher than that of the other groups, but the dry weight exhibited no significant difference. This suggested that the decrease of the fresh weight might be due to moisture loss under heat and light stresses in the late growth stage of the fruit body of C. militaris. Biological efficiency is one of significant factors to evaluate the growth of mushroom. A similar biological efficiency was obtained in this study as in previous literatures, and the values varied from 14.18 to 17.42%. It was reported that the biological efficiency of C. militaris was lower than 15% when cultivated on three different substrates (Krupodorova and Barshteyn 2015) and varied from 13.68 to 23.25% when inoculated on a 50-g brown rice medium mixed with 10-g silkworm pupae at 20 ± 1 °C for 60 days (Shrestha et al. 2012a).
Most secondary metabolites are produced after the fungus has completed its initial growth phase (Calvo et al. 2002). The cordycepin content of the fruit body increased significantly during the growth period in each group, suggesting that cordycepin was actually accumulated during the late growth stage. Both heat and light stresses for 20 days stimulate accumulation of cordycepin in the order of heat stress > light stress > double stress. When treated for 20 days under heat stress, a maximum content (5.56 ± 0.05 mg/g) was achieved, almost two times more than in the control group. It was also higher than the average cordycepin content of 2.89 ± 1.99 mg/g (Lee et al. 2017) and 0.97 ± 0.02 mg/g (Guo et al. 2016) cultivated on rice or wheat medium. Strain mating was used to produce higher cordycepin content, and the maximum was 6.63 mg/g with an average of 2.98 ± 1.41 mg/g (Kang et al. 2017). However, the method was laborious and involved steps in separating single-spore strains, mating, and screening. The cordycepin content is strain-dependent, and we have verified the effect of heat stress on the cordycepin content with different strains. It was confirmed that heat stress stimulated the cordycepin production without affecting the biological efficiency in C. militaris (Fig. S2 and S3).
Many studies have been performed on the cordycepin biosynthetic pathway (Lin et al. 2016), and Xia et al. (2017) reported the putative pathway based on gene deletion and heterologous expression experiments. The gene cluster encodes an oxidoreductase (CCM_04436), metal-dependent phosphohydrolase (CCM_04437), and ATP phosphoribosyltransferase (CCM_04438), which are responsible for cordycepin biosynthesis from adenosine. The expression levels of genes encoding CCM_04437 and CCM_04438 increased significantly in the heat stress group compared to those in the control and remained at high levels during the heat stress period, which explains the cordycepin content increase in response to heat stress. With regard to the effects of heat stress on the development of fungi, further studies have demonstrated that the rapid protective transcriptional program, including heat shock proteins and heat shock factors, plays a vital role in the heat stress response (Zhang et al. 2016), which will be our next concern.
Adenosine, a natural and ubiquitous nucleoside, has a number of biological activities that allow it to be used as a cardio-protective and therapeutic agent for chronic heart failure (Kitakaze and Hori 2000) as well as a homeostatic modulator in the central nervous system (Gomes et al. 2011). The adenosine content also increased with the growth period, except in the double stress group. The content of about 2 mg/g was similar to the amounts in our previous report (Guo et al. 2016). Heat stress or light stress had no significant effect on the adenosine content.
HEA, the first calcium antagonist derived from a biological source (Furuya et al. 1983), has been intensively investigated because of its ability to decrease inflammation (Lu et al. 2015), protect kidneys, and function as a sedative (Chai et al. 2004) and an insecticide (Fang et al. 2016). However, these studies were mainly concentrated on Isaria cicadae. The present study confirmed HEA production in the fruit body of C. militaris. HEA content of the control group was lower than that of the other groups, which indicated that both heat and light stresses promoted HEA production.
Nucleosides, including adenosine, cordycepin, and HEA, represent the major active components in Cordyceps spp. (Li et al. 2006). Adenosine might be a direct precursor of cordycepin as reported in a previous study (Masuda et al. 2007), and therefore, accumulation of adenine or adenosine could result in increased cordycepin production. The positive correlation observed between the cordycepin and adenosine contents in this study support the hypothesis that adenosine is the precursor of cordycepin.
C. militaris is also a rich source of carotenoids, and its carotenoid content is higher than that of other known mushrooms (Yang et al. 2014). Many reports have confirmed that light is necessary for carotenoid production in C. militaris (Dong et al. 2013; Yang and Dong 2014). However, light stress at 6000 lx may inhibit the accumulation of carotenoids in the C. militaris fruit body, as demonstrated in this study. Colletotrichum gloeosporioides is also reported to produce less carotenoids under an excessive light intensity (Fu and Wang 2005).
Observations of the physical appearance and carotenoid content demonstrated that heat stress at 25 °C could stimulate the accumulation of carotenoids in the fruit body of C. militaris. The maximal content (4204.688 ± 19.887 μg/g) was achieved after being treated for 20 days, almost two times in amount of that of the control and that in our previous report (Guo et al. 2016). It has been reported that growth and carotenoid production have different optimal temperatures in many species. At a higher growth temperature (33 °C), cellular accumulation of lutein in Muriellopsis sp. was increased by up to sixfold, but the volumetric level was higher at 28 °C (Del Campo et al. 1999). The maximum production of carotenoids Sporobolomyces ruberrimus occurred at 19 °C and the minimum at 27 °C, but the maximum growth rate was at 27 °C (Razavi and Marc 2006). The carotenoid biosynthesis pathway is still unknown in C. militaris, and identification of this mechanism is an ongoing project in our research group.
References
Ahn YJ, Park SJ, Lee SG, Shin SC, Cho DH (2000) Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J Agric Food Chem 48(7):2744–2748
Calvo AM, Wilson RA, Bok JW, Keller NP (2002) Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev 66(3):447–459
Chai YQ, Wei ZM, Chen ZA, Li XL, Liu YG, Wang GE (2004) N6-(2-hydroxyethyl)-adenosine’ application in the preparation of analgesic drugs. China, ZL200410094511.0. (in Chinese)
Chang ST, Miles PG (2004) Mushrooms: cultivation, nutritional value, medicinal effect and environmental impact. CRC Press LLC 38(4):688–692
Chen C, Bau T, Bao HY (2013) Chemical composition analysis of cultured Cordyceps militaris. Food Sci 34(11):36–40 (in Chinese)
Chen AH, Wang YL, Shao Y, Huang B (2017) A novel technique for rejuvenation of degenerated caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), a valued traditional Chinese medicine. Int J Med Mushrooms 19(1):87–91
Cunningham KG, Manson W, Spring FS, Hutchinson SA (1950) Cordycepin, a metabolic product from cultures of Cordyceps militaris (Linn.) Link. Nature 166(4231):949
Das SK, Masuda M, Hatashita M, Sakurai A, Sakakibara M (2008) A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett Appl Microbiol 47(6):534–538
Das SK, Masuda M, Sakurai A, Sakakibara M (2010) Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia 81(8):961–968
Del Campo JA, Moreno J, Rodriguez H, Vargas MA, Rivas J, Guerrero MG (1999) Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76(1):51–59
Dong CH, Yao YJ (2011) On the reliability of fungal materials used in studies on Ophiocordyceps sinensis. J Ind Microbiol Biotechnol 38(8):1027–1035
Dong JZ, Wang SH, Ai XR, Yao L, Sun ZW, Lei C, Wang Y, Wang Q (2013) Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J Func Foods 5(3):1450–1455
Fang M, Chai YQ, Chen GJ, Wang HD, Huang B (2016) N6-(2-hydroxyethyl)-adenosine exhibits insecticidal activity against Plutella xylostella via adenosine receptors. PLoS One 11(9):e0162859
Fu MJ, Wang XJ (2005) Accumulation of carotenoid in Colletotrichum gloeosporioides induced by blue light. Acta Microbiol Sin 45(5):795–797
Furuya T, Hirotani M, Matsuzawa M (1983) N6-(2-hydroxyethyl)-adenosine, a biologically active compound from cultured mycelia of Cordyceps and Isaria species. Phytochemistry 22(11):2509–2512
Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808(5):1380–1399
Guo MM, Guo SP, Yang HJ, Bu N, Dong CH (2016) Comparison of major bioactive compounds of the caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), fruiting bodies cultured on wheat substrate and pupae. Int J Med Mushrooms 18(4):327–336
Huang SJ, Lin CP, Mau JL, Li YS, Tsai SY (2015) Effect of UV-B irradiation on physiologically active substance content and antioxidant properties of the medicinal caterpillar fungus Cordyceps militaris (Ascomycetes). J Med Mushrooms 17(3):241–253
OncoVista Inc. (2008) A phase I/II study of cordycepin plus pentostatin in patients with refractory TdT-positive leukemia. Clinical Trials. https://clinicaltrials.gov/show/NCT00709215. Accessed 2 Jan 2018
Kang N, Lee HH, Park I, Seo YS (2017) Development of high cordycepin-producing Cordyceps militaris strains. Mycobiology 45(1):31–38
Kitakaze M, Hori M (2000) Adenosine therapy: a new approach to chronic heart failure. Expert Opin Investig Drugs 9(11):2519–2535
Kojima M, Kimura N, Miura R (2015) Regulation of primary metabolic pathways in oyster mushroom mycelia induced by blue light stimulation: accumulation of shikimic acid. Sci Rep 5:8630. https://doi.org/10.1038/srep08630
Kornerup A, Wanscher JH (1978) Methuen handbook of colour. EyreMethuen, London
Krupodorova TA, Barshteyn VY (2015) Alternative substrates for higher mushrooms mycelia cultivation. J Biosci Bioeng 4(3):339–347
Lee HH, Kang N, Park I, Park J, Kim I, Kim J, Kim N, Lee JY, Seo YS (2017) Characterization of newly bred Cordyceps militaris strains for higher production of cordycepin through HPLC and URP-PCR analysis. J Microbiol Biotechnol 27(7):1223–1232
Leonardi P, Iotti M, Zeppa SD, Lancellotti E, Amicucc A, Zambonelli A (2017) Morphological and functional changes in mycelium and mycorrhizas of Tuber borchii due to heat stress. Fungal Ecol 29:20–29
Li SP, Yang FQ, Tsim KW (2006) Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal 41(5):1571–1584
Lian TT, Dong CH, Yang T, Sun JD (2014) Three types of geranylgeranyl diphosphate synthases from the medicinal caterpillar fungus, Cordyceps militaris (Ascomycetes). Int J Med Mushrooms 16(2):115–124
Lim LT, Lee CY, Chang ET (2012) Optimization of solid state culture conditions for the production of adenosine, cordycepin, and D-mannitol in fruiting bodies of medicinal caterpillar fungus Cordyceps militaris (L.:Fr.) Link (Ascomycetes). Int J Med Mushrooms 14(2):181–187
Lin S, Liu ZQ, Xue YP, Baker PJ, Wu H, Xu F, Teng Y, Brathwaite ME, Zheng YG (2016) Biosynthetic pathway analysis for improving the cordycepin and cordycepic acid production in Hirsutella sinensis. Appl Biochem Biotechnol 179(4):633–649
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and 2-∆∆C(T) method. Methods 25:402–408
Lu ZL, Kong XX, Lu ZM, Xiao MX, Chen MY, Zhu L, Shen YM, Hu XY, Song SY (2014) Para-aminobenzoic acid (PABA) synthase enhances thermotolerance of mushroom Agaricus bisporus. PLoS One 9(3):e91298
Lu MY, Chen CC, Lee LY, Lin TW, Kuo CF (2015) N6-(2-hydroxyethyl)-adenosine in the medicinal mushroom Cordyceps cicadae attenuates lipopolysaccharide-stimulated pro-inflammatory responses by suppressing TLR4-mediated NF-kB signaling pathways. J Nat Prod 78(10):2452–2460
Mao XB, Zhong JJ (2004) Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol Prog 20(5):1408–1413
Masuda M, Urabe E, Honda H, Sakurai A, Sakakibara M (2007) Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzym Microb Technol 40:1199–1205
Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2017) Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174(11):1290–1324
Oh TJ, Hyun SH, Lee SG, Chun YJ, Sung GH, Choi HK (2014) NMR and GC-MS based metabolic profiling and free-radical scavenging activities of Cordyceps pruinosa mycelia cultivated under different media and light conditions. PLoS One 9(6):e90823
Razavi SH, Marc I (2006) Effect of temperature and pH on the growth kinetics and carotenoid production by Sporobolomyces ruberrimus H110 using technical glycerol as carbon source. Iran J Chem Chem Eng 23(3):59–64
Shrestha B, Han SK, Lee WH, Choi SK, Lee JO, Sung JM (2005a) Distribution and in vitro fruiting of Cordyceps militaris in Korea. Mycobiology 33(4):178–181
Shrestha B, Han SK, Yoon KS, Sung JM (2005b) Morphological characteristics of conidiogenesis in Cordyceps militaris. Mycobiology 33(2):69–76
Shrestha B, Han SK, Sung JM, Sung GH (2012a) Fruiting body formation of Cordyceps militaris from multi-ascospore isolates and their single ascospore progeny strains. Mycobiology 40(2):100–106
Shrestha B, Zhang WM, Zhang YJ, Liu XZ (2012b) The medicinal fungus Cordyceps militaris: research and development. Mycol Prog 11(3):599–614
Song C, Chen Q, Wu XL, Zhang JX, Huang CY (2014) Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr Microbiol 69(5):611–616
Sung JM, Park YJ, Lee JO, Han SK, Lee WH, Choi SK, Shrestha B (2006) Selection of superior strains of Cordyceps militaris with enhanced fruiting body productivity. Mycobiology 34(3):131–137
Tuli HS, Sharma AK, Sandhu SS, Kashyap D (2013) Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci 93(23):863–869
Wu CY, Liang ZC, Tseng CY, Hu SH (2016) Effects of illumination pattern during cultivation of fruiting body and bioactive compound production by the caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes). Int J Med Mushrooms 18(7):589–597
Xia YL, Luo FF, Shang YF, Chen PL, Lu YZ, Wang CS (2017) Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem Biol 24:1–11
Xiao JH, Chen DX, Liu JW, Liu ZL, Wan WH, Fang N, Xiao Y, Qi Y, Liang ZQ (2004) Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. J Appl Microbiol 96(5):1105–1116
Xie CY, Gu ZX, Fan GJ, Gu FR, Han YB, Chen ZG (2009) Production of cordycepin and mycelia by submerged fermentation of Cordyceps militaris in mixture natural culture. Appl Biochem Biotechnol 158(2):483–492
Xu CP, Kim SW, Hwang HJ, Choi JW, Yun JW (2003) Optimization of submerged culture conditions for mycelia growth and exo-biopolymer production by Paecilomyces tenuipes C240. Process Biochem 38(7):1025–1030
Yan XT, Bao HY, Bau T (2010) Isolation and identification of one natural pigment from cultured Cordyceps militaris. Mycosystema 29(5):777–781
Yang T, Dong CH (2014) Photo morphogenesis and photo response of the blue-light receptor gene Cmwc-1 in different strains of Cordyceps militaris. FEMS Microbiol Lett 352(2):190–197
Yang T, Sun JD, Lian TT, Wang WZ, Dong CH (2014) Process optimization for extraction of carotenoids from medicinal caterpillar fungus, Cordyceps militaris. Int J Med Mushrooms 16(2):125–135
Yang T, Guo MM, Yang HJ, Guo SP, Dong CH (2016) The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl Microbiol Biotechnol 100(2):743–755
Yu L, Zhao J, Li SP, Fan H, Hong M, Wang YT, Zhu Q (2006) Quality evaluation of Cordyceps through simultaneous determination of eleven nucleosides and bases by RP-HPLC. J Sep Sci 29(7):953–958
Zhang W, Tang YJ (2008) A novel three-stage light irradiation strategy in the submerged fermentation of medicinal mushroom Ganoderma lucidum for the efficient production of ganoderic acid and Ganoderma polysaccharides. Biotechnol Prog 24(6):1249–1261
Zhang X, Ren A, Li MJ, Cao PF, Chen TX, Zhang G, Shi L, Jang AL, Zhao MW (2016) Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Appl Environ Microbiol 82(14):4112–4125
Zheng P, Xia YL, Xiao GH, Xiong CH, Hu X, Zhang SW, Zheng HJ, Huang Y, Zhou Y, Wang SY, Zhao GP, Liu XZ, Leger R, Wang CS (2011) Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol 12:R116
Funding
This study was funded by the National Natural Science Foundation of China (31572179, 31600054), the Coal-based Key Scientific and Technological Project from Shanxi Province (FT2014-03-01), and the Key Research and Development Program from Guangxi Province (2016AB05317).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 171 kb)
Rights and permissions
About this article
Cite this article
Jiaojiao, Z., Fen, W., Kuanbo, L. et al. Heat and light stresses affect metabolite production in the fruit body of the medicinal mushroom Cordyceps militaris. Appl Microbiol Biotechnol 102, 4523–4533 (2018). https://doi.org/10.1007/s00253-018-8899-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8899-3