Abstract
We examined the effects of abiotic (methyl jasmonate [MeJA] and salicylic acid [SA]) and biotic (yeast extract and chitosan) elicitors for improvement of bioactive compounds production on adventitious root cultures in Polygonum multiflorum. The application of yeast extract resulted in significantly (p ≤ 0.05) higher dry root biomass (9.98 g/L) and relative growth rate versus the control. Cultures treated with abiotic elicitors showed higher percentage of dry weight than the other samples. Low concentrations of all elicitors (50 μM MeJA and SA, and 50 mg/L yeast extract) improved secondary metabolite production except for chitosan, whose performance was worse than that of the control. HPLC analysis of various bioactive compounds revealed significantly higher elicitation efficiency for MeJA than for the other treatments, with an approximately 2-fold increase in root dry weight (22.08 mg/g DW) under 50 μM MeJA treatment versus the control (10.35 mg/g DW). We also investigated the feasibility of scaling up the production process by comparing shake flask cultures with 3- and 5-L balloon type bubble bioreactors (BTBB) using 50 μM MeJA as an elicitor. Growth and metabolite accumulation increased in BTBB compared with shake flask cultures. We detected a non-significant difference in biomass productivity between 3 and 5-L BTBB, but the efficiency of bioactive compound accumulation decreased with increasing volume. These findings will be useful for developing a pilot-scale P. multiflorum adventitious root cultivation process for high biomass and bioactive compound production to meet the demands for natural ingredients by the pharmaceutical and cosmetic industries without affecting the natural habitat of this plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polygonum multiflorum Thunb., a perennial vine native to China, has enormous ethno-pharmacological significance, which has led to its inclusion in Chinese Pharmacopoeia (Thiruvengadam et al. 2014; Bounda and Feng 2015). The root extracts of this herb were traditionally used as a hair dye, a liver and kidney tonic, and an anti-aging ingredient in China, Korea, and Japan (Han et al. 2015). Various investigations have also demonstrated a much wider role for bioactive compounds from P. multiflorum, which have anti-tumor, anti-diabetic, and anti-hyperlipidemic properties, besides being effective in the management of serious disorders such as cardiometabolic issues and inflammation (Hong et al. 1994; Lin et al. 2003). This plant species is widely distributed around the globe and is used in a number of herbal drugs. P. multiflorum roots contain an array of phenolic compounds including stilbenes, anthraquinones, and phospholipids (Yi et al. 2007). Since roots are the main source material for the extraction of secondary metabolites, the P. multiflorum population in its natural habitat is adversely affected when the plants are uprooted. Also, the demand for such natural ingredients for therapeutic and personal care purposes has been escalating. These metabolites are produced at very low levels, which depend on both the physiology and developmental stage of the plant (Rao and Ravishankar 2002). Besides low yields, dwindling natural plant populations, and metabolic variability, geographic and environmental factors, along with the poor supply of safe, quality ingredients, further jeopardize the use of this plant by the pharmaceutical and personal care manufacturing sectors.
Plant cell, tissue, and organ culture represents the best alternative to conventional methods for increasing the yields of bioactive compounds to meet the mounting industrial demands for such natural, low molecular weight molecules. Conventional plant tissue cultures are a feasible system for obtaining commercially important secondary metabolites while helping to conserve plant bioresources in their natural habitat; however, the low, unstable metabolic yields were a major bottleneck to further developing this technology (Bourgaud et al. 2001; Murthy et al. 2016). Besides, bioreactors as systems provide a stable yield in limited space and controlled conditions for adventitious root cultures. Thus, adventitious root cultures in bioreactor are known for their rapid growth, with stable metabolic profiles similar to those of field-grown plants (Lee et al. 2015; Murthy et al. 2016). We previously optimized various process parameters and successfully produced P. multiflorum adventitious roots in bioreactor systems (Ho et al. 2017).
Among the various biotechnological approaches, elicitation is an effective tool for improving the biosynthesis of secondary metabolites (Radman et al. 2003). Furthermore, a number of studies have employed plant defense mechanisms using elicitors to increase the yields of secondary metabolites under in vitro conditions (Zhao et al. 2005). Elicitors can primarily be grouped into biotic and abiotic compounds, depending on their nature or form. Polysaccharides of biological origin derived from microbes, seaweed, and so on are considered to be biotic elicitors, while abiotic compounds include a range of physical (thermal, radiation, and drought) and chemical (heavy metal, minerals, and hormones) stress compounds (Namdeo 2007). Specific receptors that bind to the cell membrane recognize most elicitors, which stimulate signal transduction systems by activating specific genes that in turn speed up secondary metabolism (Baenas et al. 2014). In adventitious root cultures, methyl jasmonate (MeJA) acts as a signaling molecule, while the phytohormone salicylic acid (SA) mediates the phenylpropanoid pathway and is effective in triggering the stress response through elicitation (Lu 2009; Hu et al. 2009; Lee et al. 2015). Cell wall-derived polysaccharides such as chitin and chitosan (CH) are also efficient biotic elicitors, which trigger depolarization of the cell membrane, along with a subsequent change in protein flux and an alteration in cell permeability (Ramirez-Estrada et al. 2016). However, the appropriate elicitor types and concentrations vary with plant species as well as target metabolite. It is therefore important to investigate the effects of such compounds or factors on growth and metabolic yield in commercially important medicinal plants. In the present study, we treated adventitious root cultures of P. multiflorum with various elicitors in a dose-dependent manner to evaluate their effects on root biomass and yield and the production of bioactive compounds.
Materials and methods
P. multiflorum adventitious root culture
P. multiflorum adventitious roots that were previously induced from leaf explants were maintained in suspension cultures in MS (Murashige and Skoog 1962) medium supplemented with indole-3-butyric acid (9.84 μM) and 5% sucrose (Ho et al. 2017). The root cultures were regularly subcultured at 4-week intervals and incubated in the dark at 24 ± 1 °C with shaking at 100 rpm.
Elicitor preparation
Elicitation was carried out with abiotic (MeJA, SA) and biotic (yeast extract [YE], CH) elicitors. MeJA and SA (Sigma Chemical Co., MO, USA) stock solutions (50 mM) were prepared in absolute ethanol and filter-sterilized. YE (Duchefa, the Netherlands) was dissolved in distilled water to produce stock solution (10 mg/mL) and autoclaved. CH (Sigma Chemical Co., MO, USA) stock solution (10 mg/mL) was prepared by adding glacial acetic acid dropwise at 60 °C for 15 min (final concentration, 2%; v/v). The solution was brought up to a final volume (100 mL) with distilled water before autoclaving.
Elicitor treatment
Adventitious roots were subcultured in a 250-mL flask containing 100 mL MS liquid medium to study the effects of elicitors. After 3 weeks of culture, various concentrations of elicitors (MeJA 50, 100, 200, 400 μM; SA 50, 100, 200, 400 μM; YE 50, 100, 200, 400 mg/L; and CH 50, 100, 200, 400 mg/L) were added to the culture medium. After 1 week of co-cultivation with elicitors, the adventitious roots were harvested for further analysis.
Culturing in a bioreactor system
The effect of MeJA was further verified via scale-up production of P. multiflorum adventitious roots in 3-L (2-L medium) and 5-L (3-L medium) balloon type (air lift) bubble bioreactor (BTBB) systems (Baque et al. 2012; Ho et al. 2017). Each bioreactor contained full-strength MS medium supplemented with 9.84 μM IBA, 5% sucrose, and inoculum density at 5 g/L of adventitious root fresh weight. The incubation was carried out at fixed temperature (24 ± 1 °C) and dissolved oxygen [0.1 vvm (air volume/culture volume min)] under dark conditions throughout the culture period. MeJA (50 μM) was added to the culture medium after 3 weeks of culture, and harvested after 1 week of treatment to evaluate the effect of the elicitor on adventitious root growth and bioactive compound accumulation. The production levels of bioactive compounds from in vitro-grown roots versus in situ roots from 2- and 5-year-old field-grown plants were compared. The plants were obtained from a greenhouse at the Floriculture and Biotechnology Laboratory, Chungbuk National University, Korea.
Determination of root biomass
P. multiflorum roots were harvested after 4 weeks of culture and washed with distilled water. The free surface water was removed by blotting with tissue paper, and the fresh weight (FW) was measured. The dry weight (DW) was recorded after drying at 60 °C after achieving no change in weight. The growth index, overall productivity, and relative growth rate were determined as follows:
Growth index (GI) = [Final dry weight (g) − initial dry weight (g)] / initial dry weight (g)
Relative growth rate (RGR) = [lnW2 − lnW1] / CP, where ln: natural log, W1 and W2: initial and final weights, respectively, and Cp: culture period.
Overall productivity (P, mg/L, w/v) = [metabolite content (mg/g root biomass, DW) × volumetric root biomass yield (g/L, DW)]
Quantification of total phenolic and flavonoid contents
Preparation of root extracts
Phenolic compounds were extracted from dried roots (0.5 g) by refluxing (LS-2050-S10, LS-TECH, Korea) with 15 mL 80% methanol at 80 °C for 1 h and filtered through filter paper (Advantec 110 mm, Toyo Roshi Kaisha Ltd., Japan). The final volume of the solution was brought to 15 mL using absolute methanol.
Determination of total phenolic content
The Folin-Ciocalteu colorimetric method was used to quantify total phenolics (Folin-Ciocalteu 1927). The methanolic extracts (0.05 mL) were mixed with distilled water (2.55 mL), followed by the addition of Folin-Ciocalteu reagent (0.1 mL, 2 N). After 5 min, the reagent was combined with 2.5 mL Na2CO3 solution (20%) and incubated in the dark at room temperature. The absorbance (change in color) at 760 nm after 30 min was measured with a spectrophotometer (Optizen POP, Mecasys Co., Ltd., Korea). A standard curve obtained with gallic acid (Sigma Chemical Co., St. Louis, MO, USA) was used for data comparison, and total phenolic content (TPC) was expressed as milligrams of gallic acid equivalent (GAE) per gram of DW adventitious roots.
Determination of total flavonoid content
The colorimetrical method was used to measure total flavonoid content (TFC) (Wu et al. 2006). Methanolic root extracts and (+)-catechin (Sigma Chemical Co., St. Louis, MO, USA) standard (0.25 mL) were prepared in 1.25 mL distilled water. NaNO2 (0.075 mL 5% NaNO2) was added to the solution, followed by vigorous shaking. After 6 min of reaction time, 0.15 mL AlCl3 solution (10%) was added to the sample, followed by incubation for 5 min at room temperature. The absorbance at 510 nm was measured using a spectrophotometer (Optizen POP, Mecasys Co., Ltd., Korea). The results were expressed as milligrams of (+)-catechin equivalents (CE) per gram of DW adventitious roots.
Determination of free radical scavenging (DPPH activity)
The antioxidant capacity of P. multiflorum adventitious roots was measured using the 1,1-diphenyl-2-picrylhydrazyl (DPPH, Sigma Chemical Co., St. Louis, MO, USA) method (Hatano et al. 1998). DPPH radical solution (0.8 mL 200 μM DPPH) was added to 0.2 mL methanolic root extract, while 40% methanol served as a control. The prepared solution was incubated for 5 min at room temperature, and the absorbance at 517 nm was measured using a spectrophotometer (Optizen POP, Mecasys Co., Ltd., Korea). The results were presented as follows:
Determination of phenolic compounds by HPLC
Root powder (0.5 g) was sonicated (Sonicator, Mujigae, Korea) for 3 h in 80% methanol to ensure complete extraction. The extract was filtered through filter paper (Advantec, 110 mm, Japan), and the solvent was evaporated. The dried residue was dissolved in 10% methanol and fractionated twice with 10 mL diethyl-ether/ethyl-acetate (1:1) prior to evaporation under a vacuum to dryness. The residues of both fractions were combined and dissolved in methanol prior to filtration through a membrane filter (0.2 μm pore size; Whatman, England). A photodiode array (PDA)-equipped HPLC (2690 Separations Module, Waters Chromatography, Milford, MA, USA) system was used to measure phenolic compounds. Separation was performed using a Fortis C18 column (5 μL, 150 × 4.6 mm). Acetonitrile (A) and 0.1% aqueous acetic acid (v/v) (B) was used as the mobile phase with linear gradients of 8–10% A at 0–2 min, 10–30% A at 2–27 min, 30–90% A at 27–50 min, 90–100% A at 50–51 min, 100% A at 51–60 min, and 100–8% A at 60–70 min. The column was re-equilibrated for 10 min between injections at a 1.0-mL/min flow rate, and 20-μL aliquots were injected into the HPLC at a time. Calibration plots were obtained by measuring the peak areas. UV absorption spectra and retention time were used as criteria for the identification of individual compounds.
Statistical analysis
The experiments were performed in a completely randomized design with three replicates, unless otherwise specified. Significant differences were determined by Duncan’s Multiple Range Test (DMRT) using SAS software (Version 9.4; SAS Institute, USA).
Results
Secondary metabolites help plants adapt to environmental stress, and serve as defensive or protective bioactive molecules against microorganisms, insects, and higher herbivorous predators. In the present study, we investigated the effects of elicitors on enhancing the accumulation of bioactive compounds in P. multiflorum adventitious root cultures.
Effect of types and concentrations of elicitors on biomass and bioactive compound production of P. multiflorum
Adventitious root growth
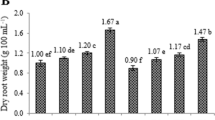
The results suggest that elicitor treatment suppresses adventitious root growth (except for YE at low concentrations) compared with control cultures (Table 1). However, no significant difference was observed in the FW of root biomass between the control and YE treatment at concentrations of 50 and 100 mg/L. The highest DW was recorded at 100 mg/L YE (9.98 g/L), followed by control root samples. The root biomass decreased with increasing elicitor concentration. Moreover, the percentage of DW was higher under most elicitor treatments than under the control, with the highest value recorded under 50 μM MeJA (13.14%) treatment. The growth index (18.96) and relative growth rates (2.32) were highest in 100 mg/L YE-treated root samples, whereas these values were otherwise adversely influenced by elicitor treatment.
Phenolic compound accumulation and antioxidant activity
Elicitation led to an increase in total phenolic and flavonoid contents compared with the control in adventitious roots of P. multiflorum, except in CH-treated samples (Fig. 1). The accumulation in phenolic compounds appeared to be dependent on the concentration of the elicitor, as the levels of these compounds decreased with increasing elicitor concentration. Among the elicitors, MeJA treatment led to the highest yields of bioactive compounds, followed by SA and YE (Fig. 1). The highest contents of phenolics (55.45 mg/g DW) and flavonoids (29.79 mg/g DW) were obtained under 50 μM MeJA treatment (Fig. 1a).
We also performed quantitative estimation of major bioactive compounds by HPLC analysis (Fig. 2). Treatment with abiotic elicitors led to significantly higher yields of total phenolic compounds compared with the control and biotic elicitor treatments. Some of the elicitors had much stronger stimulating effects on phenolic compound accumulation compared with their suppressive effects on adventitious root biomass. In particular, phenolic levels increased approximately 2-fold in adventitious root samples treated with 50 μM MeJA (22.08 mg/g DW) versus the control (10.08 mg/g DW), whereas only an ~ 20% decrease in DW occurred in these samples. In addition, analysis of the HPLC profiles of elicitor-treated root samples showed that the type of elicitor used influenced the biosynthetic capacity of adventitious roots for specific metabolites. CH treatment increased the accumulation of anthraquinones, especially physcion (more than 0.30 mg/g DW), compared with the control and other elicitor treatments. These results indicate that abiotic elicitors have stronger effects on the accumulation of bioactive compounds than biotic elicitors and that the type of elicitor used influences the biosynthesis of specific metabolites.
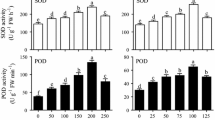
In addition, we investigated the antioxidant activity based on DPPH free radical scavenging to examine the effect of elicitors on redox potential. Figure 3 shows the changes in antioxidant potential of P. multiflorum adventitious root cultures after treatment with various elicitors. Similar to the yields of phenolic compounds, DPPH radical scavenging activity was significantly higher in elicitor-treated samples than in the control, except for CH-treated samples. We detected a positive correlation between DPPH radical scavenging activity and phenolic accumulation, which varied with elicitor type and concentration (Fig. 3). In summary, treatment with 50 μM MeJA is an effective way to enhance the biosynthesis of phenolic compounds in P. multiflorum adventitious root cultures without compromising the growth of adventitious root biomass (Fig. 4).
Application of bioreactor systems
Adventitious root growth
We attempted to scale up the production of P. multiflorum adventitious roots in 3- and 5-L balloon type bubble bioreactors (BTBB) using 50 μM MeJA as an elicitor (Fig. 5). We observed a significant (p ≤ 0.05) increase in root biomass in cultures scaled up from a shake flask to a bioreactor (Table 2). In addition, productivity increased significantly in the BTBB systems, from 7.60 g/L in flasks to 11.47 and 9.99 g/L in 3- and 5-L BTBB, respectively. However, no significant difference in productivity was observed during adventitious root cultivation in 3- versus 5-L BTBB. Our results suggest that BTBB is a suitable bioreactor system for industrial-scale production of P. multiflorum adventitious root biomass.
Schematic for improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum. a Plant in vitro. b Induced adventitious root. c Adventitious root in flask culture treated with 50 μM MeJA. d Adventitious root in the bioreactor system. e Adventitious root treated 50 μM MJ in the bioreactor. f Harvest adventitious root treated 50 μM MeJA in bioreactor
Phenolic compound production in flasks, bioreactors, and field-grown plants
The total phenolic and flavonoid contents were found to be at par in all in vitro production systems, with significantly high productivity in 3-L BTBB (621.98 mg/L) followed by 5-L BTBB and shake flask (Table 2). However, compared with in vitro cultured adventitious roots, the roots of field-grown (2- and 5-year-old) plants had significantly higher phenolic and flavonoid contents. The average bioactive contents were 1.82-fold (phenolics) and 1.5-fold (flavonoid) higher in field-grown samples than in BTBB and shake flask cultures. However, it is important to note that field cultivation of medicinal plants requires a long period to obtain sufficient yields of natural compounds, whereas these compounds can be obtained in only 4 weeks of in vitro adventitious root culture. Therefore, adventitious root culture represents an attractive method for producing commercially important metabolites in a short time without challenging the plant population in its natural habitat.
We also performed HPLC quantification of bioactive compounds from in vitro and in situ samples to investigate possible variability in their metabolic profiles (Fig. 4). Among in vitro adventitious root samples, we detected a significant difference in the yield of total phenolic compounds in shake flask versus BTBB. The bioreactor cultures showed higher accumulation of bioactive compounds (with increases of 3.12-fold for physcion, 1.89-fold for ferulic acid, 1.35-fold for p-hydroxybenzoic acid, and 2.42-fold for biochanin) than flask cultures. The metabolic yield in BTBB root samples was similar to that in 5-year-old field-grown samples. However, 2-year-old field-grown samples had significantly higher total phenolic compound levels (31.19 mg/g DW) than the other samples. In addition, some compounds, such as quercetin, kaempferol, biochanin, and naringenin, were present at lower levels in field-grown samples than in in vitro adventitious root cultures. Interestingly, higher levels of flavonol accumulation (quercetin and kaempferol) were detected in the 5-L BTBB root samples than in the other samples. Furthermore, 5-year-old root samples had significantly higher levels of anthraquinones, especially physcion (~ 13-fold higher), whereas 2-year-old root samples were rich in myricetin and ferulic acid compared with the other samples. The 2-year-old plant samples had significantly higher levels of bioactive compounds than the 5-year-old samples, except for physcion, emodin, and SA. These results indicate that anthraquinone (emodin and physcion) accumulation requires a long in situ cultivation period in P. multiflorum. Our comparison of total phenolic yields in BTBB samples versus 5-year-old field-grown plants strengthens the notion that in vitro adventitious root culture represents an effective alternative strategy for producing natural bioactive compounds without the need to harness natural bioresources.
Discussion
The metabolic profile of a plant is influenced by various microenvironmental and macroenvironmental conditions. The plant responds to its surrounding environmental factors (biotic and abiotic) through the stimulation of secondary metabolism to produce the desired compounds needed for its survival, a process known as elicitation (Gorelick and Bernstein 2014). In this study, we found that abiotic (MeJA, SA) and biotic (YE, CH) elicitors had a significant effect on root growth and the enhancement of phenolic compound accumulation in P. multiflorum adventitious root cultures. Among the various elicitor treatments, 50 μM MeJA was optimum with respect to adventitious root growth and bioactive compound accumulation. We detected a concentration-dependent effect of elicitors on root growth as well as metabolite accumulation in the present study. Elicitor treatment might have resulted in the production of reactive oxygen species (ROS) due to stress, which adversely affects root biomass. To mitigate the effects of ROS, plant tissues exhibit an increase in the production of secondary metabolites (Ali et al. 2007; Belhadj et al. 2008). The effect of MeJA on adventitious root growth was less pronounced than that of SA in the present study. Moreover, adventitious root growth was slightly influenced by increased concentrations of CH and YE.
MeJA treatment stimulates the biosynthesis of secondary metabolites in plant cell cultures via signal transduction, which speeds up enzyme catalysis, thereby leading to the formation of specific compounds such as polyphenol, terpenoids, flavonoids, and alkaloids (Mueller et al. 1993; Zhao et al. 2005). SA is another plant hormone that induces the expression of genes involved in the biosynthesis of specific plant secondary metabolites (Taguchi et al. 2001). In adventitious root cultures, the addition of MeJA and SA induces various defense mechanisms. Similar to the current findings, the adverse effects of higher concentrations of elicitors on adventitious biomass and metabolite accumulation were reported previously (Ali et al. 2007; Wu et al. 2014; Lee et al. 2015). Indeed, the biomass of adventitious root cultures of Panax ginseng (Yu et al. 2002), Hypericum perforatum (Wu et al. 2014), and Eleutherococcus koreanum (Lee et al. 2015) significantly decreased with increasing MeJA and SA concentration. Moreover, the addition of MeJA and SA to the culture medium induces defense mechanisms and stimulates the biosynthesis of various bioactive compounds in adventitious roots (Ali et al. 2007; Murthy et al. 2016). In the current study, we found that 50 μM MeJA was a suitable elicitor for increasing the production of phenolic compounds in P. multiflorum adventitious root cultures. Similarly, bioactive compound levels increase upon elicitation with MeJA and SA in Eleutherococcus sessiliflorus (Shohael et al. 2008) and Eleutherococcus koreanum (Lee et al. 2015). However, the optimal elicitor type and concentration vary among systems, pointing to the need to investigate the specificity of these compounds with respect to plant species or target bioactive compound. YE is often used as a biotic elicitor in plant secondary metabolite production. CH (deacetylated chitin) is a signaling molecule that has been intensively studied (Zhao et al. 2005; Cai et al. 2012). The stimulation of phenolic compound accumulation by biotic elicitors has also been observed in cell and adventitious root cultures. CH enhances withanolide production in Withania somnifera (Sivanandhan et al. 2012), polysaccharide elicitors enhance anthocyanin and phenolic compound production in Vitis vinifera (Cai et al. 2012), and YE increases total flavonoid accumulation (4.5-fold) in hairy root cultures of Pueraria candollei (Udomsuk et al. 2011).
The production of phenolic compounds was more pronounced in response to MeJA elicitation than to SA, YE, and CH, whereas the production of anthraquinones (emodin and physcion) increased more strongly in response to CH than to the other elicitors. Adventitious root cultures of P. multiflorum showed different patterns of phenolic compound accumulation with respect to the type of elicitor used. In a study on tanshinone accumulation in Salvia miltiorrhiza cultures, the production of cryptotanshinone increased in response to YE (34-fold), while tanshinone I production was stimulated (3.4-fold) by CH (Zhao et al. 2010). In this system, biotic elicitors more strongly stimulated the accumulation of tanshinone than MeJA and SA. Moreover, the effect of elicitors on specific plant tissues depends on the elicitor type and concentration, the plant species, the metabolite under question, and the stage and timing of elicitation (Cai et al. 2012). Elevated secondary metabolism is often negatively correlated with cell growth (Van der Plas et al. 1995). However, the results of the current study indicate that the effect of elicitors varies depending on the adventitious root biomass or specific secondary metabolite.
Secondary metabolites, particularly phenolic compounds, are involved in plant responses to biotic and abiotic stress and significantly contribute to the antioxidant activity of plant tissues (Ferrat et al. 2003). Elicitor treatment resulted in a significant increase in phenolic compound accumulation in P. multiflorum adventitious root cultures, which might be responsible for enhancing DPPH radical scavenging activity. Similarly, in Vitis vinifera cell culture, treatment with a polysaccharide elicitor increased DPPH radical scavenging activity, and a positive correlation was reported between DPPH radical scavenging activity and the accumulation of phenolic compounds (Cai et al. 2012). In hairy root suspension cultures of Solanum trilobatum, elevated levels of phenolics in roots grown in medium treated with MeJA were correlated with improved DPPH radical scavenging activity (Shilpha et al. 2016).
A comparative evaluation of field-grown plants versus in vitro cultures revealed high phenolic contents in the in situ samples, along with different metabolic profiles, which was expected considering the completely different growth environments in these systems. During plant growth, secondary metabolite accumulation changes depending on the environment, the developmental stage, and the roles of specific compounds during plant growth (Kim et al. 2014; Liu et al. 2016). In Panax ginseng, Liu et al. (2016) reported that the total ginsenoside content rapidly increased from the first to fourth year of growth. During the fourth year, the contents of Rg1, Re, and Rf increased 2.5-, 2.7-, and 3-fold, respectively, but the content of Rg3 significantly decreased. Bioreactor culture systems represent more advanced technology than flask cultures, enabling the production of high levels of secondary metabolites from plant cell, tissue, and organ cultures (Murthy et al. 2014; Wu et al. 2014). Based on previous data, 50 μM MeJA was used in bioreactor culture. We found that P. multiflorum adventitious roots grown in a bioreactor had higher biomass and secondary metabolite production than those in flask culture when treated with 50 μM MeJA. Wu et al. (2014) found that the hypericin content is enhanced by the addition of 350 μM MeJA during adventitious root culture in Hypericum perforatum. Lee et al. (2015) reported that 50 μM MeJA is the optimal elicitor for biomass and bioactive compound production in Eleutherococcus koreanum. Therefore, the current and previous studies indicate that adventitious root culture in a bioreactor system supplied with an elicitor is a good alternative to field cultivation, yielding high biomass and high bioactive compound levels in a short time (4 weeks), along with enhanced secondary metabolite production.
In conclusion, adventitious root cultures induced from P. multiflorum leaf explants are influenced by both abiotic and biotic elicitor treatments. At low concentrations, all of the elicitors examined improved secondary metabolite production. Treatment with 50 μM MeJA resulted in the highest accumulation of bioactive compounds without compromising root growth. HPLC quantification of various bioactive compounds also revealed the significantly high elicitation efficiency of MeJA. Scale-up of the production process from shake flasks to BTBB led to high levels of growth as well as metabolite accumulation. Thus, the culture system developed in this study can be replicated on a large scale for industrial production of P. multiflorum adventitious roots with high biomass and high bioactive compound accumulation. This alternative system for the production of natural ingredients can be used to help meet the demands for natural ingredients by the pharmaceutical and cosmetic industries without affecting the natural habitat of P. multiflorum.
References
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension culture. Molecules 12(3):607–621. https://doi.org/10.3390/12030607
Baenas N, Garcia-Viguera C, Moreno DA (2014) Elicitation: a tool for enriching the bioactive composition of foods. Molecules 19(9):13541–13563. https://doi.org/10.3390/molecules190913541
Belhadj A, Teleg N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Merillon JM (2008) Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46(4):493–499. https://doi.org/10.1016/j.plaphy.2007.12.001
Baque MA, Moh SH, Lee EJ, Zhong JJ, Paek KY (2012) Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol Adv 30(6):1255–1267. https://doi.org/10.1016/j.biotechadv.2011.11.004
Bounda GA, Feng Y (2015) Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharm Res 7:225–236
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161(5):839–851. https://doi.org/10.1016/S0168-9452(01)00490-3
Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I (2012) Polysaccharide elicitor enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 108(3):401–409. https://doi.org/10.1007/s11240-011-0051-3
Ferrat L, Pergent-Martini C, Roméo M (2003) Assessment of the use of biomarkers in aquatic plants for the evaluation of environmental quality: applications to seagrasses. Aquatic oxicology 65(2):187–204. https://doi.org/10.1016/S0166-445X(03)00133-4
Folin O, Ciocalteu V (1927) On trysonic and tryptophane determination in protein. J Biol Chem 27:627–650
Gorelick J, Bernstein N (2014) Elicitation: an underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv Agron 124:201–230. https://doi.org/10.1016/B978-0-12-800138-7.00005-X
Han MN, Lu JM, Zhang GY, Yu J, Zhao RH (2015) Mechanistic studies on the use of Polygonum multiflorum for the treatment of hair graying. BioMed Res Int. https://doi.org/10.1155/2015/651048
Hatano T, Kagawa H, Yasuhara T, Okuda T (1998) Two new flavonoids and other constituents in licorice: their relative astringency and radical scavenging effects. Chem Pharm Bull 36:2090–2097
Ho TT, Lee KJ, Lee JD, Bhushan S, Paek KY, Park SY (2017) Adventitious root culture of Polygonum multiflorum for phenolic compounds and its plot-scale production in 500 L-tank. Plant Cell Tissue Organ Cult 130(1):167–181. https://doi.org/10.1007/s11240-017-1212-9
Hong CY, Lo YC, Tan FC, Wei YH, Chen CF (1994) Astragalus membranaceus and Polygonum multiflorum protect rat heart mitochondria against lipid peroxidation. Am J Chin Med 22(01):63–70. https://doi.org/10.1142/S0192415X94000085
Hu X, Li W, Chen Q, Yang Y (2009) Early signal transduction linking the synthesis of jasmonic acid in plant. Plant Signal Behav 4(8):696–697. https://doi.org/10.4161/psb.4.8.9181
Kim YJ, Jeon JN, Jang MG, JY O, Kwon WS, Jung SK, Yang DC (2014) Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res 38(1):66–72. https://doi.org/10.1016/j.jgr.2013.11.001
Lee EJ, Park SY, Paek KY (2015) Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Pant Cell Tiss Organ Cult 120(1):1–10. https://doi.org/10.1007/s11240-014-0567-4
Lin LC, Nalawade SM, Mulabagal V, Yeh MS, Tsay HS (2003) Micropropagation of Polygonum multiflorum Thunb and quantitative analysis of the anthraquinones emodin and physcion formed in in vitro propagated shoots and plants. Biol Pharm Bull 26(10):1467–1471. https://doi.org/10.1248/bpb.26.1467
Liu J, Liu Y, Zhang ZH, YG Z, Tang ZH, Efferth T (2016) Correlation of cultivation of Panax ginseng with metabolic profiles of nine ginsenoside and mRNA expression of genes encoding major biosynthetic enzymes. Acta Physiol Plant 38(2). https://doi.org/10.1007/s11738-015-2049-7
Lu H (2009) Dissection of salicylic acid-mediated defense signaling networks. Plant Signal Behav 4(8):713–717. https://doi.org/10.4161/psb.4.8.9173
Mueller MJ, Brodschelm W, Spannagl E, Zenk MH (1993) Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc Natl Acad Sci 90(16):7490–7494. https://doi.org/10.1073/pnas.90.16.7490
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–479. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118(1):1–16. https://doi.org/10.1007/s11240-014-0467-7
Murthy HN, Dandin VS, Paek KY (2016) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15(1):129–145. https://doi.org/10.1007/s11101-014-9391-z
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacognosy Rev 1:69–79
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plant and microbial systems. Biotechnol Appl Bioc 37(1):91–102. https://doi.org/10.1042/BA20020118
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182. https://doi.org/10.3390/molecules21020182
Karla RE, Heriberto VL, Diego H, Elisabeth M, Marta G, Rosa CM, Javier P (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2). https://doi.org/10.3390/molecules21020182
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20(2):101–153
Shilpha J, Satish L, Kavikkuil LMJV, Ramesh M (2016) Methyl jasmonate elicits the solasodine production and anti-oxidant activity in hairy root cultures of Solanum trilobatum. Ind Crop Prod 71:64–64
Shohael AM, Murthy HN, Hahn EJ, Lee HL, Paek KY (2008) Increased eleutheroside production in Eleutherococcus sessiliflorus embryogenic suspension cultures with methyl jasmonate treatment. Biochem Eng J 38(2):270–273. https://doi.org/10.1016/j.bej.2007.07.010
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam M, Selvaraj N, Ganapathi A (2012) Chitosan enhances withanolides production in adventitious root cultures of Wathania somnifera (L. ). Ind Crop Prod 37(1):124–129. https://doi.org/10.1016/j.indcrop.2011.11.022
Taguchi G, Yazawa T, Hayashida N, Okazaki M (2001) Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. Eur J Biochem 268(14):4086–4094. https://doi.org/10.1046/j.1432-1327.2001.02325.x
Thiruvengadam M, Praveen N, Kim EH, Kim SH, Chung IM (2014) Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 251(3):555–566. https://doi.org/10.1007/s00709-013-0554-3
Udomsuk L, Jarukamjorn K, Tanaka H, Putalun W (2011) Improved isoflavonoid production in Pueraria candollei hairy root cultures using elicitation. Biotechnol Lett 33(2):369–374. https://doi.org/10.1007/s10529-010-0417-3
Van der Plas LHW, Eijkelboom C, Hagendoorn MJM (1995) Relation between primary and secondary metabolism in plant cell suspension-competition between secondary metabolite production and growth in model system (Morinda citrifolia). Plant Cell Tissue Organ Cult 43(2):111–116. https://doi.org/10.1007/BF00052164
Wu CH, Dewir YH, Hahn EJ, Paek KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J Plant Biol 49:193–199
Wu SQ, Yu XK, Lian ML, Park SY, Piao XC (2014) Several factors affecting hypericin production of Hypericum perforatum during adventitious root culture in airlift bioreactors. Acta Physyol Plant 36(4):975–981. https://doi.org/10.1007/s11738-013-1476-6
Yi T, Leung KS, GH L, Zhang H, Chan K (2007) Identification and determination of the major constituents in traditional Chinese medicinal plant Polygonum multiflorum Thunb by HPLC coupled with PAD and ESI/MS. Phytochem Anal 18(3):181–187. https://doi.org/10.1002/pca.963
Yu KW, Gao W, Hahn EJ, Paek KY (2002) Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem Eng J 11:211–215
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23(4):283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Zhao JL, Zhou LG, JY W (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87(1):137–144. https://doi.org/10.1007/s00253-010-2443-4
Acknowledgments
We thank Dr. Shashi Bushan (the Council of Scientific and Industrial Research (CSIR), India) for editing this manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Advanced Production Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (Grant Number 315013-4).
Author information
Authors and Affiliations
Contributions
Thanh-Tam Ho and Jong-Du Lee contributed to data acquisition and the writing of the manuscript. Cheol-Seung Jeong and Kee-Yoeup Paek participated in data interpretation and revising of the manuscript to include important intellectual content. So-Young Park made substantial contributions to the conception and design of this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ho, TT., Lee, JD., Jeong, CS. et al. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiflorum . Appl Microbiol Biotechnol 102, 199–209 (2018). https://doi.org/10.1007/s00253-017-8629-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8629-2