Abstract
On a daily basis, humans, and their colonizing microbiome, are exposed to both indoor and outdoor dust, containing both deleterious organic and inorganic contaminants, through dermal contact, inhalation, and ingestion. Recent studies evaluating the dust exposure responses of opportunistic pathogens, such as Escherichia coli and Pseudomonas aeruginosa, revealed significant increases in biofilm formation following dust exposure. In this study, the effects of dust exposure on mixed bacterial cultures as well as HT-29 co-cultures were evaluated. As it was observed in pure, single bacterial cultures earlier, neither indoor nor outdoor dust exposure (at concentrations of 100 μg/mL) influenced the growth of mixed bacterial liquid cultures. However, when in paired mixed cultures, dust exposure increased sensitivity to oxidative stress and significantly enhanced biofilm formation (outdoor dust). More specifically, mixed cultures (E. coli-Klebsiella pneumoniae, K. pneumoniae-P. aeruginosa, and E. coli-P. aeruginosa) exhibited increased sensitivity to 20 and 50 mM of H2O2 in comparison to their pure, single bacterial culture counterparts and significantly enhanced biofilm production for each mixed culture. Finally, bacterial proliferation during a eukaryotic gut cell (HT29) co-culture was significantly more robust for both K. pneumoniae and P. aeruginosa when exposed to both house and road dust; however, E. coli only experienced significantly enhanced proliferation, in HT29 co-culture, when exposed to road dust. Taken together, our findings demonstrate that bacteria respond to dust exposure differently when in the presence of multiple bacterial species or when in the presence of human gut epithelial cells, than when grown in isolation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With advances in technology and various industrial practices, chemicals such as plasticizers, pesticides, and those found in flame retardants are increasingly entering in the ecosystem (Weschler 2009). Their effect on the environment depends on the amount of chemical released, the type and concentration of chemical, and its source. Some chemicals (e.g., mercury, ozone, and arsenic) are deleterious if released into the environment even when there is no immediate or visible impact. In the worst case scenario, noxious chemicals accumulate in the food chain and persist in the environment for many years. Unfortunately, some of these harmful chemicals (both inorganic and organic) such as arsenic, chromium, mercury, lead, polychlorinated biphenyls (PCBs), and polycyclic aromatic hydrocarbons (PAHs) can be found in various dust particles (Whitehead 2011; Hogervost et al. 2007).
The presence of some of the aforementioned deleterious chemicals in indoor dust is a cause for concern on account of the increasingly long periods of time people spend indoors. Indoor chemical contamination can be derived from various sources including cooking, smoking/burning candles, deteriorating building materials, cleaning products, cosmetics, biocides, textiles, house furnishing, and electronic devices (Bergh et al. 2011). In the USA, adults spend roughly 21 h/day indoors, while children spend 17–19 h/day, justifying the need to evaluate the potentially deleterious effect of indoor dust on human health. Some studies (Berkowitz et al. 2003; Simcox 1995) have evaluated human risks of indoor dust exposure on infants and pregnant women. Not surprisingly, for infants and toddlers, the primary route of exposure was through hand-to-mouth activities, such as eating fallen food contaminated with dust (Butte and Heinzow 2002).
Beyond indoor dust, outdoor dust (including road dust), influenced by urbanization and industrialization, is known to contain a wide range of toxins and metals (Adriano 2001; Duong and Lee 2011). Metals found in outdoor dust are derived from diverse sources including atmospheric precipitation, various industrial processes, coal combustion, and vehicle emission (Ahmed and Ishiga 2006). More specifically, platinum group elements (PGEs) are rare natural elements which are being increasingly used in health and commercial products. Consequently, their concentrations in the environment have been steadily increasing (Ravindra et al. 2004). Metals typically enter the human body via three principal routes: ingestion, dermal contact, and inhalation (Garrison et al. 2014; Sahlstrom et al. 2015). In humans, metals have been shown to affect the central nervous system, especially in children (Valko et al. 2005; Yongming et al. 2006; Zheng et al. 2010; Du et al. 2013).
Our previous studies have shown that dust exposure influences bacterial growth, oxidative stress resistance, and virulence potential when bacteria were grown in pure culture (Suraju et al. 2015). More specifically, dust exposure on three representative commensals, who double as opportunistic pathogens (Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa), resulted in increased biofilm production in all three organisms following exposure to both indoor and outdoor dust; however, only E. coli exhibited increased sensitivity to oxidative stress following exposure to dust (Suraju et al. 2015).
Unfortunately, though, little is known about the physiological effect of dust exposure on bacterial co-cultures, which more accurately reflects native microbiological environments where multiple organisms co-exist and often compete with one another. Two primary types of competition can occur when two bacterial strains are co-cultured. In exploitative competition, species compete for limited nutrients while interference competition results in species directly antagonizing one another (Shoaie et al. 2013). In fact, some molecules produced by bacteria can influence the behavior and fitness of neighboring, yet unrelated, species within the same ecosystem. In some cases, they can be beneficial, but in most cases they are antagonistic (Iwase et al. 2010). The purpose of this study was to broaden our earlier preliminary findings (Suraju et al. 2015) by characterizing the impact of dust exposure (containing PGEs as well as other contaminants) on mixed-culture opportunistic bacterial pathogens that associate with the human microbiome as well as bacterial and eukaryotic cell co-cultures.
Materials and methods
Dust
House dust (SRM 2585) was purchased from the National Institute of Standards and Technology, and a list of organic components found in the house dust can be found in Table 1. Road dust (BCR 723, Sigma) was sampled in Austria in 1998, and a list of its inorganic can be found in Table 2.
Bacterial strains, and culture conditions
For all studies, either house dust (Sigma-Aldrich NIST SRM 2585) or road dust (Sigma-Aldrich BCR 723) was used at concentrations of 100 μg/mL in PBS unless otherwise noted. All dust samples were diluted in PBS and plated to ensure sterility before use. E. coli K-12 (Carolina Biological, Burlington, NC, USA—155065A), P. aeruginosa PA01 (Carolina Biological, Burlington, NC, USA—155250A), and Klebsiella pneumoniae (Carolina Biological, Burlington, NC, USA—155095A) were used in all studies. For all experiments, Brain Heart Infusion (BHI) medium (Sigma-Aldrich) was used to grow bacterial strains with agitation (250 rpm) at 37 °C. All absorbance readings were taken using a BioTek™ ELx800™ microplate reader at 595 nm.
Eukaryotic cell lines
HT-29 cells (ATCC HTB38) were cultured as previously described (Petiot et al. 2000) with some minor modifications. DMEM (Thermo Fisher 11965092) supplemented with 10% FBS (Thermo Fisher 16140071) and 5% pen-strep (Thermo Fisher 15140122) was used. Cells were incubated at 37 °C with 5% CO2. Flask medium was changed every 3 days.
Growth curve analysis
For liquid growth assays, phosphate-buffered saline (PBS) (Amresco)-suspended indoor (house) or outdoor (road) dusts were diluted into BHI broth at a final concentration of 100 μg/mL each. Saturated cultures of K. pneumoniae, E. coli, and P. aeruginosa were diluted to an optical density (595 nm) of 0.2 in a 96-well plate (final volume, 200 μL/well). For control wells, only PBS was added to each well. Growth rates were monitored every 30 min for 8 h. All growth experiments were conducted in triplicate. To enumerate and distinguish individual bacteria strains in mixed cultures, either MacConkey agar (Difco) or eosin methylene blue (EMB) agar (Difco) was used. On EMB agar, E. coli colonies were metallic green, K. pneumoniae colonies were pinkish-purple, and P. aeruginosa colonies appeared colorless.
Protein expression
Saturated bacterial cultures, grown at 37 °C with agitation, of K. pneumoniae, E. coli, and P. aeruginosa, were diluted to an optical density (600 nm) of 0.2 in 2 mL of BHI medium (Difco) in either the presence of 100 μg/mL of house, road, or no dust. Five hundred-microliter samples were removed from each growing subculture at 0-, 3-, and 6-h time points. Cells were harvested via centrifugation (14,000×g), and bacterial pellets were resuspended in 100 μL RIPA buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin) for 45 min on ice. Samples were then centrifuged at 14,000 rpm for 5 min. After determination of total protein concentration using the Bradford assay (Bio-Rad), 15 μg total protein from each sample was loaded on 10% Tris-polyacrylamide gel and then Coomassie (Bio-Rad) stained.
Oxidative stress assays
PBS-suspended indoor (house) or outdoor (road) dust was diluted into BHI broth to a final concentration of 100 μg/mL. Saturated cultures of K. pneumoniae, E. coli, and P. aeruginosa were diluted to an optical density (595 nm) of 0.2 in a 96-well plate (final volume, 200 μL). For negative control wells, only PBS was added. Following 1 h of growth, H2O2 was added to the subcultures at final concentrations of 0, 20, and 50 mM, and growth was monitored for 7 h. All experiments were conducted in triplicate.
Biofilm formation assay
For our biofilm formation assay, we employed our previously described methods (Suraju et al. 2015) with some modifications. Briefly, saturated cultures of K. pneumoniae, E. coli, and P. aeruginosa, grown in BHI, were diluted to an optical density (595 nm) of 0.2 in a 96-well plate (final volume, 200 μL/well). Microtiter plates were incubated for 24 h with agitation (~ 100 rpm) at 37 °C, after which optical densities at 595 nm were measured. Wells were washed with water and incubated with 0.1% (vol/vol) crystal violet (total volume, 250 μL/well) for 1 h at ambient temperature. Unbound crystal violet was removed by washing with water, and wells were dried overnight. Biofilm-bound crystal violet was dissolved in 250 μL of 30% acetic acid. Optical densities of solubilized crystal violet were measured at 570 nm. Biofilm produced was normalized based on relative biomass (optical densities of planktonic cells) to account for any differences in the growth rates of the various bacterial strains used. All experiments were carried out in triplicate or quadruplicate.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
HT29 cells were seeded in a 96-well plate at a density of 5000 cells/well ~ 24 h preceding the experiment. The following day, treatment of designated wells using 25, 50, and 100 μg/mL of indoor (house) or outdoor (road) dust occurred for 0, 3, 6, or 12 h. Twenty microliters of 5 mg/mL MTT was added to each well, followed by incubation at 37 °C with 5% CO2. After 4 h, medium was gently removed from each well and replaced with 100 μL of DMSO. Cells were agitated on an orbital shaker for 5 to 10 min, and absorbencies were read at 570 nm with a reference filter of 640 nm.
Bacterial co-culture with eukaryotic cells
HT29 cells were seeded into 24-well plates at densities of ~ 1 × 105/well, 24 h prior to bacterial infection. At this seeding density, monolayers were subconfluent (~ 60–80% confluency) at the time of the experiment. Bacteria were grown to saturation in BHI broth at 37 °C with agitation (~ 250 rpm), washed with 1× PBS, and diluted to optical densities (600 nm) of 1.0 in DMEM + 10% FBS. Diluted cultures of K. pneumoniae, E. coli, and P. aeruginosa, were further diluted (as necessary) to achieve multiplicities of infection of 1. Following a 30-min attachment period, each well was washed with PBS, and DMEM containing 100 μg/mL of either indoor or outdoor dust was added to each well. Viable colony plate counts were enumerated for both the 0- and 6-h end points, and fold increases over that time period were calculated. All plates were incubated for 24 h at 37 °C.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism software (version 6). All experiments were performed in triplicate, and error bars on the graphs represent standard error of the mean. For comparing sets of data, the Student’s t test was used, and statistical significance was considered for P values <0.05.
Results
Dust exposure did not influence bacterial growth in either pure or mixed culture
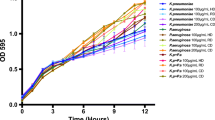
To determine the impact of dust (indoor and outdoor) exposure on bacterial growth, we first exposed pure bacterial liquid cultures of E. coli, K. pneumoniae, or P. aeruginosa to 100 μg/mL of dust (indoor and outdoor) while measuring their biomass every 30 min for 8 h (Fig. 1a–c). In agreement with our earlier findings in which the growth of E. coli, E. faecalis, and P. aeruginosa was not influenced by dust exposure for 4 h (Suraju et al. 2015), exposure to 100 μg/mL of either indoor or outdoor dust similarly did not impact bacterial growth for all three organisms (E. coli, K. pneumoniae, or P. aeruginosa) evaluated in this study during an 8-h period (Fig. 1a–c). Similarly, dust exposure (both indoor and outdoor) of 100 μg/mL had no impact on mixed-culture growth (of (1) E. coli–P. aeruginosa, (2) K. pneumoniae–E. coli, and (3) K. pneumoniae–P. aeruginosa) when compared to the same mixed cultures grown in the absence of dust (Fig. 1d–f).
Bacterial growth in response to dust exposure. Growth curve analysis of E. coli, K. pneumoniae, and P. aeruginosa in pure culture (a–c) or in mixed cultures (d–f), is shown in response to house and road dust exposure. Distribution of individual strains within a mixed culture following dust exposure is also shown (g–i). Representative experiment shown is in triplicate, and errors of the mean are also shown. Student’s t test was used to determine any statistical differences
To further evaluate the impact of dust exposure on mixed bacterial cultures, we employed selective solid medium (eosin methylene blue) plates to determine whether one specific organism, within the mixed culture, outgrew its counterpart. We observed that although bulk biomass remains unaffected, as observed in liquid cultures (Fig. 1d–f), some representatives within the mixed cultures appear to outgrow their companion strains on solid EMB selective medium. More specifically, when exposed to house dust (but not road dust), K. pneumoniae appeared to outgrow E. coli in an E. coli–K. pneumonia mixed culture (Fig. 1g). Similarly, when exposed to both house and road dust, P. aeruginosa appeared to outgrow E. coli in an E. coli–P. aeruginosa mixed culture (Fig. 1h). Interestingly, in the K. pneumoniae–P. aeruginosa mixed culture, neither organism appeared to outgrow/outcompete its companion (Fig. 1i). Taken together, employing the more refined selective solid medium enabled us to determine that, although mixed cultures experienced no alternations in net growth (as measured by biomass), dust exposure could enhance the ability of select members of the gut microbiota to expand at the expense of neighboring unrelated organisms.
Dust exposure did not grossly alter bacterial protein production
To determine whether dust exposure could grossly affect protein production in exposed bacteria, we challenged E. coli, K. pneumoniae, or P. aeruginosa with either 100 μg/mL of house, road, or no dust for 0, 3, or 4 h timepoints. At the aforementioned times, cells were harvested, lysed, loaded on polyacrylamide gels, and subjected to electrophoresis. For each of the timepoints evaluated, there was no apparent difference in E. coli’s total protein production when exposed to either 100 μg/mL of house, road, or no dust (Fig. 2). Similarly, we did not observe any dramatic alterations in protein production in either K. pneumoniae or P. aeruginosa when exposed to 100 μg/mL of house, road, or no dust for 0, 3, or 4 h timepoints (data not shown). Although, these data did not reveal any gross-level differences in protein expression, a more refined look at specific subsets of proteins and or gene expression (i.e., mRNA transcript levels) could reveal dust responsive bacterial genes and/or gene products.
Evaluation of gross level protein production in dust exposed E. coli. A 5.0-mL E. coli subculture was started at an optical density 600 nm of 0.2, and 100-μL aliquots were removed at 0-, 3-, and 6-h time points for bacterial cell harvest. After total protein quantification using the Bradford assay, 15 μg of total protein was loaded in each well of a 10% polyacrylamide gel. The gel was Coomassie stained, and protein bands and their intensities are displayed
Dust exposure increased the sensitivity of mixed bacterial cultures to oxidative stress
Earlier, we demonstrated that exposure to dust (indoor and outdoor) increased the sensitivity of E. coli, but not of E. faecalis, and P. aeruginosa, to H2O2 challenge (Suraju et al. 2015). In this study, and in agreement with our earlier finding (Suraju et al. 2015), E. coli exhibited increased sensitivity to 20 and 50 mM of H2O2 when exposed to both indoor and outdoor dust (Fig. 3 compare panels b, c to e, f, and h, i). In extending our earlier studies (Suraju et al. 2015), we also evaluated the impact of dust exposure on the oxidative stress response of K. pneumoniae (Fig. 2). Like E. coli, K. pneumoniae similarly experienced increased sensitivity to 20 mM H2O2 (compare panel Fig. 3b–h) following road dust exposure but did not experience altered sensitivity to 20 mM H2O2 when exposed to house dust (Fig. 3e). This again underscores the unique components found in each dust type as well as the unique responses of the individual bacteria. In sharp contrast to both E. coli and K. pneumoniae, P. aeruginosa demonstrated significantly better intrinsic resistance to both 20 and 50 mM H2O2 exposure (Fig. 3b, c) in the absence of dust challenge. Further, in the presence of both house (Fig. 3e, f) and road dust (Fig. 3h, i), P. aeruginosa was significantly more resistant than either E. coli or K. pneumoniae to subsequent 20 mM (Fig. 3e, h) and 50 mM (Fig. 3f, i) H2O2 challenge.
Oxidative stress resistance of single and mixed bacterial cultures. Single and mixed cultures of E. coli, K. pneumoniae, and P. aeruginosa were exposed to 0, 20, and 50 mM H2O2 without dust (a–c, respectively), with 100 μg/mL of house dust (d–f, respectively), or 100 μg/mL of road dust (g–i, respectively). Representative experiments shown are in triplicate, and errors of the mean are also shown. Student’s t test was used to determine any statistical differences. p < 0.05 was considered significant
Since bacteria live mostly in heterogeneous, mixed-culture native environments, we further investigated the sensitivity of bacterial mixed cultures to H2O2. More specifically, E. coli and K. pneumoniae, E. coli–P. aeruginosa, and K. pneumoniae–P. aeruginosa mixed cultures, initially conditioned in either house or road dust (100 μg/mL) for 1 h, were subsequently challenged with 0, 20, or 50 mM of H2O2. E. coli–K. pneumoniae mixed cultures exhibited greater sensitivity to 20 mM H2O2 when in the presence of 100 μg/mL of house dust (compare Fig. 3b, e) than the pure culture of each individual bacterial cultures (Fig. 3b, e). Interestingly, the E. coli–K. pneumoniae mixed culture was significantly more resistant to 20 mM H2O2 when conditioned/exposed to road dust than the two individually pure cultures (Fig. 3h) but not house dust (Fig. 3f). However, the aforementioned enhanced resistance was not observed when the E. coli–K. pneumoniae mixed culture was challenged with 50 mM H2O2 in the presence of road dust (Fig. 3i).
Despite P. aeruginosa being significantly the most resistant to both 20 and 50 mM H2O2 challenge in the presence of both house (Fig. 3e, f) and road (Fig. 3h, i) dust, the E. coli–P. aeruginosa mixed culture was significantly more sensitive to 50 mM of H2O2 in the presence of house dust than either of the two individually pure cultures (Fig. 3f). Similarly, in the presence of road dust, the E. coli–P. aeruginosa mixed culture was significantly more sensitive to 50 mM of H2O2 than the P. aeruginosa pure culture (Fig. 3i).
The K. pneumoniae–P. aeruginosa mixed culture exhibited significantly greater sensitivity to 20 mM of H2O2 with 100 μg/mL of road dust than K. pneumoniae and P. aeruginosa pure cultures (Fig. 3h). Similarly, when challenged with 50 mM H2O2, the K. pneumoniae–P. aeruginosa mixed culture exhibited significantly greater sensitivity than the P. aeruginosa and K. pneumoniae pure culture (Fig. 3i) Taken together, these data suggest that in a mixed culture, P. aeruginosa loses its intrinsic resistance to H2O2 challenges when otherwise grown independently, suggesting perhaps antagonistic relationships in mixed bacterial cultures.
Enhanced biofilm production in opportunistic bacterial mixed cultures exposed to dust
For our biofilm assays, we chose to employ concentrations of 100 μg/mL of indoor and outdoor dust, as we had done earlier (Suraju et al. 2015), on account of the aforementioned concentration not adversely affecting the growth kinetics of all bacteria evaluated in either mixed or pure culture conditions (Fig. 1). In agreement with our earlier findings (Suraju et al. 2015), exposure to either 100 μg/mL of indoor or outdoor dust resulted in significantly increased biofilm production in both E. coli and P. aeruginosa (Fig. 4a). Mirroring E. coli and P. aeruginosa, K. pneumoniae similarly exhibited significantly enhanced biofilm production following exposure to either 100 μg/mL of indoor or outdoor dust (Fig. 4a).
Biofilm formation of single and mixed bacterial cultures. Single cultures of E. coli, K. pneumoniae, and P. aeruginosa were exposed to 100 μg/mL of house dust, road dust, or no dust (a). Mixed cultures of E. coli, K. pneumoniae, and P. aeruginosa were exposed to 100 μg/mL of house dust or no dust (b). Mixed cultures of E. coli, K. pneumoniae, and P. aeruginosa were exposed to 100 μg/mL of road dust or no dust (c). Representative experiments shown are in triplicate, and errors of the mean are also shown. Student’s t test was used to determine any statistical differences. p < 0.05 was considered significant
Curious about whether the same phenomenon would be observed in mixed bacterial cultures, we exposed E. coli–P. aeruginosa, K. pneumoniae–P. aeruginosa, and E. coli–K. pneumonia mixed cultures to either 100 μg/mL of indoor, outdoor, or no dust. Interestingly, despite each individual pure culture exhibiting significantly increased biofilm formation following exposure to 100 μg/mL of indoor dust (Fig. 4a), only the K. pneumoniae–P. aeruginosa mixed culture demonstrated significantly enhanced biofilm formation among all mixed cultures tested. More specifically, no change in biofilm formation was observed for either the E. coli–P. aeruginosa or E. coli–K. pneumonia mixed cultures (Fig. 4b). This somewhat unexpected finding suggests that in mixed culture, E. coli–P. aeruginosa and E. coli–K. pneumonia could be antagonizing to one another in response to house dust exposure resulting in compromised biofilm production. In sharp contrast, all three of the aforementioned mixed cultures tested experienced significantly enhanced biofilm production following exposure to 100 μg/mL of outdoor dust (Fig. 4c), further underscoring the unique physiological consequences triggered by the disparate contents found within each type of dust (Suraju et al. 2015).
HT29 gut epithelial cells are not influenced by 100 μg/mL of either indoor or outdoor dust
“To determine whether any gross-level changes observed in HT29 gut epithelial- bacterial co-culture infections were a result of bacterial responses to the tested dust alone and not due to any negative influence on HT29 viability, an MTT assay was performed on HT29 cells (alone) following dust exposure to gauge dust-induced host cytotoxicity.”
More specifically, prior to conducing bacterial co-culture experiments with eukaryotic cells, we sought to determine whether our working concentrations of 100 μg/mL (of either dust type) had any deleterious effects on HT29 human colonic gut epithelial cells. To determine this, we exposed cultured HT29 cells to 25, 50, and 100 μg/mL of either house or road dust for 0, 3, 6, and 8 h. Fortunately, none of the three concentrations tested for either house (Fig. 5a) or road (Fig. 5b) dust significantly influenced HT29 cell viability at any of the times evaluated (Fig. 5). This finding enabled us to next evaluate the impact of dust exposure on bacterial HT29 co-cultures, knowing that if differences were observed they would not be due to adverse effects on either bacterial or HT29 viability.
Effect of dust on human gut epithelial HT29 cells. MTT assays were performed to determine cytotoxicity of 25, 50, and 100 μg/mL of either house dust (a) or road dust (b) on human HT29 cells. Representative experiment shown is in triplicate, and errors of the mean are also shown. Student’s t test was used to determine any statistical differences
HT29 bacterial co-cultures as a model for gut exposure to dust
When E. coli was co-cultured with HT29 cells, interestingly, exposure to 100 μg/mL of house dust did not significantly influence bacterial proliferation during the 6-h infection period; however, exposure to road dust (at the same concentration) did significantly enhance E. coli’s proliferation during the same infection period (Fig. 6a). This finding again underscores the importance of dust components in promoting bacterial responses (since the components found in road and house dust differ). Unlike E. coli, which only seemed to respond to road dust exposure in co-culture (Fig. 6a), K. pneumoniae and P. aeruginosa responded to both dust types (Fig. 6b, c). More specifically, when K. pneumoniae (in an HT29 co-culture) was exposed to both house and road dust, 1000-fold increases were observed in bacterial proliferation counts for each, both significantly higher rates of proliferation than the ~ 100-fold proliferation observed for K. pneumoniae when no dust was present in the co-culture (Fig. 6b). Similarly, when P. aeruginosa (in an HT29 co-culture) was exposed to both house and road dust, > 1500-fold increases were observed in bacterial proliferation counts for each, both significantly higher rates of proliferation than the ~ 600-fold proliferation observed for P. aeruginosa when no dust was present in the co-culture (Fig. 6c). Taken together, these data indicate that within the gut microenvironment (HT29 cells), not all resident flora representatives respond similarly to dust exposure (mimicking dust ingestion in humans). Further, the source of the dust (indoor vs. outdoor) could also influence bacterial responses and proliferation, potentially promoting some opportunistic pathogen-induced disease states.
Dust-exposed bacterial cells co-cultured with HT29 cells modeling the gut microenvironment. HT29–E. coli (a), HT29−K. pneumoniae (b), and HT29–P. aeruginosa (c) co-cultures exposed to 100 μg/mL of house, road, or no dust were evaluated for bacterial proliferation following a 6-h infection period. Representative experiments shown are in triplicate, and errors of the mean are also shown. Student’s t test was used to determine any statistical differences. p < 0.05 was considered significant. Values shown represent fold increases over time
Discussion
Various contaminants (many of them noxious) present in dust can enter the human body through inhalation, direct ingestion (e.g., consumption of vegetables grown in contaminated fields), and/or dermal contact (Bjerg et al. 2015; Garrison et al. 2014; Karottki et al. 2014; Sahlstrom et al. 2015; Qu et al. 2012). Several studies have attempted to evaluate population health risks due to heavy metal exposure such as arsenic, cadmium, lead, and mercury, through various exposure pathways, especially through the soil and food chain (MacIntosh et al. 1996; Hough et al. 2004; Baastrup et al. 2008; Albering et al. 1999; Man et al. 2010; Mari et al. 2009). While it has been easier to evaluate the effect of inhaled dust contaminants, it has been much more challenging to determine similar effects in the human gut, mainly on account of ingested dust contaminants rapidly being metabolized and excreted (Colombo et al. 2008b). With regards to the human gut, the intestinal microbiota plays a critical role in human health by enhancing host metabolism, producing some nutritional factors, providing protection against transient flora (many of which are pathogenic), and improving host immune function (Guinane and Cotter 2013). In fact, perturbations in the gut microbiata can result in serious consequences for human health, either beneficial or harmful (Ley et al. 2006a). For example, disruptions of the gut microbiota can lead to intestinal disease states including obesity (Ley et al. 2006b; Zhang et al. 2009), malnutrition (Kau et al. 2011), inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC), and Crohn’s disease (Frank et al. 2011). Further, gut microbiota disruptions can also lead to systematic diseases such as diabetes (Qin et al. 2012). Furthermore, epidemiological studies linked air pollution exposure to gastrointestinal diseases, including inflammatory bowel disease (IBD) (Kaplan et al. 2010; Ananthakrishnan et al. 2011), appendicitis (Kaplan et al. 2013), irritable bowel syndrome (Kaplan et al. 2012), and enteric infections in infants (Chen et al. 2008). Additionally, pollutant particulate matter can adulterate food and water supply in significant amounts (Beamish et al. 2011; De Brouwereet al. 2012). According to Lomer et al. (2004), 1012–1014 particles are ingested per day by an individual on a typical Western diet, with an estimated mucosal uptake of ~ 1% (109–1012 per day).
Our recent studies demonstrated that dust exposure can affect bacterial growth, oxidative stress resistance, and the virulence potential of E. coli, E. faecalis, and P. aeruginosa, through enhanced biofilm production, when in pure culture (Suraju et al. 2015). In this manuscript, we employed three representative opportunistic pathogens, E. coli, K. pneumoniae, and P. aeruginosa (which are also part of the human gut microbiota), to determine their behaviors in mixed cultures (with each other) and co-culture with human gut epithelial HT29 cells. In so doing, this approach better enables us to model how gut microbiota (in their native environment) could react when exposed to dust contaminants. In using 100-μg/mL concentrations of both house and road dust previously determined to not adversely affect the growth rates of pure bacterial cultures (Suraju et al. 2015), we similarly observed that mixed culture growth rates are also not adversely affected in liquid culture. However, when we employed selective medium (EMB) to enumerate individual bacterial colonies within the mixed cultures, we observed that although bulk biomass remains unaffected (as observed in liquid cultures), some representatives within the mixed cultures appear to outgrow their paired companion strains. More specifically, when exposed to house (but not road) dust, K. pneumoniae appeared to outgrow E. coli in an E. coli–K. pneumonia mixed culture. Similarly, when exposed to both house and road dust, P. aeruginosa appeared to outgrow E. coli in an E. coli–P. aeruginosa mixed culture.
We observed that mixed E. coli–K. pneumoniae, K. pneumoniae–P. aeruginosa, and E. coli–P. aeruginosa cultures exhibited increased sensitivity to 20 and 50 mM of H2O2 in comparison to the pure bacteria culture. Studies have shown that the Cpx and σE stress responses exist in many Gram-negative bacterial pathogens (Raivio et al. 1999; Raivio and Silhavy 2001; Ades 2004). It is possible that, only when grown independently, E. coli, K. pneumoniae, and P. aeruginosa, all Gram-negative bacteria possessing σE, Cpx, and Bae envelope stress response factors, benefit from protection against a variety of stressors, including oxidative stress (Raivio 2005); however, when grown together in mixed culture, this stress response benefit becomes over-ridden by some unknown mechanism(s).
With regards to biofilm production, the specific type of dust exposure had disparate influences on the mixed culture biofilm production relative to their pure culture counterparts. For example, while exposure to both house and road dust significantly enhanced biofilm production in all three individual cultures, only road dust exposure was able to emulate that response for all three mixed cultures evaluated (i.e., E. coli–P. aeruginosa, K. pneumoniae–P. aeruginosa, and E. coli–K. pneumonia). When exposed to house dust, only the K. pneumoniae–P. aeruginosa mixed culture produced significantly higher biofilm than the untreated mixed-culture controls. It is entirely possible that this disparity could be due, in part, to the unique contaminants found within each dust type. Another possible contributing factor could be due to bacterial competition/antagonism. In fact, some molecules produced by bacteria can influence the behavior and fitness of other species within the same ecosystem. In some cases, they can be beneficial, but in most cases they are antagonistic (Iwase et al. 2010).
In co-culture with gut HT29 cells following road dust challenge, we observed E. coli, K. pneumoniae, and P. aeruginosa all exhibiting significantly enhanced proliferation over a 6-h infection period than the untreated controls. Curiously, of the three organisms evaluated, only E. coli did not respond to house dust challenge with significantly enhanced proliferation, likely owing to the different components within each dust type (Suraju et al. 2015). Perhaps the enhanced proliferation is due, in part, to the upregulation of adhesive pili, in response to dust exposure. For example, a P. aeruginosa and Roseobacter denitrificans co-culture study showed the activation of various defense mechanisms (Conway et al. 2012), while another study revealed enhanced fermentation of mixed microbial communities (Anburajan et al. 2017). With regards to K. pneumonia, it uses two adhesive pilus structures, the mannose-sensitive type 1 pili (T1P), composed of a major fimbrial FimA subunit and a minor tip adhesin FimH, and the mannose-resistant type 3 pili (T3P or MR/K), composed of the major pilus subunit MrkA and the minor tip adhesin MrkD (Gerlach et al. 1988; Gerlach et al. 1989). Perhaps contaminants in dust (indoor and outdoor) can trigger an upregulation of these different pilus components promoting enhanced cell adhesion and subsequent proliferation in the HT29 co-cultures. Although our gross level-protein expression analysis did not reveal any dramatic differences in protein levels following dust exposure at various time points, it is still entirely possible that differences in some key stress-response factor levels could be at play, accounting for at least some of the physiological responses we have observed. In the future, it would be useful to combine and monitor some of the changes in gene expression of our study’s suggested factors and proteins (e.g., pili, σE, Cpx, and Bae factors) with these types of physiological and growth experiments. Ultimately, future studies will need to be conducted evaluating meta-transcriptomics coupled with mixed bacterial cultures co-cultured with HT29 cells to more accurately reflect changes to the diversity found in the human microbiome in response to dust contaminants.
References
Ades SE (2004) Control of the alternative sigma factor σE in Escherichia coli. Curr Opin Microbiol 7:157–162
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York
Ahmed F, Ishiga H (2006) Trace metal concentration in street dusts of Dhaka city, Bangladesh. Atmos Environ 40:3835–3844
Albering HJ, van Leusen SM, Moonen EJC, Hoogewerff JA, Kleinjans JCS (1999) Human health risk assessment: a case study involving heavy metal soil contamination after the flooding of the river Meuse during the winter of 1993–1994. Environ Health Perspect 107:37–43
Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K (2011) Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis 17(5):1138–1145
Anne Petiot A, Ogier-Denis E, Blommaart EFC, Alfred J, Meijer AJ, Codogno P (2000) Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275(2):992–998
Anburajan P, Park JH, Sivagurunathan P, Pugazhendhi A, Kumar G, Choi CS, Kim SH (2017) Mixed-culture H2 fermentation performance and the relation between microbial community composition and hydraulic retention times for a fixed bed reactor fed with galactose/glucose mixtures. J Biosci Bioeng S1389-1723(16):30578–30573
Baastrup R, Sørensen M, Balstrøm T, Frederiksen K, Larsen CL (2008) Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Perspect 116:231
Beamish LA, Osornio-Vargas AR, Wine E (2011) Air pollution: an environmental factor contributing to intestinal disease. J Crohns Colitis 5(4):279–286
Bergh C, Torgrip R, Emenius G, Östman C (2011) Organophosphate and phthalate esters in air and settled dust—a multi-location indoor study. Indoor Air 21(1):67–76
Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS (2003) Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect 111(1):79–84
Bjerg A, Ronmark EP, Hagstad S, Eriksson J, Andersson M, Wennergren G, Toren K, Ekerljung L (2015) Gas, dust, and fumes exposure is associated with mite sensitization and with asthma in mite-sensitized adults. Allergy 70(5):604–607
Butte W, Heinzow B (2002) Pollutants in house dust as indicators of indoor contamination. Rev Environ Contam Toxicol Rev Environ Contam Toxicol 175:1–46
Chen H, Goldberg MS, Villeneuve PJ (2008) A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health 23(4):243 9gertrud7
Colombo C, Monhemius AJ, Plant JA (2008) Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicol Environ Saf 71(3):722–730
Conway CA, Esiobu N, Lopez JV (2012) Co-cultures of Pseudomonas aeroginosa and Roseobacter denitrificans reveal shifts in gene expression levels compared to solo cultures. ScientificWorldJournal 2012:120108
De Brouwere K, Buekers J, Cornelis C, Schlekat CE, Oller AR (2012) Assessment of indirect human exposure to environmental sources of nickel: oral exposure and risk characterization for systemic effects. Sci Total Environ 419:25–36
Du Y, Gao B, Zhou H, Ju X, Hao H, Yin S (2013) Health risk assessment of heavy metals in road dusts in urban parks of Beijing, China. Procedia Environ Sci 18:299–309
Duong TT, Lee B (2011) Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. J Environ Manag 92(3):554–562
Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, et al. (2011) Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis 17:179–184
Garrison VH, Majewski MS, Konde L, Wolf RE, Otto RD, Tsuneoka Y (2014) Inhalable desert dust, urban emissions, and potentially biotoxic metals in urban Saharan–Sahelian air. Sci Total Environ 500–501:383–394
Gerlach GF, Allen BL, Clegg S (1988) Molecular characterization of the type 3 (MR/K) fimbriae of Klebsiella pneumoniae. J Bacteriol 170:3547–3553
Gerlach GF, Clegg S, Allen BL (1989) Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol 171:1262–1270
Guinane CM, Cotter PD (2013) Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol 6(4):295–308
Hogervorst J, Plusquin M, Vangronsvel J, Nawrot T, Cuypers A, Van Hecke E, Roels HA, Carleer R, Staessen J (2007) House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res 103(1):30–37
Hough RL, Breward N, Young SD, Crout NMJ, Tye AM (2004) Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environ Health Perspect 112(2):215–221
Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465(7296):346–349
Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ (2010) The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol 105(11):2412–2419
Kaplan GG, Tanyingoh D, Dixon E, Johnson M, Wheeler AJ, Myers RP, Bertazzon S, Saini V, Madsen K, Ghosh S (2013) Ambient ozone concentrations and the risk of perforated and nonperforated appendicitis: a multicity case-crossover study. Environ Health Perspect 121(8):939–943
Kaplan GG, Szyszkowicz M, Fichna J, Rowe BH, Porada E, Vincent R, Madsen K, Ghosh S, Storr M (2012) Non-specific abdominal pain and air pollution: a novel association. PLoS One 7(10):e47669
Karottki DG, Beko G, Clausen G, Madsen AM, Andersen ZJ, Massling A, Ketzel M, Ellermann T, Lund R, Sigsgaard T, Moller P, Loft S (2014) Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle-aged subjects. Environ Int 73:372–381
Kau A, Ahern P, Griffin N, Goodman A, Gordon J (2011) Human nutrition, the gut microbiome and the immune system. Nature 474:327–336
Ley R, Peterson D, Gordon J (2006a) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848
Ley R, Turnbaugh P, Klein S, Gordon J (2006b) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023
Lomer MC, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RP, Powell JJ (2004) Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br J Nutr 92(6):947–955
MacIntosh DL, Spengler JD, Ozkaynak H, Tsai L, Ryan PB (1996) Dietary exposures to selected metals and pesticides. Environ Health Perspect 2:202–209
Man YB, Sun XL, Zhao YG, Lopez BN, Chung SS (2010) Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environ Int 36:570–576
Mari M, Nadal M, Schuhmacher M, Domingo JL (2009) Exposure to heavy metals and PCDD/Fs by the population living in the vicinity of a hazardous waste landfill in Catalonia, Spain: health risk assessment. Environ Int 35:1034–1039
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Qu CS, Ma ZW, Yang J, Yang L, Jun Bi J, Huang L (2012) Human exposure pathways of heavy metals in a lead zinc mining area, Jiangsu Province, China. PLoS One 7(11):e46793
Raivio TL (2005) Envelope stress responses and gram-negative bacterial pathogenesis. Mol Microbiol 56(5):1119–1128
Raivio TL, Silhavy TJ. (2001) Periplasmic stress and ECF sigma factors. Annu Rev Microbiol. 55:591–624
Raivio TL, Popkin DL, Silhavy TJ. (1999) The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 181(17):5263–72.
Ravindra K, Bencs L, Van Grieken R (2004) Platinum group elements in the environment and their health risk. Sci Total Environ 318(1–3):1–43
Sahlstrom LM, Sellstrom U, de Wit CA, Lignell S, Darnerud PO (2015) Estimated intakes of brominated flame retardants via diet and dust compared to internal concentrations in a Swedish mother–toddler cohort. Int J Hyg Environ Health 218(4):422–432
Shoaie S, Karlsson F, Mardinoglu A, Nookaew I, Bordel S, Nielsen J (2013) Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci Rep 3:2532
Simcox NJ (1995) Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ Health Perspect 103(12):1126–1134
Suraju MO, Lalinde-Barnes S, Sanamvenkata S, Esmaeili M, Shishodia S, Rosenzweig JA (2015) The effects of indoor and outdoor dust exposure on the growth, sensitivity to oxidative-stress, and biofilm production of three opportunistic bacterial pathogens. Sci Total Environ 538:949–958
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Weschler CJ (2009) Changes in indoor pollutants since the 1950s. Atmos Environ 43(1):153–169
Whitehead T (2011) Estimating exposures to indoor contaminants using residential dust. J Expo Sci Environ Epidemiol 21:549–564
Yongming H, Peixuan D, Junji C, Posmentier ES (2006) Multivariate analysis ofheavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ 355(1–3):176–186
Zhang H, DiBaise J, Zuccolo A, Kudrna D, Braidotti M, Yu Y (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 106:2365–2370
Zheng N, Liu J, Wang Q, Liang Z (2010) Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, northeast of China. Sci Total Environ 408(4):726–733
Acknowledgements
We would like to thank Dr. McClean from the University of Houston for sharing all bacterial strains with us. We would also like to thank Mohammed Suraju, Shari Galvin, Hyun-Min Hwang, and Daniel Vrinceanu for stimulating discussions and thoughtful criticism of our work. Finally, we would like to thank Nkem Azu for her assistance with the MTT assay performed. This work was supported by the National Science Foundation RISE (HRD-1345173; awarded to JR and SS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by the National Science Foundation (HRD-1345173).
Conflict of interest
The authors declare that she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Bado, M., Kwende, S., Shishodia, S. et al. Impact of dust exposure on mixed bacterial cultures and during eukaryotic cell co-culture infections. Appl Microbiol Biotechnol 101, 7027–7039 (2017). https://doi.org/10.1007/s00253-017-8449-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8449-4