Abstract
Staphylococcus aureus (S. aureus) biofilms are clinically serious and play a critical role in the persistence of chronic infections due to their ability to resist antibiotics. The inhibition of biofilm formation is viewed as a new strategy for the prevention of S. aureus infections. Here, we demonstrated that minimum inhibitory concentrations (MICs) of aloe-emodin exhibited no bactericidal activity against S. aureus but affected S. aureus biofilm development in a dose-dependent manner. Further studies indicated that aloe-emodin specifically inhibits the initial adhesion and proliferation stages of S. aureus biofilm development. Scanning electron microscopy (SEM) indicated that the S. aureus ATCC29213 biofilm extracellular matrix is mainly composed of protein. Laser scanning confocal microscope assays revealed that aloe-emodin treatment primarily inhibited extracellular protein production. Moreover, the Congo red assay showed that aloe-emodin also reduced the accumulation of polysaccharide intercellular adhesin (PIA) on the cell surface. These findings will provide new insights into the mode of action of aloe-emodin in the treatment of infections by S. aureus biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus (S. aureus) is the most common pathogen in cases of bacteraemia, endocarditis and sepsis. It is also involved in a variety of biofilm-associated infections such as those in heart implants and bloodline catheters, superficial skin infections and otitis media (Agarwal and Jain 2010; Thornton et al. 2013). Management of biofilm infections is extremely difficult due to the fact that bacterial cells embedded in biofilms exhibit inherent resistance to anti-microbial agents because they have a dormant phenotype; thus, biofilms can radically reduce cell metabolism and contribute to the bacterial persistence in chronic infections. In addition, biofilms represent a reservoir that can disseminate S. aureus infections to other sites in the host body, leading to recurrent infection and increased fatalities in cases of the most recalcitrant infections (Lewis 2010; Waters et al. 2016). Thus, it is important to identify new therapeutic strategies that interfere with biofilm development.
The development of bacterial biofilms can be divided into four stages: initial adhesion, proliferation, maturation and diffusion (Boles and Horswill 2011; Otto 2008b). In the initial stage, the planktonic S. aureus cells attach reversibly to a solid living or non-living substratum. The specific interaction between so-called MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) and human matrix proteins such as fibrinogen or fibronectin is most likely of much greater importance for the initiation of biofilm formation on biotic materials (Foster et al. 2014). In the absence of human matrix molecules, S. aureus may attach to abiotic surfaces through electrostatic and hydrophobic interactions (Kennedy and O’Gara 2004). The negatively charged teichoic acids and the major autolysin, AtlA, also have been shown to be involved in cell attachment to polystyrene and glass surfaces (Biswas et al. 2006; Houston et al. 2011; Gross et al. 2001).
After attaching to a surface, the adherent bacteria begin to divide, accumulate and form microcolonies (Stoodley et al. 2008). When a sufficient nutrient source is present and microcolonies are formed, the biofilm undergoes the maturation stage. This stage is characterized by intercellular aggregation and the establishment of a more complex mature biofilm with a more three-dimensional appearance and mushroom-like cell towers that surround fluid-filled channels, which are equipped to aid the flow of nutrients into the interior of the biofilm (Otto 2008a).
In the final stage of development, single cells or larger cell clusters can be dispersed from the biofilm via mechanical forces such as flow in a blood vessel and via detachment factors such as surfactants or enzymes that destroy the matrix. Detachment is crucial for dissemination of bacteria to new niches.
Targeting the different developmental stages of biofilm formation, three principal strategies have been developed: inhibition of attachment, disruption of biofilm architecture and signal transduction interference (Chung and Toh 2014). Several distinct inhibitor classes have been identified including synthesized small molecules or plant-derived natural compounds (Brackman and Coenye 2015; Mogosanu et al. 2015), silver ions and nanoparticles (Jia et al. 2017), matrix-targeting enzymes (Itoh et al. 2005), a cell wall-degrading enzyme from S. aureus bacteriophage (Kelly et al. 2012), synthetic anti-biofilm peptides (Pletzer and Hancock 2016) and anti-biofilm polysaccharides (Rendueles et al. 2013).
Currently, research work in our group mainly focuses on identifying new potential anti-infection small molecule compounds from medicinal herbs that target key bacteria virulence factors such as SrtA and α-haemolysin (Hla)in S. aureus and listeriolysin O in Listeria monocytogenes (Qiu et al. 2012; Wang et al. 2015a; Wang et al. 2015b). The capacity to form biofilms is now considered to be one of the important virulence traits in several Staphylococcus bacteria. By detecting the inhibition of biofilm formation, we have screened anti-biofilm molecules from TCM that have detoxification effects. Several compounds from the medicinal herb rhubarb (Rheum officinale) have been identified.
Chinese rhubarb (R. officinale) is one of the most well-known traditional Chinese herbal medicines and has been used for the treatment of various diseases including constipation, jaundice, gastrointestinal haemorrhage and ulcers for over 2000 years (Tang et al. 2012; ZHENG et al. 2013). In recent years, this rhubarb has also been shown to have anti-bacterial, anti-oxidant, anti-cancer, anti-angiogenesis and anti-inflammation effects (Hu et al. 2014).
Anthraquinone derivatives including aloe-emodin, chrysophanol, emodin and physcion are the primary active constituents of rhubarb (Komatsu et al. 2006). We had a strong interest in aloe-emodin because of its relatively high S. aureus biofilm inhibitory activities (Fig. 1a). Pharmacological data indicate that aloe-emodin has a broad spectrum of biological activities such as anti-bacterial, anti-viral, hepatoprotective and anti-cancer effects (Park et al. 2009); however, its effect on anti-biofilm activity is seldom reported. In the present study, we report the effect of aloe-emodin on biofilm formation by S. aureus on different materials and elucidate the primary mechanisms that are responsible for this kind of action.
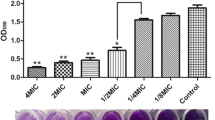
a The anti-biofilm activities of anthraquinone compounds against S. aureus were determined by crystal violet staining. b The anti-biofilm activities (OD595) of aloe-emodin against S. aureus were determined. The statistical significance was determined using one-way analysis of variance (ANOVA) (*P < 0.05; **P < 0.001). c Chemical structures of aloe-emodin and related compounds
Materials and methods
Bacterial strains, growth conditions and materials
S. aureus ATCC29213 (National Center for Medical Culture Collections) was used in this study, as it is proficient in biofilm formation (Abouelhassan et al. 2014). The strains were subcultured in brain-heart infusion (BHI) solution (Oxoid, Basingstoke, UK) that contained 3% NaCl and 0.5% glucose and were incubated for 12 h at 37 °C. Aloe-emodin and other compounds were obtained from the Changchun Baotaike Biotech Company (Changchun, China). Sytox green, fluorescein isothiocyanate (FITC), Syto 63 and Texas Red-concanavalin A (ConA) were obtained from Invitrogen-Molecular Probes (Invitrogen-Molecular Probes, Oregon).
Antibacterial activity assays
The minimum inhibitory concentration (MIC) of aloe-emodin was determined by a microtitre broth dilution method, as described in the CLSI guidelines (Kim et al. 2015). In brief, serial twofold dilutions of aloe-emodin in fresh BHI broth were undertaken, and S. aureus ATCC29213 was added at 1 × 105 CFU/ml to sterile 96-well polystyrene plates (Costar 3595; Corning Life Sciences). The assay plates were incubated at 37 °C for 24 h, and the bacterial growth inhibition assays were monitored by measuring the absorbance at 600 nm.
Crystal-violet screening assay for biofilm inhibitors
Biofilm inhibitors screening was based on a high-throughput assay described previously (Freeman et al. 1989). Overnight-cultured bacteria were diluted 1:100 into fresh BHI that was supplemented with 3% NaCl and 0.5% glucose. Then, 200 μl cultures were transferred into sterile 96-well polystyrene plates and covered with 20% rabbit blood plasma at 4 °C overnight. Aloe-emodin and other compounds were added to the assay plates at different concentrations, and the plates were incubated for 12 h without shaking at 37 °C. Then, the supernatants were removed, and the wells were washed twice with PBS. The wells were stained with crystal violet for 15 min, and the plates were thoroughly washed with PBS solution and then dissolved in 200 μl of 100% ethanol for 30 min at room temperature. The biofilms were quantified using a microplate reader (Infinite®F500, Tecan, Shanghai, China) at OD595. The biofilm formation of SrtA, Fnbps and SarA knockdown strains was based on methods described previously.
Confocal microscopy
S. aureus ATCC29213 biofilms were evaluated by confocal laser-scanning microscopy (Olympus, Shanghai, China). As described above, the biofilm was formed on glass with or without aloe-emodin at different concentrations (Hochbaum et al. 2011). The assay plates were incubated at 37 °C for 3, 6, 12 or 24 h. After culturing, the wells were washed twice with 2 ml of PBS. Sytox green (0.5 μM) was added to PBST (PBS supplemented with 0.5% Triton X-100), and the combination was added to assay plates that were then shaken for 30 min. FITC (0.001%) or ConA (50 μg/ml) and Syto 63 (100 μM) were added to PBS, which was added to the well without shaking. The supernatants were removed, and the wells were washed several times with PBS. Confocal microscopy images were observed using NIS-Elements C version 3.2 (Nikon eclipse).
Assay of anti-biofilm effects on different surfaces
The assay was based on a previously described method (Opperman et al. 2009). The biofilm adhered to glass, polystyrene, polyvinyl-chloride (PVC) and silicone. The ability of aloe-emodin to inhibit biofilm attachment to PVC and polystyrene was assayed using 96-well polyvinyl chloride (PVC) assay plates and 96-well polystyrene assay plates, respectively. Assays of aloe-emodin inhibiting biofilm attachment to glass and silicone were performed in six-well assay plates that contained glass and silicone disks, respectively. All assays were conducted with or without aloe-emodin at different concentrations and were incubated for 12 h at 37 °C.
Effect of aloe-emodin on growth cycle of biofilm formation
S. aureus ATCC29213 biofilm formation was established as described above (Opperman et al. 2009). The assay plates were inoculated with or without 128 μg/ml aloe-emodin at various times. After inoculation, the biofilms were quantified as described previously.
Primary attachment assay
The static biofilm primary attachment assay was performed as described above (Cue et al. 2015). Bacteria were adhered for 1 h at 37 °C, washed with PBS and stained with crystal violet, and the OD595 of the samples was measured.
Identification of the main components of S. aureus biofilms
To determine the main components of the S. aureus ATCC29213 in BHI that was supplemented with 3% NaCl and 0.5% glucose (Mazmanian et al. 2002), the biofilm formation assay was carried out as previously described but without aloe-emodin (Opperman et al. 2009). After 12 h of incubation at 37 °C, the plates were washed with PBS, followed by the separate addition of 2 mg/ml DNase I (Sigma-Aldrich, Tokyo, Japan), 100 μg/ml proteinase K and 10 mM sodium metaperiodate; sterile BHI medium with sodium metaperiodate butter (50 mM sodium acetate buffer, pH 4.5) and saline was used as the control. After their incubation for 2 h at 37 °C without shaking, the plates were washed twice with PBS, and the biofilms were evaluated by crystal violet staining.
Scanning electron microscopy
S. aureus ATCC29213 biofilms were formed on six-well plates that contained glass coverslips and were examined using scanning electron microscopy (SEM) (Chu et al. 2016). After 12 h of incubation at 37 °C, the glass coverslips were washed with PBS and fixed with 2.5% glutaraldehyde at 4 °C for 12 h. Then, the wells were dehydrated with ethanol (60, 70, 80, 90, 95 and 100%) and coated with a 10-nm layer of gold/palladium. Scanning electron microscopy images were observed using a Hitachi S-5000 field emission SEM.
Congo red agar method
The Congo red agar method was used as previously reported (Chu et al. 2016). Briefly, the medium was composed of 37 g/l Tryptone Soy Broth (TSB), 50 g/l sucrose and 0.8 g/l Congo red. Congo red dye was autoclaved (121 °C for 15 min separately from other medium components) and was added when the agar had cooled to 55 °C. S. aureus ATCC29213 was incubated on Congo red agar plates with or without aloe-emodin (32, 64 and 128 μg/ml) for 24 h at 37 °C.
Autolysis assays
The assays were performed as described previously (Opperman et al. 2009). S. aureus ATCC29213 was incubated on medium with or without aloe-emodin (64 and 128 μg/ml) for 12 h at 37 °C. Then, the cultures were harvested by centrifugation, washed twice with ice-cold deionized water and suspended in 50 ml of 0.05% Triton X-100 solution (50 mM Tris-HCl [pH 7.5]). The cultures were then incubated at 30 °C with shaking. Samples were taken every 30 min, and their OD600 was measured.
Statistical analysis
The Student’s t test was performed using SPSS 13.0 software, and the data were expressed as the mean ± standard deviation. Results with P < 0.05 and P < 0.001 were considered statistically significant.
Results
Aloe-emodin inhibited biofilm formation by S. aureus ATCC29213 without affecting planktonic cell growth
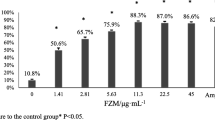
We have conducted screening of phytochemicals with anti-biofilm activity against S. aureus ATCC29213 in 96-well polystyrene plates that were covered with 20% rabbit freeze-dried plasma. Five anthraquinone compounds from R. officinale (aloe-emodin, emodin, physcoin, chrysophanol and aloin) inhibited S. aureus ATCC29213 biofilm formation at different concentrations (Fig. 1c). Among these compounds, aloe-emodin has the most potential (Fig. 1a). The results showed that aloe-emodin at 16 μg/ml markedly inhibited S. aureus ATCC29213 biofilm formation (Fig. 1a). Further evaluation showed that 32 μg/ml of aloe-emodin was required to inhibit biofilm formation by ≥85% (Fig. 1b).
Confocal laser microscopy was used to analyse changes in biofilm formation on glass and was in-line with biofilm data that were obtained using 24-well polystyrene plates. The fluorescent images indicated that in the control group, the biofilm gradually increased as time progressed from 3 to 24 h and aloe-emodin dose-dependently inhibited S. aureus ATCC29213 biofilm formation (Fig. 2a).
To verify whether aloe-emodin inhibited S. aureus ATCC29213 biofilm formation by affecting bacterial growth, the anti-microbial activity of aloe-emodin was investigated by determining the MICs. As shown in Fig. 2b, the MICs of aloe-emodin against S. aureus ATCC29213 were >1024 μg/ml. These findings show that the reduced biofilm formation caused by aloe-emodin was due to its anti-biofilm activity and not to its anti-microbial activity.
Aloe-emodin inhibits biofilm formation on abiological surfaces
S. aureus can develop biofilms on the surfaces of different materials. To determine whether aloe-emodin inhibits biofilm formation on an abiological surface, the ability of aloe-emodin to inhibit biofilm formation of S. aureus ATCC 29213 on glass, polystyrene, polyvinyl chloride (PVC) and silicone was determined. Aloe-emodin exhibited significant anti-biofilm activity on polystyrene and PVC surfaces. At a concentration of 32 μg/ml, this compound almost completely inhibited biofilm formation on PVC surfaces. When the concentration of aloe-emodin was greater than 64 μg/ml, it inhibited biofilm formation on other abiological surfaces (Fig. 3). The results showed that aloe-emodin not only inhibits biological surface biofilm formation but also inhibits biofilm formation on abiological surfaces.
Aloe-emodin inhibited the initial adhesion and proliferation stages of biofilm development
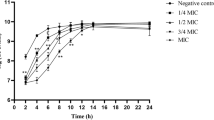
To identify the biofilm development stages of S. aureus ATCC29213 under the above culture conditions (0.5% glucose, 3% NaCl), we performed tests based on the method previously described (Otto 2013). The results showed that the period from 0 to 1 h was the bacterial adhesion stage, 1–12 h was the bacterial proliferation stage, 12–72 h was the biofilm maturation stage and the biofilm diffusion stage occurred after 72 h (Fig. 4a).
To gain insight into the preliminary mode of action of aloe-emodin anti-biofilm activity, aloe-emodin (128 μg/ml) was added to the cultures of S. aureus ATCC29213 at different time points during the development of the biofilm. The influence on biofilm formation was measured after a 24-h incubation. Addition of aloe-emodin immediately after inoculation (0 h) resulted in 90% inhibition of biofilm formation (Fig. 4b). Aloe-emodin reduced the biofilm gradually when added at 1, 4 and 8 h into biofilm development. After 12 h of incubation, the biofilm culture was completely resistant to aloe-emodin (Fig. 4b). These results indicated that aloe-emodin would terminate the biofilm growth process when added during the initial adhesion and the proliferation stages.
To further test the effect of aloe-emodin on bacterial adhesion, the anti-adhesion activity of different concentrations of the compound was measured after 1 h of incubation. These results suggested that aloe-emodin reduces more than 50% of bacterial adhesion at concentrations of approximately 4 μg/ml with an inhibition rate of 65% (Fig. 4c). Collectively, these data showed that aloe-emodin significantly inhibited the attachment step in biofilm formation.
S. aureus ATCC29213 biofilm extracellular matrix is mainly composed of protein
The extracellular matrix composition of S. aureus biofilm is different under different culture conditions (Arce Miranda et al. 2011). To identify whether S. aureus biofilms are polysaccharide intercellular adhesin (PIA)-dependent or PIA-independent under these culture conditions, a biofilm detachment assay was carried out as previously described (Boles and Horswill 2008; Tetz et al. 2009). According to the results, the amount of residual biofilm after treatment with proteinase K, DNase I and sodium periodate was approximately 10, 35 and 50%, respectively (Fig. 5a). This result indicates that protein is the main component of the S. aureus ATCC29213 biofilm extracellular matrix.
a Enzymatic hydrolysis of biofilm. The amount of residual biofilm after treatment with or without proteinase K, DNase I and sodium periodate was measured at OD595. The statistical significance was calculated using one-way analysis of variance (ANOVA) (*P < 0.05; **P < 0.001). b SEM analysis of S. aureus ATCC29213 biofilms. SEM images of biofilms formed by S. aureus ATCC29213 that had been incubated for 6, 12 and 24 h. Scale bars in the figures above represent 5 μm and scale bars in the figures below represent 20 μm and it was equally divided into ten units and each unit represents 0.5 or 2 μm, respectively
According to a previous study, S. aureus cells form highly dense aggregates without any detectable extracellular matrix when grown in a protein-mediated biofilm, and PIA/PNAG-dependent biofilm cells are embedded in an abundant extracellular material (Vergarairigaray et al. 2009). SEM of the biofilm carried out at different time points showed that the cells in the biofilm were smooth and there was not a visible filament that was attached to the bacterial surface (Fig. 5b). The detailed results further suggested that S. aureus ATCC29213 biofilm develops in a PIA-independent mode and that protein represents the main extracellular matrix under these culture conditions.
Aloe-emodin inhibits biofilm formation by reducing the production of extracellular proteins
The effect of aloe-emodin on extracellular biofilm proteins was studied by confocal microscopy. Syto63 dye stained the intracellular DNA red, and the FITC dye stained the extracellular proteins green. The control strain was surrounded by obvious green fluorescence, representing the extracellular matrix protein in the biofilm. After treatment with aloe-emodin (128 μg/ml), the surrounding green fluorescence decreased significantly (Fig. 6a).
a Confocal analysis of extracellular proteins. Syto 63 dye stained the intracellular DNA red, and the FITC dye stained the extracellular proteins green. b Autolysis assays of eDNA. Autolysis activity was monitored as the change in OD600 over time. c Congo red staining of PIA. S. aureus ATCC29213 was grown on Congo red medium and incubated with various concentrations of aloe-emodin
Autolytic activity from cell subpopulations caused the release of extracellular DNA (eDNA), which promotes cell adhesion at the biofilm maturation stage. This eDNA contributes to the structure of the S. aureus biofilm matrix and to both cell-cell and cell-surface interactions (Mann et al. 2008; Rice et al. 2007). The effect of aloe-emodin on the autolysis of bacteria indirectly proved its effect on eDNA. The results showed that the treatment group and the control group were not significantly different (Fig. 6b). These results suggest that aloe-emodin has no effect on eDNA release from S. aureus ATCC29213 at 64 or 128 μg/ml concentrations.
Congo red can react with bacterial biofilm surface PIA, causing the colonies to become black. The present study showed that aloe-emodin treatment significantly reduced the number of black colonies in a dose-dependent manner (Fig. 6c). This result indicated that aloe-emodin inhibited the production of PIA from bacterial biofilms.
Discussion
S. aureus is a leading cause of nosocomial infections due in large part to rising rates of resistance to diverse antibiotics. The formation of a biofilm represents one of the mechanisms of drug resistance in S. aureus, because it is a physical barrier to antibiotics and results in differences in metabolism that further restrict antibiotic efficacy (Hochbaum et al. 2011). With the emergence of multidrug-resistant S. aureus, the need for more effective treatment of biofilm-associated infections has become imperative.
As natural products exhibit a large number of interesting structures, they have played an important role as a major source of new drugs for human therapy in persistent infections (Baker et al. 2007). Many categories of compounds from plants exhibit anti-biofilm activity against S. aureus in a variety of biofilm models while not causing significant bacterial growth inhibition. These compounds include, for example, oregano oil (Nostro et al. 2007), tea tree oil (Kwieciński et al. 2009), cinnamaldehyde (Jia et al. 2011), chelating pyrethroids (Payne et al. 2013), ginkgolic acids and Ginkgo biloba extract (Lee et al. 2014), gallic acid (Liu et al. 2016) and ellagic acid rhamnoside (Fontaine et al. 2017). Although these agents show great potential in the treatment of biofilm-associated infections, their mechanisms of action remain unclear.
The development of bacterial biofilms can be divided into four stages: initial adhesion, proliferation, maturation and diffusion. S. aureus biofilms are often encased in a matrix of self-produced extracellular polymeric substances (EPSs) that contain varying amounts of cell wall-associated proteins, PIA and eDNA (Arciola et al. 2012; Hochbaum et al. 2011). However, the composition of the matrix varies greatly under different growth conditions (Beenken et al. 2003).
We investigated the mode of action of aloe-emodin during the biofilm developmental stages and on matrix composition. We found that aloe-emodin terminated biofilm growth processes when it was added at the initial adhesion and proliferation stages (Fig. 6). We therefore determined the mechanism of aloe-emodin action against biofilms. The results indicated that aloe-emodin inhibits extracellular protein production and accumulation of PIA, leading to inhibition of biofilm formation by S. aureus.
In the initial adhesion stage, primary attachment to the biotic surface of the host tissues and to synthetic surfaces coated with plasma proteins is achieved by cell wall-anchored (CWA) proteins of S. aureus. Numerous surface proteins such as fibrinogen/fibronectin-binding proteins (FnBPA and FnBPB), clumping factors A and B (ClfA and ClfB), surface proteins C and G (SasC and SasG) and serine aspartate repeat protein (SdrC, SdrD and SdrE) are individually implicated in binding host matrix components to initiate cell adherence and biofilm matrix formation in S. aureus (Foster et al. 2014; Speziale et al. 2014).
After they attach to a surface, the adherent S. aureus cells will begin to grow, multiply and form microcolonies (Stoodley et al. 2008). Staphylococci produce several factors that could facilitate biofilm accumulation by stabilizing cell-to-cell interactions shortly after the initial attachment. Some of the CWA proteins such as the FnBPs, ClfB and SdrC proteins play dual roles in both attachment and accumulation (Speziale et al. 2014). In addition, protein A (Merino et al. 2009), SasC (Schroeder et al. 2009) and Bap (Cucarella et al. 2001) have all shown a propensity to aid in biofilm accumulation. Similarly, PIA has been implicated in early accumulation stage of S. aureus biofilm formation (Arciola et al. 2015).
Most of these surface proteins share a common cell wall-targeting motif (LPXTG) and are anchored to the cell wall envelope by sortase A (SrtA), which catalyses the covalent attachment of these proteins to the penta-glycine cross-linker component of the peptidoglycan (Mazmanian et al. 1999). Several categories of small molecule inhibitors of SrtA showed significant anti-biofilm activity by inhibiting the transpeptidase activity of SrtA and interfering with the functional display of surface proteins (Hochbaum et al. 2011). We have assessed the inhibitory activities of aloe-emodin against SrtA in vitro through a fluorescence resonance energy transfer (FRET) assay (Hochbaum et al. 2011). This compound did not show any potential transpeptidase inhibitory activity at concentrations up to 64 μg/ml (data not shown). This result indicated that aloe-emodin could not interfere with cell wall anchoring of surface proteins and that its biofilm development inhibition effects resulted from down-regulation of expression of certain surface proteins or direct blocking of adhesion of those proteins to other matrix components. Our future research will focus on identifying the precise molecular mechanism by which aloe-emodin inhibits S. aureus biofilm formation.
To conclude, aloe-emodin was effective at preventing S. aureus biofilm formation in a dose-dependent manner even at subinhibitory concentrations. Therefore, aloe-emodin can be used to prevent formation of biofilms on the surfaces of medical devices. We believe that this molecule is a potential novel treatment against S. aureus biofilm-related infections.
References
Abouelhassan Y, Garrison AT, Burch GM, Wong W, Th NV, Rd HR (2014) Discovery of quinoline small molecules with potent dispersal activity against methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis biofilms using a scaffold hopping strategy. Bioorg Med Chem Lett 24(21):5076–5080
Agarwal A, Jain SA (2010) Medical significance and management of staphylococcal biofilm. Fems Immunology & Medical Microbiology 58(2):147–160
Arce Miranda JE, Sotomayor CE, Albesa I, Paraje MG (2011) Oxidative and nitrosative stress in Staphylococcus aureus biofilm. FEMS Microbiol Lett 315(1):23–29
Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33(26):5967
Arciola CR, Campoccia D, Ravaioli S, Montanaro L (2015) Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Frontiers in Cellular & Infection Microbiology 5(7):7
Baker DD, Chu M, Oza U, Rajgarhia V (2007) The value of natural products to future pharmaceutical discovery. ChemInform 39(13):1225
Beenken KE, Blevins JS, Smeltzer MS (2003) Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 71(7):4206
Biswas R, Voggu L, Simon UK, Hentschel P, Thumm G, Götz F (2006) Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett 259(2):260–268
Boles BR, Horswill AR (2008) Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4(4):e1000052
Boles BR, Horswill AR (2011) Staphylococcal biofilm disassembly. Trends Microbiol 19(9):449–455
Brackman G, Coenye T (2015) Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des 21(1):5–11
Chu M, Zhang MB, Liu YC, Kang JR, Chu ZY, Yin KL, Ding LY, Ding R, Xiao RX, Yin YN (2016) Role of berberine in the treatment of methicillin-resistant Staphylococcus aureus infections. Sci Rep 6:24748
Chung PY, Toh YS (2014) Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathog Dis 70(3):231–239
Cucarella C, Solano C, Valle J, Amorena B, Lasa Í, Penadés JR (2001) Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183(9):2888–2896
Cue D, Junecko JM, Lei MG, Blevins JS, Smeltzer MS, Lee CY (2015) SaeRS-dependent inhibition of biofilm formation in Staphylococcus aureus Newman. PLoS One 10(4):e0123027
Fontaine BM, Nelson K, Lyles JT, Jariwala PB, Garcíarodriguez JM, Quave CL, Weinert EE (2017) Identification of ellagic acid rhamnoside as a bioactive component of a complex botanical extract with anti-biofilm activity. Front Microbiol 8:496
Foster TJ, Geoghegan JA, Ganesh VK, Höök M (2014) Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12(1):49
Freeman DJ, Falkiner FR, Keane CT (1989) New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol 42(8):872–874
Gross M, Cramton SE, Gotz F, Peschel A (2001) Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun 69:3423–3426
Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R (2011) Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol 193(20):5616
Houston P, Rowe SE, Pozzi C, Waters EM, O’Gara JP (2011) Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infection & Immunity 79(3):1153–1165
Hu B, Zhang H, Meng X, Wang F, Wang P (2014) Aloe-emodin from rhubarb (Rheum officinale) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol 153(3):846–853
Itoh Y, Wang X, Hinnebusch BJ, Romeo T (2005) Depolymerization of beta-1,6-N-acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 187(1):382
Jia P, Xue YJ, Duan XJ, Shao SH (2011) Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol 53(4):409–416
Jia M, Chen Z, Guo Y, Chen X, Zhao X (2017) Efficacy of silk fibroin–nano silver against Staphylococcus aureus biofilms in a rabbit model of sinusitis. Int J Nanomedicine:2933–2939
Kelly D, Mcauliffe O, Ross RP, Coffey A (2012) Prevention of Staphylococcus aureus biofilm formation and reduction in established biofilm density using a combination of phage K and modified derivatives. Lett Appl Microbiol 54(4):286–291
Kennedy CA, O’Gara JP (2004) Contribution of culture media and chemical properties of polystyrene tissue culture plates to biofilm development by Staphylococcus aureus. J Med Microbiol 53(11):1171–1173
Kim ES, Kang SY, Kim YH, Lee YE, Choi NY, You YO, Kim KJ (2015) Chamaecyparis obtusa essential oil inhibits methicillin-resistant Staphylococcus aureus biofilm formation and expression of virulence factors. J Med Food 18(7):810
Komatsu K, Nagayama Y, Tanaka K, Ling Y, Basnet P, Meselhy MR (2006) Development of a high performance liquid chromatographic method for systematic quantitative analysis of chemical constituents in rhubarb. Chemical & Pharmaceutical Bulletin 54(7):941
Kwieciński J, Eick S, Wójcik K (2009) Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int J Antimicrob Agents 33(4):343–347
Lee JH, Kim YG, Ryu SY, Cho MH, Lee J (2014) Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157:H7 and Staphylococcus aureus biofilm formation. Int J Food Microbiol 174:47–55
Lewis K (2010) Persister cells. Annu Rev Microbiol 64(64):357
Liu MH, Wu XX, Li JK, Liu L, Zhang RG, Shao DY, Du XD (2016) The specific anti-biofilm effect of gallic acid on Staphylococcus aureus by regulating the expression of the ica operon. Food Control 73:613-618
Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW (2008) Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4(6):e5822
Mazmanian SK, Liu G, Ton-That H, Schneewind O (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285(5428):760
Mazmanian SK, Ton-That H, Su K, Schneewind O (2002) An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci 99(4):2293–2298
Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I (2009) Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol 191(3):832
Mogosanu GD, Grumezescu AM, Huang KS, Bejenaru LE, Bejenaru C (2015) Prevention of microbial communities: novel approaches based natural products. Curr Pharm Biotechnol 16(2):94–111
Nostro A, Sudano RA, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56(Pt 4):519
Opperman TJ, Kwasny SM, Williams JD, Khan AR, Peet NP, Moir DT, Bowlin TL (2009) Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrob Agents Chemother 53(10):4357–4367
Otto M (2008a) Staphylococcal biofilms. Curr Topics Microbiol Immunol 322(6):207
Otto M (2008b) Staphylococcal biofilms. Curr Topics Microbiol Immunol 322(6):207–228
Otto M (2013) Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64(1):175
Park MY, Kwon HJ, Sung MK (2009) Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci Biotechnol Biochem 73(4):828
Payne DE, Martin NR, Parzych KR, Rickard AH, Underwood A, Boles BR (2013) Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect Immun 81(2):496–504
Pletzer D, Hancock RE (2016) Anti-biofilm peptides: potential as broad-spectrum agents. J Bacteriol 198(19):2572
Qiu JZ, Niu XD, Dong J, Wang DC, Wang JF, Li HG, Luo MJ, Li ST, Feng HH, Deng XM (2012) Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of α-hemolysin. J Infect Dis 206(2):292
Rendueles O, Kaplan JB, Ghigo JM (2013) Antibiofilm polysaccharides. Environ Microbiol 15(2):334–346
Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW (2007) The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104(19):8113–8118
Schroeder K, Jularic M, Horsburgh SM, Hirschhausen N, Neumann C, Bertling A, Schulte A, Foster S, Kehrel BE, Peters G (2009) Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation. PLoS One 4(10):e7567
Speziale P, Pietrocola G, Foster TJ, Geoghegan JA (2014) Protein-based biofilm matrices in Staphylococci. Front Cell Infect Microbiol 4(4):171
Stoodley P, Nistico L, Johnson S, Lasko LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S (2008) Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am 90(8):1751
Tang MJ, Tan LP, Liu ZH, Li TY (2012) The review of pharmacological activities of rhubarb. China Trop Med 12(7):886-889
Tetz GV, Artemenko NK, Tetz VV (2009) Effect of DNase and antibiotics on biofilm characteristics. Antimicrob Agents Chemother 53(3):1204
Thornton RB, Wiertsema SP, Kirkham LAS, Rigby PJ, Vijayasekaran S, Coates HL, Richmond PC (2013) Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media—a potential treatment target. PLoS One 8(2):e53837
Vergarairigaray M, Valle J, Merino N, Latasa C, García B, Mozos IRDL, Solano C, Toledoarana A, Penadés JR, Lasa I (2009) Relevant role of Fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77(9):3978–3991
Wang JF, Qiu JZ, Tan W, Zhang Y, Wang HS, Zhou X, Liu S, Feng HH, Li WH, Niu X, Deng XM (2015a) Fisetin inhibits Listeria monocytogenes virulence by interfering with the oligomerization of listeriolysin O. J Infect Dis 211(9):1376
Wang L, Bi CW, Cai HJ, Liu BR, Zhong BX, Deng XM, Wang TD, Xiang H, Niu XD, Wang DC (2015b) The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front Microbiol 6:1031
Waters EM, Rowe SE, O’Gara JP, Conlon BP (2016) Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog 12(12):e1006012
Zheng QX, Wu HF, Guo J, Nan HJ, Chen SL, Yang JS, Xu XD (2013) Review of rhubarbs: chemistry and pharmacology. Chin Herb Med 5(1):9–32
Acknowledgements
This work was supported by The National Key Technology R&D Program (No.2016YFD05013) and the China Education Department of Jilin Province Science and Technology Research Project of “13th Five-Year” ([2016] No. 194).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article was conducted without the use of human or animal participants.
Rights and permissions
About this article
Cite this article
Xiang, H., Cao, F., Ming, D. et al. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. Appl Microbiol Biotechnol 101, 6671–6681 (2017). https://doi.org/10.1007/s00253-017-8403-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8403-5