Abstract
The Camptotheca acuminata cell suspension cultures were established to produce the well-known antitumor monoterpene indole alkaloid camptothecin (CAM). Most CAM was present in the broth of the C. acuminata cell suspension cultures. The CAM production was evidenced to be attenuated when the C. acuminata cell suspension cultures were continuously subcultured and grown under identical axenic conditions. A practical cryopreservation and recovery procedure was established to maintain the C. acuminata cell suspension cultures. Biotic and abiotic elicitors were administrated to the C. acuminata cell suspension cultures to restore and enhance CAM production. Of them, sorbitol, a well-known hyperosmotic stressor, was proven to be the most effective elicitor that stimulates a ∼500-fold increase of CAM production. The committed biosynthetic precursors of CAM, tryptamine and secologanin, were feed to the C. acuminata cell suspension cultures and the CAM production is not remarkably increased. However, N 1-acetylkynuramine (NAK), an important metabolite of kynuramine pathway, was isolated and identified from the cell suspension cultures feeding with tryptamine. The present work provides an efficient method to produce CAM and NAK using the C. acuminata cell suspension cultures. The biotransformation of tryptamine to NAK sheds lights on the biosynthetic formation of the pyrroloquinoline moiety of CAM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Camptotheca acuminata, a deciduous tree native to China (Qin and Chamlong 2007), is one of the two main plant resources of camptothecin (CAM, 1, Fig. 1a), the third largest well-known plant-derived anticancer drug in the global market (Demain and Vaishnav 2011). Identified from C. acuminata in 1966, CAM is a complex pentacyclic pyrroloqinoline alkaloid with antitumor bioactivity (Wall et al. 1966). Two decades later, CAM was proven to induce protein-linked DNA breakage via mammalian DNA topoisomerase I (Hsiang et al. 1985). CAM derivatives, chemically transformed from CAM (Thomas et al. 2004), such as topotecan and irinotecan, were clinically used as efficient anticancer drugs against a number of tumor types such as ovarian, small lung, and refractory ovarian cancers (Demain and Vaishnav 2011; Sirikantaramas et al. 2007). As the starting material of the clinically used CAM-type drugs, CAM, a monoterpene indole alkaloid, was found in many plant species of the asterid clade (Shaanker et al. 2008). However, CAM production was mainly dependent on the extraction and purification from C. acuminata in China and Nothapodytes foetida in India (Lorence and Nessler 2004). The heavy demand for CAM derivatives has resulted in destructive harvesting of C. acuminata, the CAM-producing plant in China, and it was claimed to be a protected plant species in China (http://www.gov.cn/gongbao/content/2000/content_60072.htm). Thus, identifying new sources of CAM and novel production methods are urged in the science community (Bhalkar et al. 2016; Deepthi and Satheeshkumar 2016; Pu et al. 2013; Pu et al. 2015; Vasanthakumari et al. 2015; Venugopalan and Srivastava 2015; Venugopalan et al. 2016).
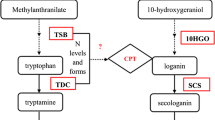
Putative biosynthetic pathways of a camptothecin (CAM) and b N 1-acetylkynuramine (NAK). The key ring-opening (C2–C7 double bound cleavage) and following ring-closing (C2–C6 double bond formation) steps involved in the biosynthesis of CAM were highlighted in blue rectangular frame. The carbon-carbon bonds of the indole moieties will be cleaved in the biosynthesis of CAM and NAK were highlighted in red
Plant tissue culture was proven to be a well-controlled, convenient, and sustainable system for efficient production of the pharmaceutically important plant-derived natural products, for instance, the callus and cell suspension cultures of C. acuminata were established in the 1970s for sustainable production of CAM (Sakato et al. 1974). The first reported CAM yield from the cell culture was 2.54 μg g−1 dry weight (DW). While the CAM contents in the field plants ranged from 0.2 to 5.0 mg g−1 DW (López-Meyer et al. 1994). Since then, different research groups developed various methods to produce CAM and the CAM yields ranged from 0.1 μg g−1 DW to 2.36 mg g−1 DW in the callus cultures and from 40 μg g−1 DW to 5.0 mg g−1 DW in the cell suspension cultures, respectively (Supplementary Table S1). The hairy root cultures of C. acuminata were shown to produce CAM at a level of 1.0 mg g−1 DW that is equal to and sometimes greater than the CAM yields of the plant roots (Supplementary Table S1). The callus and the cell suspension cultures of N. foetida were proven to produce a small amount of CAM (Supplementary Table S1). This alkaloid production level was 100∼1000-fold lower than that from the original plants (Fulzele et al. 2001). CAM could be produced in the cell cultures of Ophiorrhiza mungos (Supplementary Table S1). However, the alkaloid was not detected in the cell cultures of O. pumila, another well-known CAM-producing plant (Kitajima et al. 1998).
The elicitation treatment of plant tissue cultures is one of the most effective strategies for improving secondary metabolite production in cell suspension cultures (Cai et al. 2012; Wang and Wu 2013). A few elicitors such as salicylic acid, methyl jasmonate, abscisic acid, and metal ions were applied to the C. acuminata callus and cell suspension cultures. However, the CAM production was not increased remarkably in the treated cultures (Supplementary Table S1). Herein, the C. acuminata cell suspension cultures were initiated from the calli that originated from the seedlings of C. acuminata to produce CAM. The time-course analyses of the C. acuminata cell suspension cultures and the CAM contents showed that most CAM was present in the fermentation broth of the cell suspension cultures. The CAM production of the C. acuminata cell suspension cultures was witnessed to be attenuated when they were continuously subcultured and fermented under the same axenic conditions. Thus, a practical procedure for cryopreservation and recovery was established to maintain the C. acuminata cell suspension cultures. A series of biotic and abiotic elicitors were prepared and applied to the C. acuminata cell suspension cultures to restore and/or enhance the CAM production. Of them, sorbitol, a well-known hyperosmotic stressor, was shown to be the most effective elicitor that stimulates a ∼500-fold increase of the CAM production of the C. acuminata cell suspension cultures. The CAM production is not remarkably increased when tryptamine, a committed biosynthetic precursor of CAM, was feed to the C. acuminata cell suspension cultures. However, N 1-acetylkynuramine (NAK, 2, Fig. 1b), an important metabolite of kynuramine pathway, was isolated and identified from the C. acuminata cell suspension cultures feeding with tryptamine. The present work provides an efficient method to produce CAM and NAK in the cell suspension cultures of C. acuminata. The structural characterization of NAK from the biotransformation of tryptamine sheds lights on the biosynthetic formation of the pyrroloquinoline moiety of CAM (Fig. 1).
Materials and methods
Plant materials and seedlings growth
The C. acuminata seeds collection and seedlings growth were performed according to the reported procedures (Qu et al. 2015).
Callus induction and maintenance
The callus-inducing media are MS media (Murashige and Skoog 1962) supplemented with 30 g L−1 of sucrose, different plant growth regulators (Supplementary Table S2), and 0.35% phytogel. The pH value of the media was adjusted to 5.8 before autoclaving. The autoclaved media were poured into the sterile petri dishes. The leaves, stems, and roots of the 24-day-old seedlings of C. acuminata cultured under axenic conditions were cut into pieces (∼1 cm in length) and placed on the solid callus-inducing media in the petri dishes. Then the petri dishes containing the explants were incubated at 25 °C and 70% humidity in the dark.
Five~six weeks later, the cellular clumps consisting of small green calli grown at the verge of the explants were carefully excised and placed on the callus maintenance medium [MS medium supplemented with 30 g L−1 of sucrose, 2.0 mg L−1 of 1-naphthaleneacetic acid (NAA), 0.25 mg L−1 of 6-benzylaminopurine (6-BA), and 0.35% phytogel. The pH value of the medium was adjusted to 5.8 before autoclaving). The petri dishes containing the calli were incubated in a growth chamber with an illuminance of 25 μmol m−2 s−1 with 16-h photoperiod, 25 °C, and 70% humidity. The resulting calli were subcultured and maintained on the same conditions at 5∼6-week intervals.
Initiation, growth, and maintenance of cell suspension culture
The 5th generation C. acuminata calli (∼5 g fresh weight) were transferred into a 250-mL Erlenmeyer flask containing 100 mL of the cell suspension medium (MS medium supplemented with 30 g L−1 of sucrose, 0.5 mg L−1 of NAA, and 2 mg L−1 of 6-BA. The pH value of the medium was adjusted to 5.8 before autoclaving) (Liu et al. 2010). The resulting flasks were placed on an orbital shaker at 125 rpm under continuous light (25 μmol m−2 s−1) at 25 °C. Some small round cell aggregates were formed in the liquid medium after culturing 3∼5 days. The cell aggregates were collected and transferred into freshly prepared cell suspension media. The resulting cultures were subcultured in the same conditions every 10 days. To determine the contents of CAM and the weight of the biomass, the cell suspension culture were harvested every day and analyzed via the following methods.
Secondary metabolites extraction
The calli were harvested and dried at 60 °C to constant weight. The dried calli were finely ground and extracted three times with MeOH (10 mL MeOH per gram of calli). The organic solution was evaporated at 50 °C under reduced pressure to remove MeOH. The residue was suspended in 10 mL of H2O and extracted three times with a mixture of CHCl3 and MeOH (4:1) to give the crude calli extract. To prepare the crude extract of the cell suspension cultures, the fermentation mixture was filtered to afford the supernatant and cell pellets. The supernatant was extracted three times with a mixture of CHCl3 and MeOH (4:1) to give the crude extract. The cell pellets were extracted by using the same procedure for the secondary metabolites extraction from the calli.
Analyses, verification, and quantification of CAM
These experiments were performed according to the reported procedures (Pu et al. 2013).
Cryopreservation and recovery of cell suspension culture
According to the established protocols (Grout 2007; Yin et al. 2012), the C. acuminata cell suspension cultures (3 mL) were transferred into 100 mL of the pregrowth MS medium (MS medium supplemented with 80 g L−1 of sucrose, 0.5 mg L−1 of NAA, and 2 mg L−1 of 6-BA) and incubated at 25 °C in the dark on a gyratory shaker at 125 rpm. Three days later, 8 mL of the C. acuminata cell suspension cultures were concentrated by centrifugation at 4000 rpm and the concentrated cell suspension cultures, typically of 1 mL volume, were transferred into the sterile cryogenic vials. An equal volume of cryoprotectant MS medium supplemented with 20% DMSO, 10% DMSO − 10% glycerol, 20% DMSO − 16% sucrose, 20% DMSO − 16% glucose, or 20% DMSO − 20% glucose (v/v) was added to the concentrated suspension cultures. The resulting suspension cultures were mixed gently and incubated for an additional 30 min at room temperature. The cryogenic vials containing the cultures were placed into a refrigerator at 4 °C for 60 min, 0 °C for 30 min, −20 °C for 2 h, and subsequently maintained in an ultralow temperature refrigerator (−80 °C) for long-term cryopreservation.
To recover the cryopreserved cultures, the cryogenic vials containing the C. acuminata cell suspension cultures were put into a clean water bath (35∼40 °C) and flipped gently until the cultures were thawed. The mixture was concentrated by centrifugation at 4000 rpm and the cells were suspended and washed twice with the freshly prepared cell suspension media. Then the recovered cell suspension cultures (3 mL) were transferred to the freshly prepared cell suspension media and incubated at 25 °C in the dark for 3 days and then exposed at an illuminance of 25 μmol m−2 s−1 on a gyratory shaker at 125 rpm for 12 days. The cell suspension cultures were harvested on day 15. The biomass and the CAM contents were determined using the methods described above.
Culture media screening for CAM production
Ten liquid media (Supplementary Table S3), including various modified MS media, B5, LS, and DS media with different supplements, were prepared to grow and ferment the cell suspension cultures of C. acuminata. The CAM production of these fermentation cultures was determined following the abovementioned procedures.
Elicitors preparation and administration
Yeast polysaccharide extract was prepared from yeast extract by EtOH precipitation. In brief, 20 g of yeast extract was dissolved in 100 mL of distilled water and then mixed with 400 mL of EtOH. The resulting mixture was allowed to precipitate at 4 °C in a refrigerator for 4 days. The precipitate was re-dissolved in 100 mL of distilled water and subjected to a second round of EtOH precipitation. The final gummy precipitate was dissolved in 50 mL of distilled water and stored at 4 °C. The concentration of yeast polysaccharide was represented by total carbohydrate content that was determined by the anthrone test using sucrose as a reference standard (Dubois et al. 1956; Ge and Wu 2005). Chitosan solution was prepared by dissolving 0.5 g of crab shell chitosan (C3646, Sigma) in 1 mL of glacial acetic acid at 55∼60 °C. Distilled water was added to the acidic solution of chitosan to reach a final volume of 50 mL. Methyl jasmonate and jasmonic acid were dissolved in 95% EtOH and sterilized by filtering through a microfilter (0.22 μm). Salicylic acid, sorbitol, and the metal salts, including CoCl2, AgNO3, CaCl2, CuSO4, Li2SO4, MnSO4, and Ce(NH4)2(NO3)6, were dissolved in distilled water to the desired concentration. The pH value of the all prepared solution was adjusted to 5.8. The solutions were autoclaved at 121 °C for 15 min and stored at 4 °C in a refrigerator prior to use.
All elicitors were administered to the C. acuminata cell suspension cultures on day 3. Especially, for ultraviolet exposure, the flasks containing the C. acuminata cell suspension cultures of 3 days were thoroughly exposed in the ultraviolet-B for 1, 30, and 60 min, respectively. For ultrasound (300 w) treatment, the flasks containing the cell suspension cultures of 3 days were dipped into the ultrasonic water bath to a depth at which the level of the liquid in the flasks was ∼1.0 cm below the level of water. The temperature of the ultrasonic water bath was maintained at 25 ± 0.5 °C during the exposure. The exposure period was fixed at 1, 2, and 5 min, respectively. Both the treated and the control cell suspension cultures were incubated and harvested on days 9 and 15, respectively. The cultures were filtered and the biomass was weighed. The CAM of the fermentation broth was extracted and quantitated following the abovementioned procedures.
Biotransformation of tryptamine and isolation of NAK
The C. acuminata cell suspension cultures were incubated following the procedure mentioned above. Tryptamine (0.5 mM) was added to the C. acuminata cell suspension cultures on day 3. The resin XAD16 (10%) was added to the cultures on day 9 to adsorb the target product. The cultures were harvested by filtration on day 15. The resins were washed with H2O and then eluted with acetone. The organic solution was evaporated under reduced pressure to give 3.7 g of crude residue. The crude residue was separated by column chromatograph on silica gel (200∼300 mesh, Qingdao Haiyang Chemical Co., Ltd., China), eluting with petroleum ether–EtOAc (20: 1, 10: 1, 5: 1, 2: 1, 1: 1, v/v, each 500 mL) and CHCl3–MeOH (5: 1, 1: 1, 1: 2, 0: 1, v/v, each 500 mL), to afford fractions A∼ O. HPLC-DAD analysis suggested that compound 2 presented in fractions A∼C. Compound 2 (2 mg, retention time 24.57 min) was obtained from the semi-preparative HPLC separation of the fraction B. The HPLC was equipped with a Luna C18 column (250 × 10 mm, 10 μm), eluted with a linear gradient solvents A (0.1% formic acid in H2O) and B (0.1% formic acid in MeOH) from 10 to 55% B in 20 min, from 55 to 60% B in 10 min, from 60 to 98% B in 10 min, and continued at 98% B for an additional 5 min, at a flow rate of 3 mL min−1 at room temperature, and monitored by a diode array detector.
N 1-Acetylkynuramine (NAK, 2): yellow powder, 1H NMR (400 MHz, CD3OD): δ 7.77 (dd, J = 8.2, 1.4 Hz, 1 H), 7.25 (ddd, J = 8.5, 7.0, 1.5 Hz, 1 H), 6.75 (dd, J = 8.4, 0.8 Hz, 1 H), 6.61 (ddd, J = 8.2, 7.0, 1.2 Hz, 1 H), 3.55 (t, J = 6.5 Hz, 2 H), 3.19 (t, J = 6.5 Hz, 2 H), 1.93 (s, 3 H); 13C NMR (150 MHz, CD3OD): δ 201.92, 173.37, 152.70, 135.46, 132.22, 118.55, 118.34, 116.20, 39.28, 36.39, 22.52; HRESIMS: m/z 229.0952 [M + Na]+ (calcd for C11H14N2NaO2, 229.0947, error −2.1 ppm). The 1- and 2-D NMR spectra were included in Supplementary Fig. S8 - S12.
Results
Calli induction and cell suspension cultures initiation
Different plant pieces from the seedlings of C. acuminata were incubated on the callus-inducing MS30 media supplemented with different plant growth regulators (Supplementary Table S2) in the dark. Five∼six weeks later, some nascent C. acuminata calli were found at the verge of the plant pieces. The C. acuminata calli grown on different media showed different colors (Supplementary Table S2). The plant growth regulators have little effects on the fresh cell weight of the calli. However, the CAM yields ranged from trace to 4.5 μg g−1 DW (Supplementary Table S2). Thus, the CAM-producing calli from the MS30 medium supplemented with 2 mg L−1 of NAA and 0.25 mg L−1 of 6-BA were excised, transferred, and incubated on the callus maintenance medium with 16-h photoperiod. The CAM-producing calli were maintained and subcultured on the same conditions at 5∼6-week intervals. The CAM-producing calli became friable after five times subculturing, indicating a time point for the initiation of cell suspension cultures. The friable calli were transferred into the cell suspension medium and incubated under continuous exposure of light. The cell suspension culture was maintained by transferring them into freshly prepared cell suspension medium every 10 days (Supplementary Fig. S1).
CAM production of the calli and the cell suspension cultures
The secondary metabolites were extracted from the calli and the cell suspension cultures of C. acuminata, respectively. HPLC-DAD analysis showed that a product with identical retention time (panel II, Fig. 2a) and UV profile (panel II, Fig. 2b) to those of the standard CAM (panel I, Fig. 2a; panel I, Fig. 2b) was detected in the crude extracts of the C. acuminata calli. The product with same retention time (panel III, Fig. 2a) and UV profile (panel III, Fig. 2b) was also present in the extracts of the cell suspension cultures originated from the C. acuminata calli.
CAM production of the calli and the cell suspension cultures of C. acuminata. HPLC-DAD analyses (a), UV profiles (b), and HRESIMS data (c) of standard CAM (panel Is), the extracts of the calli (panel IIs), and the cell suspension cultures (panel IIIs). The diamond indicates CAM. The red vertical line indicates that the retention times of the corresponding products of the calli and the cell suspension cultures matched very well with that of the standard CAM
HPLC-DAD-HRMS analyses of the crude extracts were performed to confirm the CAM-producing capability of the calli and the cell suspension cultures of C. acuminata. The molecular ions at m/z 349.1154 ([M + H]+) (panel II, Fig. 2c) and 349.1149 ([M + H]+) (panel III, Fig. 2c) of the target products from the extracts of the calli and the cell suspension cultures, respectively, are consistent with the molecular ion at m/z 349.1164 ([M + H]+) of the standard CAM (panel I, Fig. 2c), which confirmed that both the calli and the cell suspension cultures can produce CAM.
Time-course of the cell suspension cultures
The C. acuminata cell suspension cultures exhibited a rapid exponential growth within an initial 4 days, a stationary phase from day 5 to day 9, and a declining phase from day 10 to day 19 (Supplementary Fig. S2). The maximum biomass reached on day 8. While quantitated by using a 5-point calibration curve of standard CAM at 373 nm (r 2 = 0.9999), the CAM contents of the cell suspension culture remained at a very low level from day 1 to day 12 and then dramatically increased on day 13 to a maximum of 2.03 mg L−1 in the broth (Supplementary Fig. S2). Only a very low level of CAM was detected in the cell pellets (Supplementary Fig. S2). Thus, most CAM was secreted into the broth on day 13 and the optimum harvest point is on day 13 in view of the highest CAM accumulation in the C. acuminata cell suspension cultures.
CAM production attenuation and cryopreservation of the cell suspension cultures
Previous works on the CAM-producing endophytes suggested that CAM production will be attenuated with subsequent successive subculturing and fermentation (Pu et al. 2013; Pu et al. 2015). A similar CAM production attenuation was observed when the C. acuminata cell suspension cultures were successively subcultured and grown in the same axenic conditions (Supplementary Fig. S3). The first- and second-generation C. acuminata cell suspension cultures were shown to produce ∼2 mg L−1 of CAM. However, ∼1.5 μg L−1 of CAM was present in the seventh-generation cultures (Supplementary Fig. S3).
In view of the abovementioned CAM production attenuation problem, a practical procedure for cryopreservation and recovery of the C. acuminata cell suspension culture was developed on the basis of the previous reports (Grout 2007; Yin et al. 2012). Different combinations of DMSO, glycerol, sucrose, or glucose were supplemented into the cryoprotectant MS media. HPLC-DAD analyses showed that the optimum cryopreservation conditions are MS medium supplemented with 10% DMSO and 8% glucose (Supplementary Fig. S4).
Effects of medium compositions and elicitors on the CAM production
The biomass and the CAM contents of the C. acuminata cell suspension cultures grown in different media (Supplementary Table S3) were determined, respectively (Supplementary Fig. S5). The media compositions have little effects on the growth of the C. acuminata cell suspension cultures. However, the CAM contents varied in different media. The MS medium supplemented with 30 g L−1 of sucrose, 0.5 mg L−1 of NAA, 2.0 mg L−1 of 6-BA, 0.1 mg L−1 of 2,4-dichlorophenoxyacetic acid (2,4-D), and 40.0 mM NH4 +/NO3 − (5:1), was set as the optimum medium to perform the following experiments in view of the highest accumulation of CAM (Supplementary Fig. S5).
The elicitors (Supplementary Table S4), including metal ions (Cu2+, Mn2+, Ca2+, Ce3+, Co2+, Ag+, and Li+), plant response-signaling compounds (methyl jasmonate, salicylic acid, and jasmonic acid), polysaccharides (yeast extract and chitosan), hyperosmotic stressor (sorbitol), biosynthetic precursors (secologanin and tryptamine), ultraviolet and ultrasound and natural light, adsorbent resins (HP20 and XAD16), and CAM-producing microorganisms [Paenibacillus polymyxa LY214 (Pu et al. 2015) and Trichoderma atroviride LY357 (Pu et al. 2013)] were applied to the C. acuminata cell suspension cultures on day 3. Most elicitation treatments have little effects on the pH values of the broth of the C. acuminata cell suspension cultures (Fig. 3a). However, the pH values decreased sharply to ∼4 when high concentration of chitosan was added to the broth of the C. acuminata cell suspension cultures. In general, the cell biomass of 15 days was lower than that of 9 days (Fig. 3b). Regarding the CAM production, most elicitation treatments have little effects on the CAM production (Fig. 3c). The C. acuminata cell suspension cultures treated with Cu2+, Ca2+, Co2+, Li+, salicylic acid, methyl jasmonate, secologanin, and T. atroviride LY357 showed a slightly higher CAM yields than that of the control group (Fig. 3c). However, when exposed in natural light, the CAM production of the C. acuminata cell suspension cultures increased ∼20-fold than that of the control group (Fig. 3c). A significant 200∼300-fold increase of CAM production was observed when the C. acuminata cell suspension cultures treated with sorbitol, a well-known hyperosmotic stressor (Fig. 3c).
Effects of biotic and abiotic elicitors on the pH values (a), the cell weights (b), and the production of CAM (c) of the sixth-generation C. acuminata cell suspension cultures. The cells were grown in the MS30 medium supplemented with 0.5 mg L−1 of NAA, 0.5 mg L−1 of 6-BA, 0.1 mg L−1 of 2,4-D, and 40.0 mM NH4 +/NO3 − (5:1)
Different concentrations of sorbitol were added into the C. acuminata cell suspension cultures to determine the optimum concentration of sorbitol (Supplementary Fig. S6). The mode of action of sorbitol showed a concentration-dependent pattern (Supplementary Fig. S6). The CAM production of the seventh-generation C. acuminata cell suspension cultures was increased ∼500-fold when 50 g L−1 of sorbitol was added into the C. acuminata cell suspension cultures, compared with that of the control (Supplementary Fig. S6).
Biotransformation of tryptamine and structure characterization of NAK
HPLC-DAD analyses showed that a novel product with UV absorption at 373 nm (panel II, Fig. 4b) was detected when the C. acuminata cell suspension cultures feeding with tryptamine were harvested on day 9 (panel III, Fig. 4a). The product with identical retention time (panel IV, Fig. 4a) and the same UV profile (panel III, Fig. 4b) to those of the novel product observed on day 9 was increased when the C. acuminata cell suspension cultures feeding with tryptamine were harvested on day 15. To separate and purify the target novel product, 18 L of the C. acuminata cell suspension culture were feed with tryptamine and fermented for 15 days. The product was purified as yellow powder. Its molecular formula, C11H14N2O2, was provided from the [M + Na]+ ion at m/z 229.0952 in its HRESIMS and the 13C NMR data. A 1,2-disubstituted phenyl was deduced from the 1H NMR signals at δ 7.77 (dd, J = 8.2, 1.4 Hz, 1 H), 7.25 (ddd, J = 8.5, 7.0, 1.5 Hz, 1 H), 6.75 (dd, J = 8.4, 0.8 Hz, 1 H), and 6.61 (ddd, J = 8.2, 7.0, 1.2 Hz, 1 H), which was confirmed by the 1H -1H COSY experiments (Supplementary Fig. S7). A - CH2 - CH2 - moiety was concluded from the 1H NMR signals at δ 3.55 (t, J = 6.5 Hz, 2 H) and 3.19 (t, J = 6.5 Hz, 2 H) and their cross correlations with each other in the 1H -1H COSY experiments (Supplementary Fig. S7). An acetyl group was concluded from the 1H NMR signal at δ 1.93 (s, 3 H) and the 13C NMR signals at δ 22.52 and 173.37, which was confirmed by the HMBC correlation of 1.92 (s, 3 H) / 173.37 (C) (Supplementary Fig. S7). The key HMBC correlations (Supplementary Fig. S7) of 3.55 (t, J = 6.5 Hz, 2 H, H-1′) / 173.37 (C-1′′), 201.92 (C-3′); 7.77 (dd, J = 8.2, 1.4 Hz, 1 H, H-6) / 201.92 (C-3′); and 6.61 (ddd, J = 8.2, 7.0, 1.2 Hz, 1 H, H-5) / 118.55 (C-1) concluded the structure of the product as N 1-acetylkynuramine (NAK, 2, Fig. 1b) (Asolkar et al. 2004).
Biotransformation of tryptamine in the cell suspension cultures of C. acuminata. a HPLC-DAD analyses of tryptamine (panel I), the cell suspension culture without the addition of tryptamine (panel II), and the cell suspension culture feeding with tryptamine harvested on day 9 (panel III) and on day 15 (panel IV). b The UV profiles of tryptamine (panel I) and the target product from biotransformation on day 9 (panel II) and on day 15 (panel III). The circle indicates tryptamine and the diamond indicates NAK. The red line indicates that the retention times of the target products of the cell suspension culture feeding with tryptamine harvested on day 9 and on day 15 matched very well
Discussion
Plant-derived natural products/secondary metabolites had been thought to be synthesized as a common response of plants to biotic and/or abiotic stresses and thus were exceedingly inducible by elicitors using plant cell, tissue, and organ cultures (Giri and Zaheer 2016). Elicitation treatments led to a 1.0- to 2230-fold enhancement of the secondary metabolites among the plant species studied (Giri and Zaheer 2016). However, in the case of CAM production, a few classes of elicitors were applied to the C. acuminata calli and cell suspension cultures and their CAM enhancement was limited (Supplementary Table S1). Herein, we reported for the first time that CAM production of the C. acuminata cell suspension cultures will be attenuated with subsequent successive subculturing of the C. acuminata cell suspension cultures (Supplementary Fig. S3). However, the CAM production of the attenuated C. acuminata cell suspension cultures could be restored and enhanced by biotic and/or abiotic elicitors (Fig. 3). Among them, sorbitol was proven to be the most effective elicitor that stimulates a concentration-dependent mode increase of the CAM production (Supplementary Fig. S6). A 5.0 ± 0.2 mg L−1 of CAM was reached when the sixth-generation C. acuminata cell suspension cultures were treated with 50 g L−1 of sorbitol on day 3 and 20% XAD16 was added into the cultures on day 9, compared with the ∼10 μg L−1 of CAM of the sixth-generation C. acuminata cell suspension cultures. The results suggested that, in addition to its roles as a carbon source and an osmotic regulator, sorbitol may also act as a chemical signal to directly or indirectly alter gene expression involved in CAM biosynthesis (Feng et al. 2011).
NAK, a rare natural product, was firstly isolated and identified at ∼0.26 mg L−1 from the fermentation broth of Janibacter limosus in 2004 (Asolkar et al. 2004). As an important metabolite of the kynuramine pathway (Fig. 1b), NAK was suggested to be originated from the degradation of tryptamine (Hardeland et al. 2009). NAK derivatives and analogues have shown their pharmaceutically importance in scavenging of free radicals and prevention of protein destruction as antioxidant (Ressmeyer et al. 2003) and neural nitric oxide synthase inhibitor (Entrena et al. 2005). NAK was also a versatile organic synthetic building block in the chemical synthesis of some biological active alkaloids (Khalil et al. 2016; Skyler and Heathcock 2001). In this work, a 28 mg L−1 of NAK could be obtained from the biotransformation of tryptamine in the C. acuminata cell suspension cultures, which is an alternative biological preparation of NAK. Additionally, the structure characterization of NAK supported that the ring-opening (C2–C3 double bound cleavage) of the indole moiety was catalyzed by a putative indole-2,3-dioxygenase (Fig. 1b). The results suggested that a functionally similar enzyme may be involved in the similar ring-opening (C2–C7 double bound cleavage) of the putative biosynthetic formation of the pyrroquinoline moiety of CAM (Fig. 1a) (Hutchinson et al. 1974; Sheriha and Rapoport 1976), which will be a useful clue to investigate the putative encoding genes for the biosynthesis of CAM.
References
Asolkar RN, Schröder D, Heckmann R, Lang S, Wagner-Döbler I, Laatsch H (2004) Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1. J Antibiot 57:17–23
Bhalkar BN, Patil SM, Govindwar SP (2016) Camptothecine production by mixed fermentation of two endophytic fungi from Nothapodytes nimmoniana. Fungal Biol 120:873–883 and references therein
Cai Z, Kastell A, Knorr D (2012) Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep 31:461–477
Deepthi S, Satheeshkumar K (2016) Enhanced camptothecin production induced by elicitors in the cell suspension cultures of Ophiorrhiza mungos Linn. Plant Cell Tissue Organ Cult 124:483–493 and references therein
Demain AL, Vaishnav P (2011) Natural products for cancer chemotherapy. Microb Biotechnol 4:687–699
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 68:350–356
Entrena A, Camacho ME, Carrión MD, López-Cara LC, Velasco G, León J, Escames G, Acunña-Castroviejo D, Tapias V, Gallo MA (2005) Kynurenamines as neural nitric oxide synthase inhibitors. J Med Chem 48:8174–8181
Feng X, Zhao P, Hao J, Hu J, Kang D, Wang H (2011) Effects of sorbitol on expression of genes involved in regeneration of upland rice (Oryza sativa L.). Plant Cell Tissue Organ Cult 106:455–463
Fulzele DP, Satdive RK, Pol BB (2001) Growth and production of camptothecin by cell suspension cultures of Nothapodytes foetida. Planta Med 67:150–152
Ge X, Wu J (2005) Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci 168:487–491
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult 126:1–18
Grout BWW (2007) Cryopreservation of plant cell suspensions. In: Day JG, Stacey GN (eds) Methods in molecular biology, Cryopreservation and Freeze-Drying Protocols, vol 368, 2nd edn. Humana Press Inc., Totowa, NJ, pp 153–161
Hardeland R, Tan DX, Reiter RJ (2009) Kynuramine, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 47:109–126
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878
Hutchinson CR, Heckendorf AH, Daddona PE, Hagaman E, Wenkert E (1974) Biosynthesis of camptothecin. I. Definition of the overall pathway assisted by carbon-13 nuclear magnetic resonance analysis. J Am Chem Soc 96:5609–5611
Khalil IM, Barker D, Copp BR (2016) Bioinspired syntheses of the pyridoacridine marine alkaloids demethyldeoxyamphimedine, deoxyamphimedine, and amphimedine. J Org Chem 81:282–289
Kitajima M, Fischer U, Nakamura M, Ohsawa M, Ueno M, Takayama H, Unger M, Stockigt J, Aimi N (1998) Anthraquinones from Ophiorrhiza pumila tissue and cell cultures. Phytochemistry 48:107–111
Liu F, Peng K, Peng Z, Xia S, Xiao L (2010) Establishment of the cell suspension culture system of Camptotheca acuminata. J Hunan Agr Univ 36:528–530
López-Meyer M, Nesler CL, McKnight TD (1994) Sites of accumulation of the antitumor alkaloid camptothecin in Camptotheca acuminata. Planta Med 60:558–560
Lorence A, Nessler CL (2004) Camptothecin, over four decades of surprising findings. Phytochemistry 65:2735–2749
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pu X, Qu X, Chen F, Bao J, Zhang G, Luo Y (2013) Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: isolation, identification, and fermentation conditions optimization for camptothecin production. Appl Microbiol Biotechnol 97:9365–9375 and references therein
Pu X, Chen F, Yang Y, Qu X, Zhang G, Luo Y (2015) Isolation and characterization of Paenibacillus polymyxa LY214, a camptothecin-producing endophytic bacterium from Camptotheca acuminata. J Ind Microbiol Biotechnol 42:1197–1202 and references therein
Qin H, Chamlong P (2007) Camptotheca acuminata Decaisne. Flora of China 13:300–301
Qu X, Pu X, Chen F, Yang Y, Yang L, Zhang G, Luo Y (2015) Molecular cloning, heterologous expression, and functional characterization of an NADPH-cytochrome P450 reductase gene from Camptotheca acuminata, a camptothecin-producing plant. PLoS One 10:e0135397
Ressmeyer AR, Mayo JC, Zelosko V, Sáinz RM, Tan DX, Burkhard P, Antolín I, Zsizsik BK, Reiter RJ, Hardeland R (2003) Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep 8:205–213
Sakato K, Tanaka H, Mukai N, Misawa M (1974) Isolation and identification of camptothecin from cells of Camptotheca acuminata suspension cultures. Agr Biol Chem 38:217–218
Shaanker RU, Ramesha BT, Ravikanth G, Gunaga R, Vasudeva R, Ganeshaiah KN (2008) Chemical profiling of Nothapodytes nimmoniana for camptothecin, an important anticancer alkaloid: towards the development of a sustainable production system. In: Ramawat KG, Mérillon JM (eds) Bioactive molecules and medicinal plants. Springer-Verlag, Berlin and Heidelberg, pp 197–213
Sheriha GM, Rapoport H (1976) Biosynthesis of Camptotheca acuminata alkaloids. Phytochemistry 15:505–508
Sirikantaramas S, Asano T, Sudo H, Yamazaki M, Saito K (2007) Camptothecin: therapeutic potential and biotechnology. Curr Pharm Biotechnol 8:196–202
Skyler D, Heathcock CH (2001) A simple biomimetic synthesis of styelsamine B. Org Lett 3:4323–4324
Thomas CJ, Rahier NJ, Hecht SM (2004) Camptothecin: current perspectives. Bioorg Med Chem 12:1585–1604
Vasanthakumari MM, Jadhav SS, Sachin N, Vinod G, Shweta S, Manjunatha BL, Kumara PM, Ravikanth G, Nataraja KN, Shaanker RU (2015) Restoration of camptothecine production in attenuated endophytic fungus on re-inoculation into host plant and treatment with DNA methyltransferase inhibitor. World J Microbiol Biotechnol 31:1629–1639 and references therein
Venugopalan A, Srivastava S (2015) Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol Adv 33:873–887 and references therein
Venugopalan A, Potunuru UR, Dixit M, Srivastava S (2016) Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresource Technol 206:104–111 and references therein
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88:3887–3890
Wang JW, Wu JY (2013) Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Adv Biochem Engin/Biotechnol 2013:183
Yin Z, Chen L, Zhao B, Zhu Y, Wang Q (2012) Cryopreservation of embryogenic cell suspensions by encapsulation-vitrification and encapsulation-dehydation. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Methods in molecular biology, Plant Cell Culture Protocols, vol 877. Springer Science + Business Media, LLC, pp 81–93
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported in part by the KSCX2-EW-Q-6 and ZHTS-003 projects from the Chinese Academy of Sciences, the Applied and Basic Research Program of Sichuan Province (2015JY0058), and the 21172216 project from the National Natural Science Foundation of China.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Yun Yang and Xiang Pu authors contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 1159 kb).
Rights and permissions
About this article
Cite this article
Yang, Y., Pu, X., Qu, X. et al. Enhanced production of camptothecin and biological preparation of N 1-acetylkynuramine in Camptotheca acuminata cell suspension cultures. Appl Microbiol Biotechnol 101, 4053–4062 (2017). https://doi.org/10.1007/s00253-017-8153-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8153-4