Abstract
Biotransformation is a green and useful tool for sustainable and selective chemical synthesis. However, it often suffers from the toxicity and inhibition from organic substrates or products. Here, we established a hollow fiber membrane bioreactor (HFMB)-based aqueous/organic biphasic system, for the first time, to enhance the productivity of a cascade biotransformation with strong substrate toxicity and inhibition. The enantioselective trans-dihydroxylation of styrene to (S)-1-phenyl-1,2-ethanediol, catalyzed by Escherichia coli (SSP1) coexpressing styrene monooxygenase and an epoxide hydrolase, was performed in HFMB with organic solvent in the shell side and aqueous cell suspension in the lumen side. Various organic solvents were investigated, and n-hexadecane was found as the best for the HFMB-based biphasic system. Comparing to other reported biphasic systems assisted by HFMB, our system not only shield much of the substrate toxicity but also deflate the product recovery burden in downstream processing as the majority of styrene stayed in organic phase while the diol product mostly remained in the aqueous phase. The established HFMB-based biphasic system enhanced the production titer to 143 mM, being 16-fold higher than the aqueous system and 1.6-fold higher than the traditional dispersive partitioning biphase system. Furthermore, the combination of biphasic system with HFMB prevents the foaming and emulsification, thus reducing the burden in downstream purification. HFMB-based biphasic system could serve as a suitable platform for enhancing the productivity of single-step or cascade biotransformation with toxic substrates to produce useful and valuable chemicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biotransformation is a useful and green tool to produce chemicals, especially enantiopure compounds that are useful and valuable intermediates for pharmaceutical manufacture (Breuer et al. 2004; Connors et al. 1997; Gao et al. 2014; Li et al. 2013; May and Padgette 1983; Raghavan and Rathore 2009; Reetz et al. 1997; Yang et al. 2014). Despite great achievements in this field, many biotransformations still suffer from the toxic and inhibitory effects of the organic substrates and products, exhibiting unsatisfied productivities. To tackle the inhibition and toxicity problems, solid-liquid biphasic system using solid particle (e.g., resin) for adsorption and mild delivery of organic substrate and product was developed for biotransformations (Lau et al. 2002; Mei et al. 2009; Mirata et al. 2009; Park et al. 2007; Roddick and Britz 1997; Wang et al. 2011; Xue et al. 2010; Zhao et al. 2006). However, this approach often suffers from low sorption capacities, specificity, and diffusion coefficient (Praveen and Loh 2012, 2013b). Another approach to prevent the toxicity and inhibition is to use a traditional dispersive partitioning biphase (TDPB) system, where an organic solvent or ionic liquid is used as the second liquid phase to harbor the majority of the toxic substrate and product (Cornmell et al. 2008; Gao et al. 2014; Gong and Xu 2005; He et al. 2006; Kansal and Banerjee 2009; Li et al. 2011). However, the second liquid phase in TDPB system could potentially impair enzyme or cell membrane integrity (Gao et al. 2014; Pfruender et al. 2004). In addition, emulsion-promoting biosurfactant produced by the microbial cells could cause foaming and emulsification and thus incur extra burden in solvent reuse and product purification (Brandenbusch et al. 2010; Collins et al. 2015; Praveen and Loh 2012, 2013a, b, 2014). To solve these problems, membrane-based biphasic system was applied, in which the organic phase cannot directly contact biocatalyst. For example, bioconversion of the poorly soluble toluene to toluene cis-glycol employed membrane facility for solvent extraction of the product, with the direct feeding of the substrate to aqueous phase under a special “feedback control” design (Hack et al. 2000). A continuous system with the feeding of substrate to the aqueous phase for the bioconversion of toluene to 3-methylcatechol was reported with solvent extraction of the product by using membrane devices (Hüsken et al. 2002). Interestingly, biofilm was also used for an aqueous/organic biphasic system in a membrane aerated bioreactor to enhance the productivity of epoxidation of styrene (Halan et al. 2010).
Recently, hollow fiber membrane bioreactor (HFMB)-based biphasic system was developed, where the aqueous phase and the organic phase were thoroughly separated by membranes and aqueous-organic phase interface was immobilized in the membrane pores (Molinari et al. 1997; Praveen and Loh 2012, 2013a; b, 2014). Using this system, the organic phase in the shell side could extract the product from the aqueous phase containing microbial cells in the lumen side without direct contacting cells (Molinari et al. 1997; Praveen and Loh 2012, 2013a, b, 2014). HFMB-based biphasic system could provide with independent flow rate, compact design, well-controlled modular parameters, and high specific interfacial area (Praveen and Loh 2012, 2013a), where the non-dispersive operation could decrease the turbulent flow and hence reduce foaming and emulsification (Praveen and Loh 2013a, b, 2014). Furthermore, compact design of HFMB-based biphasic system enables easier modeling, optimization, scaling up, and continuous operation, where the extra mass transfer resistance owing to the membrane could be decreased by expanding the interfacial areas (Praveen and Loh 2012). Thus, HFMB-based biphasic system could provide with significant advantages over TDPB system and other biphasic system using simple membrane. So far, several applications were reported of using HFMB-based biphasic systems. For instance, it was used for phenol biodegradation in waste water pretreatment (Praveen and Loh 2012, 2013a, b, 2014). Bioconversion of a soluble and less toxic alcohol to an aldehyde was performed in a HFMB-assisted extractive system, where the reaction was conducted at the aqueous phase in the bioreactor by feeding the alcohol substrate and the product was extracted into organic phase in HFMB (Molinari et al. 1997).
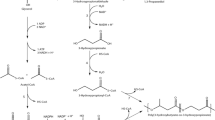
We are interested in using HFMB-based biphasic system for cascade biotransformations. Cascade biotransformations enable multi-step conversions in one pot without the labor- and time-consuming, yield-decreasing, waste-producing, and costly purification of the intermediates (Breuer et al. 2004; Buchholz et al. 2012; Chang et al. 2003a; Ladkau et al. 2014; Muschiol et al. 2015; Panke et al. 2004; Wu et al. 2014; Xu et al. 2009; Xu et al. 2011; Zhang et al. 2011, 2013). It could also provide with non-toxicity, mild reaction conditions, and high chemo- and regio-selectivities (Breuer et al. 2004; Buchholz et al. 2012; Panke et al. 2004; Wu et al. 2014; Zhang et al. 2011), thus being a green and useful tool for chemical synthesis (Liu and Li 2013; Liu et al. 2014; Wu et al. 2016a, b; Zhou et al. 2016). Similar to the simple-step biotransformations, many cascade biotransformations are also hampered by substrate or product toxicity and inhibition. For instance, dihydroxylation of styrene with recombinant cells of Escherichia coli (SSP1) coexpressing styrene monooxygenase (SMO) and Sphingomonas epoxide hydrolase (SpEH) to produce the useful and valuable (S)-vicinal diol suffers from the toxicity of styrene. Here, we report the first HFMB-based biphasic system for cascade biotransformation to enhance the productivity. Different from other reported systems, the majority of the substrate was presented in organic solvent in the shell side to avoid toxicity, the majority of the product remained in aqueous phase of the lumen side for easy product recovery, and the biotransformation was mainly performed in HFMB (Fig. 1).
Materials and methods
Chemicals, media, and strain
Styrene (≥99%), kanamycin disulfate (>98%), and isopropyl β-d-1-thiogalactopyranoside (IPTG, ≥99%) were purchased from Sigma, St. Louis, MO, USA. Tryptone and yeast extract were purchased from Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA. Unless stated otherwise, all the inorganic salts for the medium preparations were purchased from Sigma, St. Louis, MO, USA. 1-Octanol (>99%), 2-undecanone (99%), ethyl acetate (99.8%), cyclohexane (99.5%), and n-hexadecane(99%) were purchased from Sigma. n-Octane (≥99%) was purchased from Merck, Darmstadt, Germany. Luria-Bertani (LB) medium was prepared according to the reported recipe (Peschel et al. 1999). Terrific broth (TB) medium (Atsumi et al. 2008) and M9 medium (Karabika et al. 2009) were prepared according to the references. Potassium phosphate buffer (KP buffer; pH 8.0; 200 mM) was prepared to consist of 32.7 g/L of K2HPO4 and 1.63 g/L of KH2PO4. Recombinant E. coli (SSP1) coexpressing SMO and SpEH was constructed previously with pRSFduet plasmid and T7 express competent E. coli cells (Wu et al. 2014).
Analytical methods
The concentrations of styrene, (S)-1-phenyl-1,2-ethanediol, and styrene oxide in aqueous samples were analyzed by using a Waters HPLC on an Agilent Poroshell 120 EC-C18 column (150 mm × 4.6 mm; 2.7 μm). UV detection 210 nm; flow rate 0.4 mL/min; eluent acetonitrile/water (60:40, v/v); and retention time 4.8 min for (S)-1-phenyl-1,2-ethanediol, 14.8 min for styrene, and 8.2 min for styrene oxide.
The concentrations of styrene, (S)-1-phenyl-1,2-ethanediol, and styrene oxide in organic phase were analyzed by using a Perkin Elmer Clarus 600GC equipped with a flame ionization detector on an Elite-5 capillary column (30 m × 320 μm × 0.25 μm). Helium was used as the carrier gas at a flow rate of 1.0 mL/min, and the detector temperature was 250 °C. The column temperature was programmed from 120 to 300 °C at 30 °C/min and hold at 300 °C for 2 min. The following are the retention times: 3.08 min for styrene, 3.72 min for styrene oxide, and 4.71 min for (S)-1-phenyl-1,2-ethanediol.
Cell growth and specific trans-dihydroxylation activity of E. coli (SSP1)
E. coli (SSP1) cells (preserved at −80 °C) were inoculated into 20 mL LB medium containing 50 μg/mL kanamycin. Incubation was conducted at 37 °C and 250 rpm for 12 h in 125-mL tribaffled flask (SCHOTT, Elmsford, NY, USA). Two milliliter cell culture was then introduced to 50 mL TB medium containing 50 μg/mL kanamycin in a 250-mL tribaffled flask (SCHOTT, Elmsford, NY, USA). Incubation was carried out at 37 °C and 250 rpm for 2 h to reach an OD600 of 0.5–0.8, followed by adding IPTG to a final concentration of 0.25 mM for induction. The mixture was then incubated at 25 °C. Samples at different time points were taken for the analysis of the cell density and the specific activity. The cell growth experiment was continued for 16 h to establish the time courses of cell density and specific activity. For biotransformation with resting cells, E. coli cells were harvested at late exponential phase at 10 h and used as the fresh biocatalyst.
The specific activity for trans-dihydroxylation of styrene was tested in 10 mL KP buffer (200 mM, pH 8.0) containing 1.0 g cell dry weight (CDW)/L, 5 mM styrene, and glucose (2%, w/v) at 30 °C and 250 rpm for 15 min. Product formed was analyzed by HPLC, and the analytical samples were prepared as follows: 0.5 mL reaction solution was mixed with 0.5 mL ethanol containing 2 mM acetophenone as internal standard. The mixture was centrifuged at 13,000×g for 5 min, and the supernatant was separated. The specific activity was calculated in unit per gram CDW. One unit is defined as 1 μmol product formed per minute.
Toxicity of the substrate and product on E. coli (SSP1) cells
Freshly prepared E. coli (SSP1) cells were re-suspended to a cell density of 10 g CDW/L in 30 mL KP buffer (200 mM; pH 8.0). The cell suspension was divided equally into three 125-mL flasks, and each flask (SCHOTT, Elmsford, NY, USA) contains 10 mL cell suspension. Styrene and (S)-1-phenyl-1,2-ethanediol were added into two different flasks to a final concentration of 50 mM, respectively. The third flask served as the control without the addition of substrate or product. The mixtures in the three flasks were shaken at 30 °C and 250 rpm for 10 h, followed by washing with KP buffer (200 mM; pH 8.0) to remove styrene and (S)-1-phenyl-1,2-ethanediol.
The cells were then examined for specific activity on the trans-dihydroxylation of styrene by using the procedures described above. The residual activity was defined as the ratio of the specific activity of the pretreated cells to that of the control cells.
The viability of the pretreated cells was checked via inoculation of diluted cell suspension onto the LB agar plate at 37 °C for 12 h, followed by counting the number of the colonies. The cell viability was defined as the ratio of the colony number for the cells pretreated to the colony number for the control cells.
Growth of E. coli (SSP1) on different organic solvents
E. coli (SSP1) cells were inoculated in seven 250-mL tribaffled flasks (SCHOTT, Elmsford, NY, USA), each containing M9 medium without glucose (20 mL), respectively. 1-Octanol, 2-undecanone, ethyl acetate, cyclohexane, n-octane, and n-hexadecane were added to the six tribaffled flasks, respectively, with addition of each solvent at 0 h (0.02%, v/v), 6 h (0.03%, v/v), and 12 h (0.05%, v/v). The seventh tribaffled flask without adding any organic solvent served as the control. The cells were grown in tribaffled flask at 30 °C and 250 rpm for 24 h. OD600 of E. coli (SSP1) at 0, 12, and 24 h was recorded.
Biocompatibility of different organic solvents with E. coli (SSP1)
E. coli (SSP1) cells were inoculated in seven 250-mL tribaffled flasks (SCHOTT, Elmsford, NY, USA) with each one containing LB medium (20 mL). The cells in the LB medium were grown in tribaffled flask at 30 °C and 250 rpm. When reaching the exponential phase (OD600 1.0), six tribaffled flasks were added with 1-octanol, 2-undecanone, ethyl acetate, cyclohexane, n-octane, and n-hexadecane, respectively, to reach a phase ratio of 10:1 (aq./organic, v/v). The seventh tribaffled flask without adding any organic solvent served as the control. The OD600 for E. coli (SSP1) after 1 h cell growth was determined.
Determination of partitioning coefficient of styrene, styrene oxide and (S)-1-phenyl-1,2-ethanediol in organic/aqueous biphasic system
Styrene (11.5 μL), styrene oxide (11.4 μL), and (S)-1-phenyl-1,2-ethanediol (13.8 μg) were added to KP buffer (1 mL, 200 mM, pH 8.0), respectively. One milliliter of 1-octanol, 2-undecanone, ethyl acetate, cyclohexane, n-octane, or n-hexadecane was added separately to each of the KP buffer (1 mL, 200 mM, pH 8.0) containing styrene, styrene oxide, or (S)-1-phenyl-1,2-ethanediol to form different biphasic systems (1-octanol/aq., 2-undecanone/aq., ethyl acetate/aq., cyclohexane/aq., n-octane/aq., or n-hexadecane/aq.), respectively. The mixture was then shaken at 30 °C and 250 rpm for 3 h, and the concentrations of each component in the aqueous phase and the organic phase were analyzed by HPLC and GC, respectively. For HPLC analysis, 0.5 mL KP buffer was combined with 0.5 mL ethanol containing 2 mM acetophenone as internal standard, the mixture was centrifuged at 13,000×g for 1 min, and the supernatant was separated for the analysis. For GC analysis, 50 μL organic solvent was mixed with 950 μL dichloromethane. Partition coefficient was calculated as the ratio of the solute concentration in organic phase to the solute concentration in aqueous phase.
Trans-dihydroxylation of styrene with E. coli (SSP1) in monophase
Freshly prepared E. coli (SSP1) cells were suspended to a density of 10 g CDW/L in KP buffer (10 mL, 200 mM; pH 8) containing 2% (w/v) glucose. Styrene in different amount was added into 10 mL cell suspension to form mixtures with different concentrations of 5, 10, 20, and 50 mM, respectively. The trans-dihydroxylation was carried out at 30 °C and 250 rpm. During the biotransformation, samples were taken at different time points for monitoring the reaction. The product concentration was analyzed by HPLC with the sample prepared as given above.
Trans-dihydroxylation of styrene with E. coli (SSP1) in TDPB system
Freshly prepared cell pellets of E. coli (SSP1) were suspended to a density of 10 g CDW/L in 10 mL KP buffer (200 mM; pH 8) containing 2% (w/v) glucose. Then, 10 mL organic solvent (1-octanol, 2-undecanone, ethyl acetate, cyclohexane, n-octane, or n-hexadecane) containing 1 M styrene was added into 10 mL cell suspension to form a phase ratio of 1:1 (v/v). The trans-dihydroxylation was carried out at 30 °C and 250 rpm. During the biotransformation, samples were taken at different time points for analyzing the substrate and product concentrations by HPLC and GC. Analytical samples were prepared as previously described.
Trans-dihydroxylation of styrene with E. coli (SSP1) in HFMB-based biphasic systems
Polypropylene was used to fabricate HFMB (Fig. 1) with the inner fiber diameter 280 μm, average pore size 0.1 μm, fiber thickness 30 μm, and fiber length 50 cm (Praveen and Loh 2012, 2013a, 2014). The interfacial area of the organic phase and aqueous phase were manipulated by adjusting fiber numbers within the shell of the HFMB. Before the biotransformation, deionized (DI) water was pumped through the lumen side within the fibers in HFMB at flow rate of 5 mL/min for 12–18 h for wetting the membrane by using the peristaltic pump (Masterflex). After wetting, DI water was drained out of the lumen side and the organic solvent (i.e., cosolvent of the biphasic system) was pumped through the shell side of the HFMB at flow rate of 5 mL/min for 12–18 h by using the peristaltic pump (Masterflex with PTFE tubing pump head). HFMB was ready for use after draining the organic solvent. For conducting the trans-dihydroxylation of styrene, HFMB containing the appropriate fiber numbers within the shell was used to give the specific interfacial area (i.e., the ratio of interfacial area over cell density) of 0.3 and 0.15 m2/g CDW, respectively. Fifty milliliters cell suspension (5, 10, or 15 g CDW/L) in KP buffer (pH 8.0, 200 mM) and 50 mL organic solvent containing substrate (0.1–1 M) were pumped through the lumen side and shell side of the hollow fiber membrane bioreactor by the corresponding peristaltic pumps, respectively. The flow rates of the aqueous phase in lumen side and organic phase in shell side were 15 mL/min. During the trans-dihydroxylation of styrene, samples at both organic and aqueous phases were taken with regular time interval for monitoring the reaction by measuring the product concentration. The concentration of (S)-1-phenyl-1,2-ethanediol in the aqueous phase was analyzed by HPLC, and concentration of the substrate in the organic phase was analyzed by GC. The analytical samples were prepared as described previously.
Nucleotide sequence accession number
The DNA coding sequences of SMO and SpEH are available in GenBank database with the accession numbers of AF031161 and KX146840, respectively.
Results
Toxicity and inhibition of substrate and product on E. coli (SSP1)-catalyzed trans-dihydroxylation of styrene
E. coli (SSP1) (Wu et al. 2014) containing SMO and SpEH catalyzed trans-dihydroxylation of styrene to give (S)-1-phenyl-1,2-ethanediol in 98% ee. The cell growth and specific activity of E. coli (SSP1) are given in Fig. 2a. The specific trans-dihydroxylation activity reached 71.8–88.0 U/g CDW between the late exponential and the early stationary phases at 8–12 h. E. coli (SSP1) cells were harvested, and the fresh resting cells were used as robust whole cell biocatalyst for the oxidative cascade biotransformation. The inhibition of the substrate was first examined by using different substrate concentrations for the biotransformation. As shown in Fig. 2b, increase of styrene concentration from 5 to 10 mM resulted in the increase of the product titer from 5 to 8.75 mM. However, further increase of the substrate concentration to 50 mM gave a drastic reduction in production titer to 0.79 mM.
a Time course of Escherichia coli (SSP1) cell growth and specific activity for trans-dihydroxylation of styrene by E. coli (SSP1). Arrow indicates induction by adding IPTG to the concentration of 0.25 mM. b Product concentration at 24 h for the trans-dihydroxylation of styrene at different concentrations with E. coli (SSP1) (10 g CDW/L) in KP buffer (200 mM; pH 8.0) [glucose (1%, w/v) was added at 0 and 6 h, respectively] at 30 °C and 250 rpm. c Viability of E. coli (SSP1) cells after pretreatment with the substrate (50 mM) or diol product (50 mM) for 10 h. d Residual activity of E. coli (SSP1) cells after pretreatment with the substrate (50 mM) or diol product (50 mM) for 10 h. Data are the mean values with standard deviations of three replicates
The toxicity was then studied by adding styrene (50 mM) (Park et al. 2006) and (S)-1-phenyl-1,2-ethanediol (50 mM), respectively, to the fresh cell suspension (10 g CDW/L) of E. coli (SSP1) in KP buffer (200 mM, pH 8.0) and incubating at 30 °C and 250 rpm for 10 h. The cell viability was examined. As shown in Fig. 2c, only 5.8% of the cells were viable after the pretreatment with styrene, while the survival ratio for cells pretreated with (S)-1-phenyl-1,2-ethanediol was still up to 43% (Fig. 2c). Similarly, the residual activity dropped to 0.5 and 66% after the cells were pretreated with styrene and the diol product, respectively (Fig. 2d). These results demonstrated clearly that styrene was very toxic to the cells and enzyme, and (S)-1-phenyl-1,2-ethanediol was much less toxic than styrene.
Selection of suitable organic solvent for the aq./org. biphasic system for the trans-dihydroxylation of styrene
For establishing the aq./org. biphasic system, six different organic solvents (i.e., 1-octanol, 2-undecanone, ethyl acetate, cyclohexane, n-octane, and n-hexadecane) were examined for cell growth, biocompatibility, and partition coefficient (Praveen and Loh 2012, 2013a, 2014).
To examine the possibility of growing the cells on the six organic solvents, E. coli (SSP1) was inoculated in M9 medium without glucose but with each of the organic solvents as potential carbon source. OD600 after growth for 12 and 24 h was recorded for assessing the biodegradability. E. coli (SSP1) showed OD600 values of 0.09–0.24 at 12 h, which did not exceed the OD600 value of 0.24 for the control containing no organic solvent. Thus, E. coli (SSP1) cell could not grow on any of the six solvents.
To estimate biocompatibility, E. coli (SSP1) was first cultivated in LB medium, followed by adding the six organic solvents to the cell culture broth, respectively. OD600 of E. coli (SSP1) was determined as 0.68, 0.63, 1.03, 1.04, 1.97, and 2.14 for the case of 1-octanol, ethyl acetate, 2-undecanone, cyclohexane, n-octane, and n-hexadecane, respectively. The control without adding any solvent gave the highest OD600 value of 2.27. Therefore, n-hexadecane presented the highest biocompatibility among the six solvents.
Partition coefficients of styrene for 1-octanol, ethyl acetate, 2-undecanone, cyclohexane, n-octane, and n-hexadecane were determined to be 173–1731 (Table 1). These high values demonstrate that >99% of styrene was in the organic phase. Similarly, partition coefficients for styrene epoxide ranged from 58 to 289. Although these values are lower than those for styrene, the majority (>98%) of the epoxide still stayed in organic phase. On the other hand, partition coefficients of (S)-1-phenyl-1,2-ethanediol were very low (<0.01) for cyclohexane, n-octane, and n-hexadecane, suggesting >99% of the product in the aqueous phase, thus facilitating the product separation. As also shown in Fig. 2c, d, the product was much less toxic and inhibitory than the substrate. Thus, the presence of product in the aqueous phase is less troublesome than the substrate in aqueous solution.
Trans-dihydroxylation of styrene with E. coli (SSP1) in TDPB systems using various organic solvents
The trans-dihydroxylation of styrene (50 mM) to produce (S)-1-phenyl-1,2-ethanediol was conducted with E. coli (SSP1) in different TDPB systems containing each of the six solvents, and the product titer (45 mM) from aq./n-hexadecane was the highest, being 18-fold higher than the product titer (2.5 mM) of the control in aqueous phase. TDPB systems of aq./1-octanol and aq./ethyl acetate exhibited high partitioning coefficients for styrene (Table 1) but gave very low product titers of 2.39 and 2.07 mM, respectively, due to the low Log P values. n-Octane and 2-undecanone demonstrated similar partition coefficients, but aq./n-octane gave higher production titer 39.9 mM than aq./2-undecanone (19.8 mM). Aq./cyclohexane system gave 17.3 mM product. For all the TDPB system, further increase of the substrate concentration did not benefit the reaction proportionally (data not shown). Based on these results, n-hexadecane was chosen as the organic solvent to establish an HFMB-based aq./org. biphasic system for the targeted cascade biotransformation.
Trans-dihydroxylation of styrene with E. coli (SSP1) in HFMB-based biphasic systems using aq./cyclohexane and aq./n-hexadecane
To establish the HFMB-based biphasic system, deionized (DI) water and pure n-hexadecane were used for wetting the membrane prior to the biotransformation, which was to create more interfacial area within the highly porous membrane between the aqueous and organic phases for the liquid-liquid mass transportation. When wetting procedure was done, the aqueous phase of cell suspension and the organic phase of n-hexadecane containing the substrate styrene were pumped through the lumen side and shell side of the HFMB, respectively (Fig. 1). As shown in Fig. 3, the trans-dihydroxylation with E. coli (SSP1) in the HFMB-based aq./n-hexadecane biphasic system gave product in 23.5, 44.3, 75.5, and 129 mM after 24 h biotransformation with styrene concentrations of 0.1, 0.2, 0.5, and 1.0 M, respectively. The increase of product titer with substrate concentration were also observed for HFMB-based aq./cyclohexane system, where product titers of 20.2, 35.4, 65.7, and 88.4 mM were achieved with 0.1, 0.2, 0.5, and 1.0 M substrate, respectively. To evaluate the performance of a less good organic phase in HFMB system, the same reactions were carried out in aq./cyclohexane. As shown in Fig. 3, aq./n-hexadecane system gave clearly better results than aq./cyclohexane system in the presence of HFMB.
Product concentration at 24 h for trans-dihydroxylation of styrene with Escherichia coli (SSP1) (10 g CDW/L) at different substrate concentrations in HFMB-based biphasic system (specific interfacial area was 0.3 m2/g CDW) consisting of KP buffer (200 mM; pH 8.0) and n-hexadecane (white) or cyclohexane (gray) (phase ratio 1:1, v/v) at 30 °C. All concentrations and cell density were based on the volume of KP buffer. Data are the mean values with standard deviations of three replicates
Comparison of trans-dihydroxylation of styrene with E. coli (SSP1) in TDPB- and HFMB-based aq./n-hexadecane biphasic systems
Figure 4 compares the HFMB-based biphasic system for trans-dihydroxylation of styrene to TDPB system for the same reaction. The HFMB-based reaction gave 1.6-fold higher product titer (143 mM) than the titer (89.1 mM) achieved in TDPB system. As shown in Fig. 4, the TDPB system gave the product at slightly higher rate than HFMB system at the beginning; at 5–6 h, the product concentration reached ca. 80 mM for both systems; however, the reaction barely proceeded any more after 6 h for TDPB system, whereas the HFMB system maintained a comparatively high reaction rate in a long reaction period, giving 143 mM product at 35 h (Fig. 4). This signified that the whole cell biocatalyst was better protected from the toxicity and inhibition of styrene in HFMB biphasic system.
Time courses for trans-dihydroxylation of styrene (1 M) with Escherichia coli (SSP1) (10 g CDW/L) in TDPB and HFMB (specific interfacial area was 0.3 m2/g CDW)-based biphasic system containing KP buffer (200 mM; pH 8.0) and n-hexadecane (1:1, v/v) at 30 °C, respectively. All concentrations and cell density were based on the volume of KP buffer. Data are the mean values with standard deviations of three replicates
Trans-dihydroxylation of styrene with E. coli (SSP1) in HFMB-based aq./n-hexadecane system with different interfacial areas and cell densities
In principle, the mass transport barrier could be overcome by enlarging interfacial area. Thus, different interfacial areas were examined for the HFMB-based biotransformation. As shown in Fig. 5a, the final product concentration was only 87.8 mM if the apparent interfacial area was 0.15 m2/g CDW, while the final production titer was 144 mM when 0.3 m2/g CDW apparent interfacial area was used. To bring down the mass transportation barrier, membrane with higher porosity was desired, and this explained why a sufficient wetting procedure on both the lumen and the shell sides was required. Besides porosity which influenced the interfacial area, cell density also played a role in the trans-dihydroxylation of styrene with E. coli (SSP1). As shown in Fig. 5b, the product titer reached 155 mM, when using the cell density of 15 g CDW/L. This was almost 1.9-fold higher than the concentration (83.6 mM) achieved with 5 g CDW/L cells. There is no big difference of the product concentration and reaction rate when 10 or 15 g CDW/L was applied, indicating inefficient oxygenations at much higher cell density.
Time course for trans-dihydroxylation of styrene (1 M) with Escherichia coli (SSP1) in HFMB-based KP buffer (200 mM; pH 8.0)/n-hexadecane system (1:1, v/v) at 30 °C. a Using different specific interfacial areas (cell density was 10 g CDW/L). b Using different cell densities (specific interfacial area was 0.3 m2/g CDW). All concentrations and cell densities were based on the volume of KP buffer. Data are the mean values with standard deviations of three replicates
Discussion
Whole-cell biocatalysis represents a practical way of producing fine chemicals, where the enzymes inside the cells can show high stability and activity (Burton 2003; Duetz et al. 2001; Li et al. 2002; Pfruender et al. 2004; Schrewe et al. 2013; Urlacher and Eiben 2006; Urlacher and Girhard 2012; Wojaczynska and Wojaczynski 2010). For the trans-dihydroxylation of styrene to (S)-1-phenyl-1,2-ethanediol with E. coli (SSP1) cells expressing the SMO and SpEH (Chang et al. 2003b, Jia et al. 2008; Liu et al. 2006; Wu et al. 2013), the relaying of the cascade reaction between the SMO and the SpEH is efficient in the intracellular microenvironment (Fig. 1). During this cascade biotransformation of styrene, SMO-catalyzed epoxidation was the rate-limiting step, and therefore, the protection for the enzyme and electron transferring mechanism by using whole cell can benefit the overall reaction of trans-dihydroxylation to produce (S)-1-phenyl-1,2-ethanediol, a useful pharmaceutical precursor for (R)-fluoxetine (Pandey et al. 2002), chiral ligand of phosphoramidite (Gavrilov et al. 2011) and a useful auxiliary for glycosylation (Kim et al. 2005).
However, whole-cell-based trans-dihydroxylation of styrene to (S)-vicinal diol (Fig. 1) was encountered with strong substrate toxicity and inhibition, while the product appeared to be much less toxic. This can be obviously seen in Fig. 2b–d, where high substrate concentration dragged down the product titer and significantly deteriorated the viability and activity of the cells. Particularly, the sharp dropping of the product titer was observed in Fig. 2b with substrate more than 10 mM, and this could be caused by the deterioration of the membrane integrity associated with the substrate toxicity, which disrupted the electron transferring system and deactivated the enzyme. Figure 2c, d also suggested the reduction of the substrate toxicity and inhibition as the main task for enhancing the productivity in such biotransformation.
To better protect the whole cell biocatalyst from the substrate toxicity, TDPB-based aq./org. biphasic system was established by adding the organic solvent with substrate laden into the cell suspension to form biphasic system and start the reaction. Although the TDPB system with organic solvent as cosolvent had effectively enhanced the productivity of many biotransformations, this TDPB system has inherent problems such as the limited capacity for preventing the substrate toxicity, cell membrane integrity deterioration owing to the hazardous effects from organic solvent (Gao et al. 2014; Pfruender et al. 2004), and difficulties in the solvent reuse and the product purification caused by emulsification and foaming (Brandenbusch et al. 2010; Collins et al. 2015; Praveen and Loh 2012, 2013a, b, 2014). To solve the problems associated with the TDPB system, an HFMB-based aq./org. biphasic system is recommended, for the first time, to a cascade biotransformation. Here, in this work, biphasic systems with different organic solvents were examined in HFMB for the cascade biotransformation. For establishing the HFMB-based biphasic system, the main criterions to select the organic solvent are non-biodegradability, biocompatibility, partition coefficient, and Log P value (Faber 1997; Lye and Woodley 2000). Biodegradability shows the capability of the microbial cells in metabolizing the organic cosolvent, which could complicate and even compete with biotransformation. Therefore, the organic solvent should be recalcitrant to the biodegradation. Biocompatibility demonstrates the adaptability of the cells to the organic solvent since cells could be fragile, vulnerable, or deactivated in solvent with lower biocompatibility. Higher biocompatibility was desired for choosing an organic solvent for aq./org. biphasic system, where the whole cell biocatalyst could be protected for better enzymatic performance. Partition coefficient ([solute conc. in org.]/[solute conc. in aq.]) reflects the capability of the solvent in dissolving the toxic organic substrate. Solvent showing higher partition coefficient could shield more toxicity as more solute being harbored in the water-immiscible phase to separate the concentrated toxic substrate from the biocatalyst.
While E. coli (SSP1) cannot grow on any of the organic solvents used in this work, biocompatibility and partition coefficient emerged as the main concerning factors for selecting organic solvents. This is especially true for the TDPB biphasic system, where the cells and the enzymes are exposed barely to the solvents. As evidenced by TDPB systems of aq./1-octanol and aq./ethyl acetate with high partition coefficient values (Table 1), the product titers were 2.39 and 2.07 mM, probably owing to the low biocompatibility of the solvents. Again, aq./n-octane and aq./2-undecanone showed similar partition coefficient numbers but displayed different product titers of 39.9 and 19.8 mM, respectively. Based on the biocompatibility and partition coefficient, n-hexadecane was chosen as the most desired organic solvent for establishing HFMB-based biphasic system.
In addition, Log P value of the organic solvent is an important factor in constructing a HFMB-based biphasic system as higher Log P value is favored for the wellness of the cells and enzymes (Gao et al. 2014; Faber 1997; Lye and Woodley 2000). This is evidenced by the HFMB-based aq./org. biphasic systems of using n-hexadecane and cyclohexane (Fig. 3), respectively. The differences in product titers in Fig. 3 were possibly owing to Log P values (8.9 for n-hexadecane and 2.5 for cyclohexane) and hence biocompatibility. In principle, the HFMB can separate the organic solvent and aqueous phase thoroughly, while in real practices, trace amount of leakage due to the membrane damage or fouling might happen during the operation. Under this situation, the capability of HFMB in shielding the organic solvent toxicity could be hampered and organic solvent with lower biocompatibility and Log P values may cause potential risk to the biotransformation.
In the HFMB-based aq./n-hexadecane biphasic system, over 99% of the styrene was in the organic phase, leaving only a trace amount of styrene in the aqueous phase. Because of the low concentration, styrene in the aqueous phase could be quickly converted to (S)-1-phenyl-1,2-ethanediol by E. coli (SSP1) within HFMB. At the same time, styrene in n-hexadecane could be continuously stripped into the aqueous phase for biotransformation. The n-hexadecane phase in the HFMB played the role of the substrate reservoir for the controlled and mild releasing, which allowed biotransformation being carried out with better catalytic performance (Fig. 1). With the presence of the membrane, the organic solvent hardly contacted with the cells, thus allowing a better protection for the whole cell biocatalyst and higher product titer. As shown in Fig. 4, HFMB-based aq./n-hexadecane biphasic system give 143 mM product concentration at 35 h, being 1.6-fold higher than 89.1 mM obtained in the TDPB-based aq./n-hexadecane biphasic system. Since all reaction conditions (substrate concentration, cell density, and reaction time) are the same for the biotransformation with HFMB or TDPB biphasic system, HFMB biphasic system gave thus also 1.6-fold higher productivity (space-time yield) than TDPB biphasic system. As also shown in Fig. 4, TDPB-based aq./n-hexadecane biphasic system gave a higher reaction rate at the beginning. This is because that the two phases in the TDPB system could be dispersed into each other during agitation, forming emulsion and hence generating high interfacial area for diffusion, which may be beneficial for the mass transfer and helpful for the reaction at the beginning. In comparison, the membrane, being the major difference between the TDPB and HFMB systems, virtually formed a solid barrier for the mass transportation, which could bring a lower reaction rate at the beginning. There are combined toxicity/inhibition of substrate, product, and solvent in each of the two systems. Since the product concentrations in the initial 5–6 h are nearly the same in both systems and the product exists mainly in the aqueous phase, the product toxicity/inhibition should also be nearly the same for both systems. While the reaction barely proceeded any more after 6 h in TDPB system, the HFMB system maintained a comparatively high reaction rate in a long reaction period (Fig. 4). These results clearly suggested that HFMB system could significantly reduce the combined toxicity/inhibition effects of substrate and solvent by reducing the contact of the substrate and solvent with the cells. Nevertheless, this system could not reduce the toxicity/inhibition of the water-soluble product. On the other hand, the product toxicity/inhibition is much less significant than the substrate toxicity/inhibition in the current biotransformation.
The concern on mass transferring in the HFMB system could be solved by model manipulation (as shown in Fig. 5), where the interfacial area as the key factor can be deliberately increased for better reaction performances. Evidenced in Fig. 5a, final production titer increased from 87.8 to 144 mM with an apparent interfacial area expanding from 0.15 to 0.3 m2/g CDW. In addition, HFMB could quench the turbulence and provide a complete separation between aqueous and organic phases, avoiding the emulsification haunted in the TDPB system.
The HFMB system is scalable, and the operation cost could be brought down via modularization in apparatus setup (Praveen and Loh 2012, 2013a, b, 2014). The complete separation between the water phase and the organic phase in the HFMB enables the continuous operation mode and the maintaining of high activity of the cells for the biotransformation. With the endurability of the fibers, the expenses on the facility should not be a concern.
In summary, HFMB-based aq./org. biphasic system was successfully established, for the first time, for a cascade biotransformation to enhance the productivity. E. coli (SSP1) coexpressing SMO and SpEH was used in such a system for trans-dihydroxylation of styrene to produce (S)-1-phenyl-1,2-ethanediol with improved production concentration. Different from other reported biphasic system using HFMB, the organic solvent in the shell side of the HFMB harbors most of the substrate while aqueous phase contains mostly the diol product, which provides the advantages of both avoiding substrate toxicity and mitigating the product recovery burden in downstream processing. Various organic solvents were tested regarding the partitioning coefficient, biodegradability, and biocompatibility for establishing the biphasic system, and n-hexadecane was chosen as the desired organic solvent for engineering the HFMB-based aq./organic system. The HFMB-based aq./n-hexadecane system enhanced the trans-dihydroxylation production titer to 143 mM, which was 16-fold higher than the product concentration (8.75 mM) achieved by using only the aqueous phase, and 1.6-fold higher than that (89.1 mM) in using TDPB-based aq./n-hexadecane system. The combination of biphasic system with HFMB also prevented foaming and emulsification, thus facilitating downstream purification. HFMB-based biphasic system could serve as a suitable platform for conducting either single-step or cascade biotransformation in an efficient and green way. Future endeavor could be made on the investigation of the mass transfer phenomenon of the HFMB-based biphasic system and the use of the established system for growing cell biotransformation under continuous mode, with designed and controlled enzymatic activity of the thriving cells.
References
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Chou KJ, Hanai T, Liao JC (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metabolic Eng 10(6):305–311
Brandenbusch C, Bühler B, Hoffmann P, Sadowski G, Schmid A (2010) Efficient phase separation and product recovery in organic-aqueous bioprocessing using supercritical carbon dioxide. Biotech Bioeng 107(4):642–651
Breuer M, Ditrich K, Habicher T, Hauer B, Kesseler M, Sturmer R, Zelinski T (2004) Industrial methods for the production of optically active intermediates. Angew Chem Int Ed 43(7):788–824
Buchholz K, Kasche V, Bornscheuer UT (2012) Biocatalysts and enzyme technology. Wiley-Blackwell, Hoboken
Burton SG (2003) Oxidizing enzymes as biocatalysts. Trends Biotechnol 21(12):543–549
Chang D, Heringa MF, Witholt B, Li Z (2003a) Enantioselective trans dihydroxylation of nonactivated C-C double bond of aliphatic heterocycles with Sphingomonas sp. HXN-200. J Org Chem 68(22):8599–8606
Chang D, Wang Z, Heringa MF, Wirthner R, Witholt B, Li Z (2003b) Highly enantioselective hydrolysis of alicyclic meso-epoxides with a bacterial epoxide hydrolase from Sphingomonas sp. HXN-200: simple syntheses of alicyclic vicinal transdiols. Chem Commun (8):960–1
Collins J, Grund M, Brandenbusch C, Sadowski G, Schmid A, Bühler B (2015) The dynamic influence of cells on the formation of stable emulsions in organic–aqueous biotransformations. J Ind Microbiol Biotechnol 42(7):1011–1026
Connors N, Prevoznak R, Chartrain M, Reddy J, Singhvi R, Patel Z, Olewinski R, Salmon P, Wilson J, Greasham R (1997) Conversion of indene to cis-(1S),(2R)-indandiol by mutants of Pseudomonas putida F1. J Ind Microbiol Biotechnol 18(6):353–359
Cornmell RJ, Winder CL, Schuler S, Goodacre R, Stephens G (2008) Using a biphasic ionic liquid/water reaction system to improve oxygenase-catalysed biotransformation with whole cells. Green Chem 10(6):685–691
Duetz WA, Van Beilen JB, Witholt B (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol 12(4):419–425
Faber K (1997) Biotransformations in organic chemistry, 3rd edn. Springer, Berlin, pp. 300–308
Gao P, Li A, Lee HH, Wang DI, Li Z (2014) Enhancing enantioselectivity and productivity of P450-catalyzed asymmetric sulfoxidation with an aqueous/ionic liquid biphasic system. ACS Catal 4(10):3763–3771
Gavrilov KN, Zheglov SV, Gavrilova MN, Chuchelkin IV, Groshkin NN, Rastorguev EA, Davankov VA (2011) Phosphoramidites based on phenyl-substituted 1, 2-diols as ligands in palladium-catalyzed asymmetric allylations: the contribution of steric demand and chiral centers to the enantioselectivity. Tetrahedron Lett 52(43):5706–5710
Gong P-F, Xu J-H (2005) Bio-resolution of a chiral epoxide using whole cells of Bacillus megaterium ECU1001 in a biphasic system. Enzyme Microb Tech 36(2):252–257
Hack C, Woodley J, Lilly M, Liddell J (2000) Design of a control system for biotransformation of toxic substrates: toluene hydroxylation by Pseudomonas putida UV4. Enzyme Microb Tech 26(7):530–536
Halan B, Schmid A, Buehler K (2010) Maximizing the productivity of catalytic biofilms on solid supports in membrane aerated reactors. Biotech Bioeng 106(4):516–527
He J-Y, Sun Z-H, Ruan W-Q, Xu Y (2006) Biocatalytic synthesis of ethyl (S)-4-chloro-3-hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem 41(1):244–249
Hüsken LE, Oomes M, Schroën K, Tramper J, de Bont JA, Beeftink R (2002) Membrane-facilitated bioproduction of 3-methylcatechol in an octanol/water two-phase system. J Biotechnol 96(3):281–289
Jia X, Wang Z, Li Z (2008) Preparation of (S)-2-, 3-, and 4-chlorostyrene oxides with the epoxide hydrolase from Sphingomonas sp. HXN-200. Tetrahedron Asymmetry 19(4):407–415
Kansal H, Banerjee UC (2009) Enhancing the biocatalytic potential of carbonyl reductase of Candida viswanathii using aqueous-organic solvent system. Biores Technol 100(3):1041–1047
Karabika E, Kallimanis A, Dados A, Pilidis G, Drainas C, Koukkou A (2009) Taxonomic identification and use of free and entrapped cells of a new Mycobacterium sp., strain Spyr1 for degradation of polycyclic aromatic hydrocarbons (PAHs). Appl Biochem Biotech 159(1):155–167
Kim J-H, Yang H, Park J, Boons G-J (2005) A general strategy for stereoselective glycosylations. J Am Chem Soc 127(34):12090–12097
Ladkau N, Schmid A, Bühler B (2014) The microbial cell—functional unit for energy dependent multistep biocatalysis. Curr Opin Biotechnol 30(4):178–189
Lau J, Frykman S, Regentin R, Ou S, Tsuruta H, Licari P (2002) Optimizing the heterologous production of epothilone D in Myxococcus xanthus. Biotech Bioeng 78(3):280–288
Li Z, Van Beilen JB, Duetz WA, Schmid A, de Raadt A, Griengl H, Witholt B (2002) Oxidative biotransformations using oxygenases. Curr Opin Chem Biol 6(2):136–144
Li A-T, Zhang J-D, Yu H-L, Pan J, Xu J-H (2011) Significantly improved asymmetric oxidation of sulfide with resting cells of Rhodococcus sp. in a biphasic system. Process Biochem 46(3):689–694
Li A, Liu J, Pham SQ, Li Z (2013) Engineered P450pyr monooxygenase for asymmetric epoxidation of alkenes with unique and high enantioselectivity. Chem Commun 49(98):11572–11574
Liu J, Li Z (2013) Cascade biotransformations via enantioselective reduction, oxidation, and hydrolysis: preparation of (R)-δ-lactones from 2-alkylidenecyclopentanones. ACS Catal 3(5):908–911
Liu Z, Michel J, Wang Z, Witholt B, Li Z (2006) Enantioselective hydrolysis of styrene oxide with the epoxide hydrolase of Sphingomonas sp. HXN-200. Tetrahedron Asymmetry 17(1:47–52
Liu J, Wu JC, Li Z (2014) Enoyl acyl carrier protein reductase (FabI) catalyzed asymmetric reduction of the C-C double bond of α,β-unsaturated ketones: preparation of (R)-2-alkyl-cyclopentanones. Chem Commun 50(68):9729–9732
Lye G, Woodley J (2000) Advances in the selection and design of two-liquid phase biocatalytic reactors. In: Cabral JMS, Mota M, Tramper J (eds) Multiphase bioreactor design. Taylor and Francis, London, pp. 115–134
May SW, Padgette R (1983) Oxidoreductase enzymes in biotechnology: current status and future potential. Nat Biotechnol 1:677–686
Mei J, Min H, Lü Z (2009) Enhanced biotransformation of L-phenylalanine to 2-phenylethanol using an in situ product adsorption technique. Process Biochem 44(8):886–890
Mirata MA, Heerd D, Schrader J (2009) Integrated bioprocess for the oxidation of limonene to perillic acid with Pseudomonas putida DSM 12264. Process Biochem 44(7):764–771
Molinari F, Aragozzini F, Cabral J, Prazeres D (1997) Continuous production of isovaleraldehyde through extractive bioconversion in a hollow-fiber membrane bioreactor. Enzyme Microb Tech 20(8):604–611
Muschiol J, Peters C, Oberleitner N, Mihovilovic MD, Bornscheuer UT, Rudroff F (2015) Cascade catalysis—strategies and challenges en route to preparative synthetic biology. Chem Commun 51:5798–5811
Pandey RK, Fernandes RA, Kumar P (2002) An asymmetric dihydroxylation route to enantiomerically pure norfluoxetine and fluoxetine. Tetrahedron Lett 43(25):4425–4426
Panke S, Held M, Wubbolts M (2004) Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr Opin Biotech 15(4):272–279
Park JB, Bühler B, Habicher T, Hauer B, Panke S, Witholt B, Schmid A (2006) The efficiency of recombinant Escherichia coli as biocatalyst for stereospecific epoxidation. Biotechnol Bioeng 95(3):501–512
Park SW, Han SJ, Kim D-S, Sim SJ (2007) Improvement of epothilone B production by in situ removal of ammonium using cation exchange resin in Sorangium cellulosum culture. Biochem Eng J 37(3):328–331
Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F (1999) Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274(13):8405–8410
Pfruender H, Amidjojo M, Kragl U, Weuster-Botz D (2004) Efficient whole-cell biotransformation in a biphasic ionic liquid/water system. Angew Chem Int Ed 43(34):4529–4531
Praveen P, Loh K-C (2012) Two-phase biodegradation of phenol in a hollow fiber membrane bioreactor. J Environ Eng 139(5):654–660
Praveen P, Loh K-C (2013a) Simultaneous extraction and biodegradation of phenol in a hollow fiber supported liquid membrane bioreactor. J Membrane Sci 430:242–251
Praveen P, Loh K-C (2013b) Two-phase biodegradation of phenol in trioctylphosphine oxide impregnated hollow fiber membrane bioreactor. Biochem Eng J 79:274–282
Praveen P, Loh K-C (2014) Kinetics modeling of two phase biodegradation in a hollow fiber membrane bioreactor. Sep Purif Technol 122:350–358
Raghavan S, Rathore K (2009) Asymmetric synthesis of (−)-tetrahydrolipstatin. Tetrahedron 65(48):10083–10092
Reetz MT, Zonta A, Schimossek K, Jaeger KE, Liebeton K (1997) Creation of enantioselective biocatalysts for organic chemistry by in vitro evolution. Angew Chem Int Ed 36(24):2830–2832
Roddick FA, Britz ML (1997) Production of hexanoic acid by free and immobilised cells of Megasphaera elsdenii: influence of in-situ product removal using ion exchange resin. J Chem Technol Biot 69(3):383–391
Schrewe M, Julsing MK, Bühler B, Schmid A (2013) Whole-cell biocatalysis for selective and productive C–O functional group introduction and modification. Chem Soc Rev 42(15):6346–6377
Urlacher VB, Eiben S (2006) Cytochrome P450 monooxygenases: perspectives for synthetic application. Trends Biotechnol 24(7):324–330
Urlacher VB, Girhard M (2012) Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol 30(1):26–36
Wang H, Dong Q, Meng C, Shi XA, Guo Y (2011) A continuous and adsorptive bioprocess for efficient production of the natural aroma chemical 2-phenylethanol with yeast. Enzyme Microb Tech 48(4):404–407
Wojaczynska E, Wojaczynski J (2010) Enantioselective synthesis of sulfoxides: 2000–2009. Chem Rev 110(7):4303–4356
Wu S, Li A, Chin YS, Li Z (2013) Enantioselective hydrolysis of racemic and meso-epoxides with recombinant Escherichia coli expressing epoxide hydrolase from Sphingomonas sp. HXN-200: preparation of epoxides and vicinal diols in high ee and high concentration. ACS Catal 3(4):752–759
Wu S, Chen Y, Xu Y, Li A, Xu Q, Glieder A, Li Z (2014) Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase. ACS Catal 4(2):409–420
Wu S, Zhou Y, Wang T, Too H-P, Wang DI, Li Z (2016a) Highly regio-and enantioselective multiple oxy-and amino-functionalizations of alkenes by modular cascade biocatalysis. Nat Commun. doi:10.1038/ncomms11917
Wu S, Liu J, Li Z (2016b) Organic synthesis via oxidative cascade biocatalysis. Synlett DOI. doi:10.1055/s-0036-1588627
Xu Y, Jia X, Panke S, Li Z (2009) Asymmetric dihydroxylation of aryl olefins by sequential enantioselective epoxidation and regioselective hydrolysis with tandem biocatalysts. Chem Commun 12:1481–1483
Xu Y, Li A, Jia X, Li Z (2011) Asymmetric trans-dihydroxylation of cyclic olefins by enzymatic or chemo-enzymatic sequential epoxidation and hydrolysis in one-pot. Green Chem 13(9):2452–2458
Xue Y-P, Liu Z-Q, Xu M, Wang Y-J, Zheng Y-G, Shen Y-C (2010) Enhanced biotransformation of (R, S)-mandelonitrile to (R)-(−)-mandelic acid with in situ production removal by addition of resin. Biochem Eng J 53(1):143–149
Yang Y, Liu J, Li Z (2014) Engineering of P450pyr hydroxylase for the highly regio-and enantioselective subterminal hydroxylation of alkanes. Angew Chem Int Ed 53(12):3120–3124
Zhang W, Tang W, Wang ICD, Li Z (2011) Concurrent oxidations with tandem biocatalysts in one pot: green, selective and clean oxidations of methylene groups to ketones. Chem Commun 47(11):3284–3286
Zhang J, Xu T, Li Z (2013) Enantioselective biooxidation of racemic trans-cyclic vicinal diols: one-pot synthesis of both enantiopure (S, S)-cyclic vicinal diols and (R)-α-hydroxy ketones. Adv Synth Catal 355(16):3147–3153
Zhao L-Q, Sun Z-H, Zheng P, He J-Y (2006) Biotransformation of isoeugenol to vanillin by Bacillus fusiformis CGMCC1347 with the addition of resin HD-8. Process Biochem 41(7):1673–1676
Zhou Y, Wu S, Li Z (2016) Cascade biocatalysis for sustainable asymmetric synthesis: from biobased L-phenylalanine to high-value chiral chemicals. Angew Chem Int Ed 55(38):11647–11650
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Singapore-MIT Alliance through a CPE Flagship research Program and by GlaxoSmithKline (GSK) and Singapore Economic Development Board (EDB) through a Green and Sustainable Manufacturing grant (project No. 279-000-331-592).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gao, P., Wu, S., Praveen, P. et al. Enhancing productivity for cascade biotransformation of styrene to (S)-vicinal diol with biphasic system in hollow fiber membrane bioreactor. Appl Microbiol Biotechnol 101, 1857–1868 (2017). https://doi.org/10.1007/s00253-016-7954-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7954-1