Abstract
Different factors are known to influence the early gut colonization in newborns, among them the perinatal use of antibiotics. On the other hand, the effect on the baby of the administration of antibiotics to the mother during labor, referred to as intrapartum antibiotic prophylaxis (IAP), has received less attention, although routinely used in group B Streptococcus positive women to prevent the infection in newborns. In this work, the fecal microbiota of neonates born to mothers receiving IAP and of control subjects were compared taking advantage for the first time of high-throughput DNA sequencing technology. Seven different 16S rDNA hypervariable regions (V2, V3, V4, V6 + V7, V8, and V9) were amplified and sequenced using the Ion Torrent Personal Genome Machine. The results obtained showed significant differences in the microbial composition of newborns born to mothers who had received IAP, with a lower abundance of Actinobacteria and Bacteroidetes as well as an overrepresentation of Proteobacteria. Considering that the seven hypervariable regions showed different discriminant ability in the taxonomic identification, further analyses were performed on the V4 region evidencing in IAP infants a reduced microbial richness and biodiversity, as well as a lower number of bacterial families with a predominance of Enterobacteriaceae members. In addition, this analysis pointed out a significant reduction in Bifidobacterium spp. strains. The reduced abundance of these beneficial microorganisms, together with the increased amount of potentially pathogenic bacteria, may suggest that IAP infants are more exposed to gastrointestinal or generally health disorders later in age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gastrointestinal microbiota plays a very important role for the health of the host, contributing to the development of innate and adaptive immunity, host response to pathogens, bioavailability of nutrients, and host metabolic activity. Scientific evidences suggest that dysbiosis of the intestine can be directly correlated with several diseases, not only newborn pathologies, such as necrotizing enterocolitis, but also occurring at the post-weaning age up to adulthood, such as irritable bowel syndrome, obesity, celiac disease, and inflammatory bowel disease (Di Gioia et al. 2014; Azad et al. 2015; Rodríguez et al. 2015).
The main colonization of the infant begins in the first hours after birth, even if new recent evidences suggest the existence of an intrauterine transmission of maternal bacteria to fetus gut (Francino 2014). The key “educational” process of exposure to microbial infections and appropriate early colonization of the gut by beneficial microorganisms, such as intestinal strains like Bifidobacterium spp. from the mother (Jost et al. 2012; Jost et al. 2014; Makino et al. 2015) appears to be critical in the development of a well-functioning immune system, tolerant of the vast majority of microbiota members but able to mount rapid and effective inflammatory and antibody responses to invading pathogens. Although the prevalence of Actinobacteria in the newborn intestine, in particular bifidobacteria, has been recently questioned because some metagenomics studies revealed a low abundance of Bifidobacterium members compared to what was thought before, their beneficial role for the health of newborns remains indisputable (Koenig et al. 2011; Turroni et al. 2012).
It is well known that colonization in the early stages of life is influenced by several factors, the mode of delivery, the type of infant feeding, gestational age at birth, and antibiotic administration at early stage of life being the most investigated in the literature (Bezirtzoglou 1997; Fouhy et al. 2012; Azad et al. 2015). On the contrary, the use of antibiotics on the mother during labor, referred to as intrapartum antibiotic prophylaxis (IAP), routinely used in group B Streptococcus (GBS) positive women, has been taken into consideration only in a few studies. IAP was introduced in the 1990s and it allowed a reduction of incidence of GBS infection in newborns up to 80 %, thus drastically decreasing sepsis associated deaths (Al-Taiar et al. 2011). IAP consists in administration of antibiotic, mainly ampicillin, during the labor in order to reach adequate serum level in fetus and amniotic fluids. Recent studies evidenced a reduced vertical transmission of lactic acid bacteria from IAP-treated mothers to the neonates comparing the results of microbiological analysis of swabs applied both to mother’s vagina and to neonate’s oral cavity (Keski-Nisula et al. 2013) and a significant reduction of bifidobacteria in the fecal microbiota of newborns born to mothers subjected to IAP with respect to untreated mothers using qPCR (Aloisio et al. 2014). The other microbial groups examined in this study (Lactobacilllus spp., Bacteroides fragilis group, Clostridium difficile, and Escherichia coli) did not show any significant variation.
The advent of next generation sequencing (NGS) has determined a revolutionary approach in the study of complex microbial communities such as the human intestinal microbiota. NGS technologies, in particular, allowed to reveal the structure and function of the intestinal microbiota, providing high-throughput data, which instead are not provided by other approaches, such as qPCR, limited to selected bacterial species. Moreover, NGS technologies allow to perform a direct sequencing eliminating the need to clone or culture sample, thus avoiding possible biases in sampling. Recent studies analyzed the critical points that could affect the efficiency of NGS analysis, starting from the protocol used for fecal DNA extraction to the selection of primers and different bioinformatic workflows (Claesson et al. 2010; Barriuso et al. 2011; Kennedy et al. 2014; Wagner Mackenzie et al. 2015). In particular, the choice of the primers used to amplify the different hypervariable regions of 16SrRNA gene can lead to the selection of specific components of the intestinal microbiota at the expense of others. Milani et al. (2013) demonstrated that a higher number of phylotypes can be detected using primers targeted to 16S ribosomal DNA (rDNA) hypervariable regions rather than primers allowing sequencing of the whole gene. In addition, some differences have also been detected among the different hypervariable regions. The choice of multiple primers targeted to amplify seven different hypervariable regions (V2, V3, V4, V6, V7, V8, and V9) simultaneously may have the opportunity to detect a larger array of microbial groups and enable a more accurate microbial community description. In addition, the use of multiple variable regions detects more diverse bacterial populations and preserves the information from single V-regions for a multi-dimensional analysis.
This work is aimed at evaluating, for the first time, the main effects of IAP on the whole microbiome composition of newborns at 7 days after birth using a metagenomic approach. Moreover, a massive parallel sequencing targeted to seven different hypervariable regions of the 16S rRNA gene has been attempted.
Material and methods
Experimental design
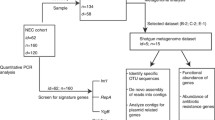
The study was performed on fecal samples obtained from 10 infants born by mothers negative to GBS and 10 infants to mothers positive to GBS, receiving IAP as described below. DNA was extracted and amplified with primers targeted to seven hypervariable regions of 16S rDNA (V2, V3, V4, V6, V7, and V8) and whole sequencing was performed with the Ion Torrent personal genome machine (PGM) platform. Data analysis was initially performed with Ion Reporter™ Software 4.0 (Life Technologies) on all the reads obtained. Then, reads relative to one hypervariable region were chosen for further bioinformatical and biostatistical analysis.
Sample collection
The study was performed on DNA extracted from feces of 20 newborns enrolled by the Neonatal Intensive Care Unit of the S. Orsola Malpighi Hospital of Bologna from April 2013 to December 2013. Inclusion criteria were born at term by vaginal delivery, birth weight adequate for gestational age (2.5–4.0 kg), exclusively breastfed (in order to reduce variability in the intestinal microbiota consequent to formula feeding), and not receiving perinatal antibiotic treatment. Fecal samples were collected from recruited newborns a 6–7 days after birth. The cohort is composed by 10 infants that were born to mothers negative to GBS after vaginal swab (control group) and 10 infants to mothers positive to GBS. The latter were treated with 2 g of intravenous ampicillin at least 4 h before delivery, followed by 1 g every 4 h during labor until delivery (IAP group) according to the institutional treatment protocol for GBS prophylaxis, derived from the Centers for Disease Control and Prevention guidelines (Verani et al. 2010).
The study was approved by the local ethics committee (Comitato Etico Indipendente dell’Azienda Ospedaliero-Universitaria di Bologna, Policlinico S. Orsola-Malpighi, document number 12/2013/U/Oss approved on March 12, 2013).
Fecal samples were frozen immediately after collection at −80 °C, in numbered screw-capped plastic containers, until they were processed for DNA extraction. Researchers carrying out DNA extraction and microbial analyses were blind to the group identity of infants (control group or IAP group).
DNA extraction
For the DNA extraction, 200 mg of newborn feces (preserved at −80 °C after collection) were used using the QIAamp DNA Stool Mini Kit (Qiagen, West Sussex, UK) with a slight modification: an additional incubation at 95 °C for 10 min of the stool sample with the lysis buffer was added to the standard protocol to improve the bacterial cell rupture (Aloisio et al. 2014). Extracted DNA was stored at −80 °C. The purity of extracted DNA was determined by measuring the ratio of the absorbance at 260 and 280 nm (Infinite®200 PRO NanoQuant, Tecan, Mannedorf, Switzerland), and the concentration was estimated by Qubit® 3.0 Fluorometer (Invitrogen, Carlsbad, CA, USA).
16S rDNA gene sequencing with the ion torrent personal genome machine and ion reporter™ analysis
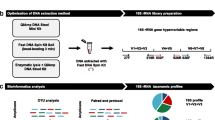
Sequencing was performed with Ion Torrent PGM platform, taking advantage from the “Ion 16S Metagenomics Sequencing Technology Access Program” (Life Technologies, South San Francisco, CA, USA). The Ion 16S™ Metagenomics kit (Life Technologies, now available as Thermo Fisher Scientific, Waltham, MA, USA, catalog number A26216) was used for the amplification of fecal 16S rDNA. The kit contained two different sets of primers targeted to seven hypervariable regions of 16S rDNA: set 1 includes three regions (V2, V4, and V8) and set 2 other four regions (V3, V6 + V7, and V9). PCR reactions were performed using the AmplitaqGold®360MM polymerase kit from Life Technologies. Each PCR mixture (50 μL) contained 25 μL of polymerase master mix, 5 μL of each set of primers, and 1 μL of appropriately diluted template DNA (5 ng/μL). The PCR was performed using the Verity Thermocycler (Applied Biosystems, Foster City, CA, USA) with the following program: initial denaturation at 95 °C for 10 min; 18 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 1 min, and extension at 72 °C for 20 s; and final extension at 72 °C for 7 min. The PCR products were purified using the magnetic beads of Agentcourt®AMPure®XP (Beckman Coulter Genomics, Brea, CA, USA) and 1 μL was analyzed with Bioanalyzer® (Agilent Technologies, Santa Clara, CA, USA) using Agilent High Sensitivity DNA Kit (Agilent Technologies) and the Bioanalyzer® software was used to determine the molar concentration (nmol/L) of target amplicons. Equimolar amounts of each of V2, V4, V8 and V3, V6 + 7, and V9 amplicons pool were then combined and pooled at the concentration of 10–100 ng in 79 μL of dilution buffer.
Libraries were prepared using Ion Plus Fragment Library Kit (Life Technologies) and Ion Xpress™ Barcode Adapters kit (Life technologies) that allow the ligation of adapters, nick-repair, and purification. Finally, the libraries were quantified with Ion Library Quantitation Kit (Life Technologies) following manufacturer’s recommendations.
Pooled libraries were subjected to emulsion PCR carried out using Ion One Touch™ 2 system and Ion PGM™ Template OT2 400 bp kit (Life Technologies). Enrichment of clonal amplified libraries was finally obtained with Ion PGM™ Enrichment Beads (Life Technologies) on an Ion One Touch ES instrument (Life Technologies). Sequencing of the retrieved templates spheres was carried out on a Ion 318™ v2 chip using Ion Torrent PGM™ system and employing the Ion Sequencing 400 kit (Life Technologies).
BAM files directly obtained from the Ion Torrent PGM output were processed using Ion Reporter™ Software 4.0 (Life Technologies). This tool provides several reads filtering parameters: (i) each read is trimmed by primer tolerating until three errors at primers’ sequence, (ii) read with length lower than 165 bp are discarded, and (iii) after primers trimming and length check, reads are placed in a hash table to get unique sequences and their abundance. On the other hand, it does not allow any cluster analysis. The alignment was performed using MicroSEQ ID and Green Genes databases in order to obtain the taxonomic identification of sequences. The same tool provided even information on relative taxonomic abundance by consensus and by primer within the dataset. Sequencing raw data were deposired as a NCBI BioProject and received the following ID: PRJNA29043, while sample accessions and metadata are in Supplementary Table S1.
OTUs clustering, phylotype identification, and statistical analysis
To further investigate the sample richness and biodiversity, operational taxonomic units (OTUs) cluster analysis have been performed based on hypervariable region V4. Sequencing reads were extracted from the total dataset using cutadapt (Martin 2011) using 515f and 806r primers (CCAGCMGCCGCGGTAA and GGACTACHVGGGTWTCTAAT, respectively) obtaining a total of 248.295 reads (11.824 reads per sample on average). Reads were analyzed using the micca pipeline (Albanese et al. 2015) (version 0.1, http://compmetagen.github.io/micca/). Forward and reverse primer trimming and quality filtering were performed using micca-preproc (parameters-q 16-l 150) truncating reads shorter than 150 nt. De novo sequence clustering, chimera filtering, and taxonomy assignment were performed by micca-otu-denovo (parameters -s 0.97 -c): operational taxonomic units (OTUs) were assigned by clustering the sequences with a threshold of 97 % pair-wise identity, and their representative sequences were classified using the RDP (Wang et al. 2007) software version 2.8. Template-guided multiple sequence alignment (MSA) was performed using PyNAST (Caporaso et al. 2010) (version 0.1) against the multiple alignment of the Greengenes database (release 13_05) filtered at 97 % similarity. Finally, a phylogenetic tree was inferred using FastTree (Price et al. 2010) software embedded in micca-phylogeny (parameters: -a template-template-min-perc75). Three samples with less than 5000 reads were discarded and sampling heterogeneity was reduced by rarefaction (5520 sequences per sample).
Results
Ion torrent experimental output
Descriptive analysis
The microbiota characterization of fecal samples taken from a cohort of 20 newborns (10 born from mothers treated with ampicillin and 10 controls) was made by metagenomic sequence analysis, targeting seven different hypervariable regions of the 16 s rDNA gene using the Ion Torrent PGM.
As expected, the amplicons’ size obtained from the Bioanalyzer analysis for the two sets of primers was between 200 and 300 bp. The total amount of reads was of 3,697,465 with a mean of 177,775 ± 36,358 reads and a median value of 172,625 reads for sample. The mean read length obtained was of 195.85 ± 5.8 bp.
Genomic sequences from Ion Torrent machine were trimmed and aligned by the Ion Reporter Software 4.0 (Life Technologies) taking advantage from the 16S Metagenomic workflow, which originated a mean of 106,195 of valid reads for sample, as set on the Ion Reporter criteria, and ∼54,323 of mapped reads.
Ion reporter global analysis for all hypervariable regions investigated
The taxonomic identification carried out by the 16S Metagenomic workflow of the Ion Reporter Software 4.0 (Life Technologies) highlighted the presence of four different phyla in all samples: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, in agreement with other metagenomic sequencing studies focused on human gut microbiome (Qin et al. 2010; Azad et al. 2013). A first global analysis with this software allowed to detect a clear difference in the gut microbial composition between the two groups (Fig.1). Particularly, we noted a marked difference in the relative abundance of Actinobacteria with a higher presence in the control group (3.8 %) than in the IAP group (0.4 %) as well as for Bacteroidetes (47.7 and 16.0 % in the control group and IAP group, respectively). On the other hand, an opposite trend was recorded for the Proteobacteria phylum which is more represented within the IAP group (54.7 %) than in the control group (15.5 %). These differences were supported by univariate ANOVA (p < 0.05). Moreover, a different distribution of gram positive and gram negative in the two groups reflects a selective trend correlated to the antibiotic treatment (Fig.2). Clearly, there is an higher abundance of gram-negative phyla within the IAP group compared to the control group.
Comparison of efficiency among different hypervariable regions
The sequencing of seven hypervariable regions of the 16S rDNA gene (V2, V3, V4, V6 + V7, V8, and V9) allowed us to evaluate the capability of each primer pair and of each sequenced region in describing the microbiota composition. The percentage of mapped reads is not homogenous among each hypervariable region as shown in Fig. 3 (in addition, Table S2 reports the number of valid and mapped reads obtained with each primer pair). In particular, the regions with the higher percentage of mapped reads are V2, V3, and V6 + V7 which represent more than 60 % of the total amount of mapped reads. On the other hand, regions V4, V8, and V9 contribute to about 30 % of mapped reads and in particular the V9 region shows the lowest percentage of mapped reads (<10 %). In addition, for each hypervariable region, we evaluated the percentage of mapped reads for each phylum in order to compare their informative power. These hypervariable regions differently explore the variability that characterize the intestinal microbiota (Fig. 3). Not all the regions could detect the four different phyla in the same way. In particular, Actinobacteria resulted to be totally absent among V3 mapped reads, whereas Bacteroidetes were not detected considering the V8 region. An extreme case is the region V9, whose data provide an extremely low level of biodiversity identifying only the Proteobacteria phylum.
IAP treatment and intestinal microbiota biodiversity: outputs obtained from the V4 region
Since the seven investigated hypervariable regions of 16S rDNA revealed a different discriminant ability to detect the four phyla, we considered as appropriate to conduct a more detailed investigation on data obtained on one region in order to avoid the primers bias effect on the results interpretation. The V4 region was chosen for its sensitivity to detect all the four phyla and for the acceptable number of reads sequenced. In addition, the V4 region is frequently used in metagenomic studies targeted to gut microbiota (Biagi et al. 2014; Huda et al. 2014; Mejía-León et al. 2014; Dareng et al. 2015). A dataset of 248.295 reads was generated with a mean coverage of 11.824 per sample. After rarefaction, a total amount of 87 OTUs was obtained at 97 % of identity.
The microbial richness and biodiversity have been assessed by two non-parametric indexes: Chao1 and Shannon, respectively, (Fig. 4). The control group showed a more complex microbial profile compared to the IAP group in term of richness, as evident from the Alpha diversity in Fig. 4a and confirmed by the higher value of Chao1 index. Differently, IAP group is characterized by a lower score of Chao1 and Shannon indexes (P < 0.01 and P < 0.05, respectively), indicating reduced level of richness and biodiversity within this group (Fig. 4b, c).
To explore the beta-diversity between the two groups of newborns, weighted and unweighted UniFrac distances were calculated (Fig. 5). No significant difference was observed for the Bray-Curtis index (P > 0.05, data not shown). On the contrary, an interesting phylogenetic and relative abundance difference is deducible from unweighted UniFrac distance (P < 0.05, Fig.5c). Data distribution using principal coordinate analysis (PCoA) shows segregation along axis1 for both UniFrac indices, indicating a separation in two clusters due to IAP treatment (Fig. 5a). Moreover, apart cluster division, a clear correlation between PCoA groups and the relative abundances of some genera is appreciable (Fig. 5b). To better characterize the variability of the microbial intestinal composition of the two groups of newborns, the taxonomic identification at family level was carried out (Fig. 6). The two microbial ecosystems show a remarkable different profile with respect to family/genus level in term of phylotypes and their relative abundance. In particular, within the IAP group, it is possible to appreciate a lower number of bacterial families with some extreme cases composed almost exclusively by Enterobactericeae family members (Proteobacteria) that can reach over 90 % of relative abundance and a few samples are even characterized by the presence of Streptococcaceae family (with an average of 13 %, Table 1).
a Principal Coordinates Analysis (PCoA) highlights the division into two major clusters along axis1 for most part of sample, due to IAP treatment, b correlation between cluster analysis and specific bacterial gener, c Unweighted Unifrac test indicates that intragroup distances are major than intergroup distances (p value = 0.02)
On the other hand, the control group shows a more complex profile in term of biodiversity with a more equal distribution at family/genus level. The latter group largely enriched in Bacteroidaceae which reach an average of 42 % (Table 1). Apart from these differences in bacterial family distribution, Wilcox test underlines a substantial distinction between the two groups within Actinobacteria phylum and, particularly, for the Bifidobacterium genus (P < 0.05, see Supplementary material, Table S3) as an important component of newborns gut microbiota which is absent or present in low levels in the IAP group.
Discussion
This study was aimed at evaluating the effects of IAP against GBS on newborn gut microbiota composition with a metagenomic approach. NGS techniques have revolutionized the way of studying the microbiota richness and diversity allowing a combination of the advantages of other molecular techniques such as real-time PCR, FISH, RFLP, and DGGE. In particular, NGS allow a massive parallel amplicon sequencing with the possibility of screening multiple samples in the same experiment at high sequencing depth. There is still no consensus for the use of a particular 16S rDNA region since sources of bias are numerous factors such as insufficient coverage of primers (Hong et al. 2009; Mao et al. 2012), primer-template mismatches (Sipos et al. 2007; Smith et al. 2002), unequal amplification (Polz and Cavanaugh 1998; Reysenbach et al. 1992; Suzuki et al. 1998), and differential efficiency of annealing (Sipos et al. 2007). Therefore, the choice of a multiplex primers approach based on seven hypervariable regions of 16S rDNA constitutes an innovative, unexplored up to now, strategy in metagenomic protocols. The different hypervariable regions of the 16S rDNA gene which show a different accuracy of microbial identification at various taxonomic level, with the V3 and V4 being as the less biased and the most used for human gut microbiota studies (Milani et al. 2013; Cai et al. 2013). Hence, the choice of the primer sets and covered regions can deeply affect the profile of gut composition in terms of detected biodiversity and relative abundance (Claesson et al. 2010; Kumar et al. 2011; Schloss et al. 2011; Soergel et al. 2012; De Filippo et al. 2010; Rodríguez et al. 2015).
As a matter of fact, a preliminary analysis of the Ion Torrent outputs considering the set of primers used a difference in accuracy and performance of each region considered was clearly observed. Particularly, the V4 and V6 + V7 regions were the most accurate both for number of assigned reads per sample and for the OTUs distribution, while the other regions seem to underestimate the diversity content. For some of these regions, as for example, the V8 or the V9, this result could be expected as these regions are used as highly informative for environmental microbial communities (Sundarakrishnan et al. 2012; ZL et al. 2013). In addition, the V3 region, even if is widely used in various studies and has shown a high number of assigned reads (Milani et al. 2013; Cai et al. 2013), does not detect all the phyla as the V4 and V6 + V7 do. For these reasons, the V3 region can be considered as less informative for the study of the human gut microbiota. In the light of these evidences, in parallel to the outputs obtained by the simultaneous analysis of the seven amplified regions, further and more detailed analyses on the samples considered in this study were carried out on the V4 region.
The various diversity indexes used to study microbial community variation revealed a different microbial composition between the two groups (IAP and control). This assumption is confirmed by PCoA analysis based on Weighted and Unweighted UniFrac distances at genus level which underlined the presence of two distinct groups. Particularly, the control group is characterized by higher indices of richness and evenness as confirmed from both alpha and beta-diversity. On the contrary, fecal samples from newborns born to mothers receiving IAP showed an incredibly poor microbial biodiversity, with a high predominance of Enterobacteriaceae and low level of Bifidobacterium members. The low amount of Bifidobacterium spp. seems to clearly distinguish the IAP group with respect to the control one, as clearly appears from Wilcox test, while the higher presence of Enterobacteriaceae found only in some samples within the IAP group is not enough to outline a clear tendency linked to antibiotic treatment. The changes related to IAP treatment are in agreement with the antimicrobial spectrum of ampicillin which is active mainly against gram-positive bacteria, and this activity may explain the overabundance of gram-negative bacteria, mainly Enterobacteraceae. It is interesting to note that this microbial family includes some potentially pathogenic microorganisms such as E. coli, Salmonella spp., Shigella spp., Enterobacter spp., and Klebsiella spp., that, in a microbiota with a reduced biodiversity like that of the IAP group newborns, could reach a substantial bacterial concentration and may cause health problems of varying extent from atopic disorders until more severe disease such as gastroenteritis (Abrahamsson et al. 2012; de Weerth et al. 2013).
Therefore, the results obtained within this study also confirmed remarkable differences in the bifidobacteria counts, as previously shown in Aloisio et al. (2014), with a decrement due to IAP treatment. For a long time, bifidobacteria have been considered the predominant component of newborn intestinal microbiota, whereas recent metagenomic studies have found that their presence is quantitatively less important than previously thought (Koenig et al. 2011). Not many studies have been focused on the microbiota at the very early stage of life; however, this study points out that in healthy newborns at 7 days of life, Bifidobacterium spp. constitutes about 4 % of the total microbiota and that they are the main target of the perinatal antibiotic treatment in addition to the action against GBS. Further studies are necessary to study the impact of such a drastic Bifidobacterium reduction on the newborns, considering that it is well known that the genus Bifidobacterium is associated with important beneficial functions for the health and development of the infant with a pivotal role in the prevention of several pathologies (Turroni et al. 2012; Di Gioia et al. 2014), and its reduction can lead to health disorders later in age.
In conclusion, the overall experimental outcome showed that IAP has a notable impact on gut microbiota of newborn, reducing microbial biodiversity, allowing colonization of Enterobacteriaceae, and reducing the amount of Actinobacteria.
References
Abrahamsson TL, Jakobsson HE, Andersson AF, Björkst B, Engstrand L, Jenmalm MC (2012) Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 129:434–440
Albanese D, Fontana P, De Filippo C, Cavalieri D, Donati C (2015) MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci Rep 5:9743
Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D (2014) Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-streptococcus activity of Bifidobacterium strains. Appl Microbiol and Biotechnol 98:6051–6060
Al-Taiar A, Hammoud M, Thalib L, Isaacs D (2011) Pattern and etiology of culture-proven early onset neonatal sepsis: a five year prospective study. Int J Infec Diseases 15:631–634
Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Radha Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL (2013) Gut microbiota of healty Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185:385–394
Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane PJ, Turvey SE, Subbarao P, Becker AB, Scott JA, Kozyrskyj AL (2015) Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 45:632–643
Barriuso J, Valverde JR, Mellado RP (2011) Estimation of bacterial diversity using next generation sequencing of 16S rDNA: a comparison of different workflows. BMC Bioinformatics 12:473
Bezirtzoglou E (1997) The intestinal microflora during the first weeks of life. Anaerobe 3:173–177
Biagi E, Candela M, Centanni M, Consolandi C, Rampelli S, Turroni S, Severgnini M, Peano C, Ghezzo A, Scurti M, Salvioli S, Franceschi C, Brigidi P (2014) Gut microbiome in down syndrome. PLoS one 9:e112023
Cai L, Ye L, Tong AH, Lok S, Zhang T (2013) Biased diversity metrics revealed by bacterial 16 s pyrotags derived from different primer sets. PLoS one 8:e53649
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Anderson GL, Knight R (2010) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Claesson MJ, Wang Q, O’Sullivan O, Greene-Diniz R, Cole JR, Ross RP, O’Toole PW (2010) Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res 38:e200
Dareng EO, Ma B, Famooto AO, Akarolo-Anthony SN, Offiong RA, Olaniyan O, Dakum PS, Wheeler CM, Fadrosh D, Yang H, Gajer P, Brotman RM, Ravel J, Adebamowo CA (2015) Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect 11:1–15
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696
de Weerth C, Fuentes S, Puylaert P, de Vos WM (2013) Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 131:e550–e558
Di Gioia D, Aloisio I, Mazzola G, Biavati B (2014) Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol 98:563–577
Fouhy F, Ross RP, Fitzgerald G, Stanton C, Cotter P (2012) Composition of the early intestinal microbiota, knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 3:203–220
Francino MP (2014) Early development of the gut microbiota and immune health. Pathogens 4:769–790
Hong S, Bunge J, Leslin C, Jeon S, Epstein SS (2009) Polymerase chain reaction primers miss half of rRNA microbial diversity. ISME J 3:1365–1373
Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, Stephensen CB (2014) Stool microbiota and vaccine responses of infants. Pediatrics 134:e362–e372
Jost T, Lacroix C, Braegger CP, Chassard C (2012) New insights in gut microbiota establishment in healthy breast fed neonates. PLoS one 7:e44595
Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C (2014) Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 16:2891–2904
Kennedy N, Walker A, Berry S, Duncan S, Farquarson F, Louis P, Thompson T, Ahmad T, Satsangi J, Flint H, Parkhill J, Lee C, Hold G (2014) The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS one 9:e88982
Keski-Nisula L, Kyynäräinen HR, Kärkkäinen U, Karhukorpi J, Heinonen S, Pekkanen J (2013) Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr 102:480–485
Koenig JE, Scalfone N, FrickerAD SJ, Knight R, Angenent LT, Ruth EL (2011) Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108:4578–4585
Kumar PS, Brooker MR, Dowd SE, Camerlengo T (2011) Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS one 6:e20956
Makino H, Martin R, Ishikawa E, Gawad A, Kubota H, Sakai T, Oishi K, Tanaka R, Ben-Amor K, Knol J, Kushiro A (2015) Multilocus sequence typing of bifidobacterial strains from infant’s faeces and human milk: are bifidobacteria being sustainably shared during breastfeeding? Benef Microbes 6:563–572
Mao DP, Zhou Q, Chen CY, Quan ZX (2012) Coverage evaluation of universal bacterial primers using the metagenomic datasets. BMC Microbiol 12:66
Martin M (2011) Cut adapt removes adapter sequences from high-throughput sequencing reads. EMB Net J 17:10–12
Mejía-León ME, Petrosino JF, Ajami NJ, Domínguez-Bello MG, de la Barca AMC (2014) Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep 4:e3814
Milani C, Hevia A, Foroni E, Duranti S, Turroni F (2013) Assessing the fecal microbiota: An optimized ion torrent 16S rRNA Gene-Based Analysis protocol. PLoS one 8:e68739
Polz MF, Cavanaugh CM (1998) Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol 64:3724–3730
Price MN, Dehal PS, Arkin AP (2010) FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS one 5:e9490
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, MetaHIT Consortium, Bork P, Ehrlich SD, Wang J (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65
Reysenbach AL, Giver LJ, Wickham GS, Pace NR (1992) Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol 58:3417–3418
Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC (2015) The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 26:26050
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS one 6:e27310
Sipos R, Szekely AJ, Palatinszky M, Revesz S, Marialigeti K, Nikolausz M (2007) Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbiol Ecol 60:341–350
Smith S, Vigilant L, Morin PA (2002) The effects of sequence length and oligonucleotide mismatches on 5′ exonuclease assay efficiency. Nucleic Acids Res 30:e111
Soergel DA, Dey N, Knight R, Brenner SE (2012) Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J 6:1440–1444
Sundarakrishnan B, Pushpanathan M, Jayashree S, Rajendhran J, Sakthivel N, Jayachandran S, Gunasekaran P (2012) Assessment of microbial richness in pelagic sediment of andaman sea by bacterial tag encoded FLX titanium amplicon pyrosequencing (bTEFAP). Indian J Microbiol 52:544–550
Suzuki M, Rappe MS, Giovannoni SJ (1998) Kinetic bias in estimates of coastal picoplankton community structure obtained by measurements of small-subunit rRNA gene PCR amplicon length heterogeneity. Appl Environ Microbiol 64:4522–4529
Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O’Toole PW, van Sinderen D, Marchesi JR, Ventura M (2012) Diversity of bifidobacteria within the infant gut microbiota. PLoS one 7:e36957
Verani J, McGee L, Schrag S (2010) Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 59:1–32
Wagner Mackenzie B, Waite DW, Taylor MW (2015) Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front Microbiol 6:e130
Wang Q, Garrity GM, Tiedje JM, Cole RJ (2007) Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Yan ZL, Zheng XW, Han BZ, Han JS, Nout MJ, Chen JY (2013) Monitoring the ecology of Bacillus during daqu incubation, a fermentation starter, using culture-dependent and culture-independent methods. J Microbiol Biotechnol 23:614–622
Acknowledgments
We would like to thank Life Technologies (Thermo Fisher Scientific) for the valuable support to our work. A special acknowledgment is for Dr. Raffaldi Fabio for his excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local ethics committee as indicated in the text.
Funding
This study was funding by the University of Bologna, Fundamental Research Oriented Program (grant number J61J10000790001).
Conflict of interest
All the authors declare that they have no conflict of interest.
Informed consent
“Informed consent was obtained from all individual participants included in the study.”
Additional information
Irene Aloisio and Andrea Quagliariello contributed equally
Electronic supplementary material
ESM 1
(PDF 49 kb)
Rights and permissions
About this article
Cite this article
Aloisio, I., Quagliariello, A., De Fanti, S. et al. Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol 100, 5537–5546 (2016). https://doi.org/10.1007/s00253-016-7410-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7410-2