Abstract
A novel high-throughput strategy was developed to determine the calcium precipitation activity (CPA) of mineralization bacteria used for self-healing of concrete cracks. A bacterial strain designated as H4 with the highest CPA of 94.8 % was screened and identified as a Bacillus species based on 16S rDNA sequence and phylogenetic tree analysis. Furthermore, the effects of certain influential factors on the microbial calcium precipitation process of H4 were evaluated. The results showed that lactate and nitrate are the best carbon and nitrogen sources, with optimal concentrations of approximately 25 and 18 mM, respectively. The H4 strain is able to maintain a high CPA in the pH range of 9.5–11.0, and a suitable initial spore concentration is 4.0 × 107 spores/ml. Moreover, an ambient Ca2+ concentration greater than 60 mM resulted in a serious adverse impact not only on the CPA but also on the growth of H4, suggesting that the maintenance of the Ca2+ concentration at a low level is necessary for microbial self-healing of concrete cracks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increasing requirements for environment-friendly concrete maintenance, bacteria-based self-healing technology for concrete has become a research hotspot in the field of civil engineering in recent decades. Self-healing of concrete cracks by bacteria is based on microbial-induced calcium carbonate (CaCO3) precipitation, which is a common phenomenon in the natural environment. Certain mineralization bacteria can make use of carbon source to produce CO2 or CO3 2−, which subsequently reacts with calcium ions to form calcium carbonate precipitate on the surface of concrete cracks, thus sealing the concrete cracks (Douglas and Beveridge 1998). At the same time, the metabolism of mineralization bacteria creates an alkaline environment, which further favours the process of calcium precipitation. Moreover, bacteria also provide nucleation sites for calcium precipitation. In addition to the ability to induce calcium precipitation, self-healing bacteria should be alkaliphilic and spore-forming due to the harsh environment inside the concrete (De Muynck et al. 2010).
Bacteria-based self-healing technology has been widely studied as a promising approach for reducing the high maintenance and repair costs of concrete infrastructure. Various spore-forming bacteria were used in previous studies. Two main types of carbonate-generating pathways are involved in bacteria-based crack healing, including oxygen-independent enzymatic hydrolysis and oxygen-dependent respiration. Bang et al. (2010) and Wang et al. (2012, 2014a, 2014b, 2014c) used the urease-producing bacteria Sporosarcina pasteurii and Lysinibacillus sphaericus. Urea hydrolysis has been demonstrated to induce continuous formation of dense calcium carbonate crystals, but the ammonium ions released from urea lysis might have a negative effect on the environment and human health (De Muynck et al. 2010; Jonkers et al. 2010). Recently, a non-ureolytic self-healing system that uses organic acid salt (e.g. calcium lactate, calcium glutamate) instead of urea as the CO2/CO3 2− donor was proposed by Jonkers et al. (2010) to avoid such drawbacks. Various facultative aerobes or aerobes such as Bacillus cohnii, Bacillus alkalinitrilicus and Bacillus pseudofirmus have been proven to produce CaCO3 in this respiration-based crack healing system (Wiktor and Jonkers 2011).
Regardless of the metabolic pathway involved, the calcium precipitation activity (CPA) of bacteria is the key factor for improving the effectiveness of self-healing even though the performance of calcium precipitation is influenced by selected other factors such as the type of nutrients, bacterial cell concentration, Ca2+ source, pH, etc. (Okwadha and Li 2010; Lee et al. 2006). However, due to the absence of a suitable evaluation strategy, the CPA values of various bacteria have not been measured and compared in previous studies. Certain researchers evaluated the self-healing effectiveness by roughly measuring the width of the repaired crack, but given the complexity of the microenvironment inside the concrete, this measurement technique is far from accurate and cannot exactly reflect the actual ability of bacteria to induce the formation of calcium carbonate. Therefore, establishing an effective method to detect the CPA of bacteria will aid in screening of bacterial strains with high CPA and also will facilitate optimization of the bacterial-induced calcium precipitation process.
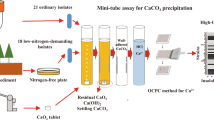
The current study is the first to establish a high-throughput assay for determination of the CPA of bacteria used for self-healing of concrete cracks. Based on the established strategy, the bacterial strain with the highest CPA was screened and identified. Furthermore, the effects of such influential factors as carbon source, nitrogen source, spore dosage, Ca2+ concentration and pH on the performance of calcium precipitation were investigated. This study provides a foundation for further application of bacterial self-healing of concrete cracks.
Materials and methods
Establishment of a high-throughput CPA assay
The CPA assay was based on the detection of free Ca2+ concentration via the O-cresolphthalein complexone (OCPC) method. The detailed steps are described as follows. Spores of bacterial strains were prepared by sporulation on 2× SG medium agar plates (Ghosh et al. 2015) supplemented with 50 mM of Na2CO3 and NaHCO3 as a pH buffer. Spores were harvested by scraping from the agar surface, purified and stored in water at 4 °C, as described by Ramirez-Peralta et al. (2012). All spores used in this work were free of cells or germinated spores as determined by phase-contrast microscopy. An amount of 10 μl spores at a concentration of 1 × 109 spores/ml (determined by direct spore counting with a Helber bacterial counting chamber) was used to inoculate 100 μl of calcium precipitation medium (CPM) in the well of a 96-well microplate I (Corning Inc., USA) to ensure that the final spore concentration in CPM was 1 × 108 spores/ml. The CPM contained (mM) l-sodium lactate 40, NaNO3 23.5, MgCl2 1, KH2PO4 0.13, CaCl2 20, inosine 2.5, NaCl 409, Na2SO4 28.2, KCl 9.08, KBr 0.82, H3BO3 0.42, NaF 0.07, SrCl2 0.09 and 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) 100. The pH of the CPM was adjusted to 10.5 with 6 M NaOH. For each strain, an additional well inoculated with sterilized spores (inactivated strain) was used as a control. Both experimental and control samples were assessed in triplicate. Incubation was performed in a CO2-free vacuum desiccator at 30 °C for 7 days. The CO2-free environment in the vacuum desiccators was obtained by vacuumization of the desiccators and continuous supply of a N2-O2 (4:1, v:v) gas mixture. Vacuum grease was applied to the flanges to ensure an airtight seal. After 7 days, 3.75 μl supernatant from each culture was obtained by centrifugation and transferred into the corresponding well of another 96-well microplate II. An amount of 300 μl calcium assay solution containing 0.04 mM OCPC, 3.50 mM 8-hydroxyquinoline and 500 mM 2-amino-2-methyl-1-propanol was added into each well. The reaction was performed at 30 °C for 10 min. After reaction, the free Ca2+ concentration was measured at 575 nm using a 96-well plate spectrometer (SpectraMax 190, Molecular Devices, US). The CPA was calculated as follows:
where C C and C E are the average residual Ca2+ concentrations of the control and the experimental samples, respectively, after 7 days of incubation. In all CPA assay experiments, the spore concentrations in CPMs were set at 1 × 108 spores/ml.
Microorganism isolation
Sediment samples were separately collected from the Shenzhen Futian mangrove reservation district of China. Each sediment sample was blended with 50 ml 0.1 M Na2CO3-NaHCO3 solution (pH of approximately 9.7) in a 250-ml flask and shaken at 150 rpm and 30 °C for 30 min. The sediment suspensions were subsequently heated in a water bath at 70 °C for 30 min to kill all vegetative cells and reproductive spores, leaving only dormant endospores to develop potential colony-forming units. An amount of 5 ml 25 % yeast extract solution was added into each flask as a germinant to induce the germination of dormant endospores at 150 rpm and 30 °C for 30 min. The germinable spores were harvested by centrifugation (3000×g, 5 min) and washed four times with Na2CO3-NaHCO3 solution to remove the yeast extract. Subsequently, the germinable spores were resuspended, serially diluted with Na2CO3-NaHCO3 solution and plated onto an LNC (lactate-nitrate-carbonate) agar plate, which contained (mM) l-sodium lactate 80, NaNO3 23.5, KH2PO4 0.13, FeSO4 0.06, NaCl 409, MgCl2 53.3, CaCl2 20, Na2SO4 28.2, KCl 9.08, KBr 0.82, H3BO3 0.42, NaF 0.07 and SrCl2 0.09. Amounts of 10 ml trace element solution SL6 (Pfennig 1974) and 15 g agar were added per litre of LNC. The initial pH of the agar medium was approximately 11.0. The plates were held in an incubator at 30 °C for 7 days to allow the appearance of colonies. The colonies were further purified via repeated streaking. The CPA values of all isolated strains were measured by the high-throughput assay established above. The strain with the highest CPA value, designated as H4, was screened for further evaluation.
Identification of the isolated strain H4
The genomic DNA of the H4 strain was extracted using the Direct PCR kit for Microorganisms (TaKaRa BioInc., China) according to the manufacturer’s instructions and was used as the template for PCR amplification of 16S rDNA. A partial 16S rDNA region was amplified using the primers F27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR amplification was performed for 30 cycles in an iCycler Thermal Cycler (Bio-Rad Laboratories Inc., Hercules, USA). Each cycle consisted of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min. The amplified DNA fragments were sequenced at Invitrogen Life Technologies and analysed by a similarity search in the EzTaxon database using a combination of BLAST and the robust pairwise sequence alignment algorithm. The 16S rRNA gene sequence of H4 and the members of its closely related validly published type strains were retrieved from the EzTaxon server (http://eztaxon-e.ezbiocloud.net) and aligned using the MEGA software version 4 (Kim et al. 2012; Tamura et al. 2007). A phylogenetic tree was constructed using the neighbour-joining method. Furthermore, bootstrap analysis was performed to assess the confidence limits of the branching.
Morphology of the calcium precipitate
Calcium precipitates were crystallized in the non-CO2 system for 7 days at 30 °C. A 24-well plate was used as an incubation container, and a 5 mm × 5 mm silicon slice was placed on the bottom of each well to collect precipitates. In the experimental group, 50 μl H4 spore suspension (1 × 109 spores/ml) were added into each well containing 1 ml CPM. The silicon slice was submerged into the CPM with a depth of approximately 8 mm. After 7 days, silicon slices with deposited crystals were carefully removed from the wells, washed with deionized water and dried in air. In the control group, no H4 spores were inoculated in the CPM. The resultant crystal was sputter coated with Au for the scanning electron microscopy (SEM) analysis and energy dispersive spectrometer (EDS) analysis. SEM and EDS analysis were performed on a FEI Quanta 250 microscope scanning electron microscope (FEI Inc., Hillsboro, OR, USA) with 5 and 20 kV accelerating voltages for SEM and EDS analysis, respectively. Nonbiogenic sample of calcium carbonate was also analysed by EDS as the reference.
For X-ray diffraction (XRD) analysis, 2.5 ml H4 spores suspension (1 × 109 spores/ml) was added to a Petri dish containing 50 ml CPM and incubated as described above. After 7 days, the precipitated particles were collected by centrifugation (6000×g, 10 min) and washed four times with water to remove the medium. The particles were dried at room temperature. The mineralogy of the formed particles was determined by X-ray diffraction (XRD, D8 ADVANCE, Bruker) using a Cu Kα radiation source (λ = 1.5406 Å). The XRD data in an overall range of 3° to 60° (2θ) were collected with a step size of 0.02°.
Optimization of the bacterial calcium precipitation process
Influential factors in the process of bacteria-induced calcium precipitation include the carbon source, nitrogen source, spore concentration, Ca2+ concentration and pH. Glucose, sucrose, soluble starch, sodium formate and sodium acetate were respectively prepared at 40 mM for replacement of the sodium lactate in CPM to optimize the carbon source. For the nitrogen source, ammonium chloride, ammonium nitrate, urea, beef extract, yeast extract and tryptone were respectively prepared at 23.5 mM to replace the sodium nitrate. After the best carbon source and nitrogen source were determined, the effects of pH, spore dosage and initial Ca2+ concentration were further evaluated. Incubation was performed at 30 °C for 7 days. The CPA was measured as described above, except for the spore dosage experiment, in which the initial spore concentrations in CPM were adjusted to the desired values. During the experiment, all measurements were performed in triplicate.
Statistical analysis
Results are expressed as the mean value ± the standard deviation. Statistical comparison is performed using one-way analysis of variance. A probability (P) value less than 0.05 is considered statistically significant.
The 16S rDNA sequence of H4 was submitted to NCBI GenBank with an accession number of KP728996.
Results
High-throughput CPA assay for self-healing bacteria
The high-throughput CPA assay is performed in a 96-well microplate based on the OCPC-calcium colorimetric reaction. OCPC is a metallochromic dye used to measure free Ca2+ in biochemical and physiological studies (Neelamegam et al. 2010; Nejadnik et al. 2014; Alghamdi et al. 2014). The chromophore of OCPC reacts with free Ca2+ in the solution to form a purple complex. The intensity of the colorimetric reaction is proportional to the Ca2+ concentration and can thus be spectrophotometrically determined at 575 nm. During the assay, an initial Ca2+ concentration of 20 mM was applied to simulate the pore solution inside the concrete, where the free Ca2+ concentration is relatively low due to the poor solubility of calcium hydroxide (Dong et al. 2015).
Our results obtained a good proportion between Ca2+ concentration and OD575. A linearized standard curve was derived from 0 to 4 mM Ca2+ with r 2 of 0.9997. The equation of the standard curve was described by the following equation:
One important item that should be taken into consideration during the assay is the effect of ambient CO2 on free Ca2+ measurement. Our experiment found that the degree of influence was dependent on the pH of the solution. If the pH was less than 8.0, the effect was negligible (data not shown). However, with increasing alkalinity, ambient CO2 caused a significant decline in the free Ca2+ concentration, even if no bacteria were inoculated. Figure 1 clearly shows that at pH 10.5, CO2 in the open system consumed almost all free Ca2+ within 20 h, whereas in the CO2-free system, the free Ca2+ concentration remained at nearly the same level over 24 h. Even after 200 h, the total decline of free Ca2+ was less than 20 %. Therefore, establishment of a CO2-free system is necessary for the CPA evaluation of alkaliphilic self-healing bacteria.
Isolation of the calcium-precipitating bacteria
Thirteen morphologically different alkaliphilic strains that are capable of inducing calcium precipitation were obtained during the screening process. The CPAs of the resultant 13 strains were detected using the established high-throughput assay, and the results are shown in Fig. 2. It should be noted that even if isolated from the same area, the strains exhibit varying calcium precipitation abilities. Among the strains, the CPAs of strain H4, H3 and M29 are significantly higher than other strains, and H4 shows the highest CPA of 94.8 %, which was nearly 20 times higher than that of M95. Therefore, H4 is selected for the further experiments.
Identification of H4
The phylogenetic identity of H4 was determined by 16S ribosomal RNA sequencing. The 16S rDNA sequence of H4 was submitted to NCBI GenBank with an accession number of KP728996. The strain was deposited in the China General Microbiological Culture Collection Center (CGMCC) with a deposit number of 9629. A comparison of 16S rRNA gene sequence of H4 with published sequences available in the EzTaxon database revealed that H4 is 99.46 % similar to B. pseudofirmus DSM 8715(T). In the phylogenetic tree of H4 with other closely related strains established in Fig. 3, H4 is near B. pseudofirmus DSM 8715(T).
Morphology and identification of the precipitate
As shown in the SEM images (Fig. 4a, c), a large amount of crystals grew in the bacteria-inoculated medium. Two types of morphological crystals were observed, single irregular spheres and irregular branches/aggregates of spheres (Fig. 4a). Both types of crystals had rough surfaces with many deformed-lamellar rhombohedra (Fig. 4c). Single irregular spherical crystals with a size of 20–40 μm agglomerated into irregular branches. Moreover, the crystals showed evidence of bacterial involvement. Rod-shaped and round holes (1–4 μm) were found on the surface of the crystals (Fig. 4c), which presumably occurred in the space occupied by the bacterial cells or spores. These holes in the crystals also suggested that bacteria served as nucleation sites during the mineralization process. Element composition analysis via energy dispersive spectroscopy (EDS) revealed that the crystal is primarily composed of calcium, carbon and oxygen with a weight ratio closely matching that of CaCO3, indicating that the crystal is CaCO3 (Fig. 4e). Furthermore, the XRD analysis shown in Fig. 5 confirmed that the crystal induced by H4 is calcite.
For comparison, in bacteria-free medium, only fusiform and amorphous crystals were formed, and the amount of formed crystals was much smaller than that in bacteria-inoculated medium (Fig. 4b, d). Furthermore, no sign of bacteria involvement was observed during the production of crystals.
Optimization of the calcium-precipitating process
Carbon and nitrogen sources
Carbon and nitrogen sources are important influential factors for cell growth and metabolism. However, a good carbon/nitrogen source for cell growth is not necessarily favourable for a specific metabolic pathway. For example, glucose and yeast extract are generally the best carbon and nitrogen sources for growth of most bacterial strains, but they might inhibit the production of selected secondary metabolites.
In our experiment, carbon sources such as lactate and acetate and nitrogen sources such as nitrate and urea are preferable to other carbon/nitrogen sources in terms of calcium precipitation induction. Among these materials, lactate and nitrate confer the highest CPA on H4 (Fig. 6a, b). The effect of the concentration of carbon and nitrogen sources on calcium precipitation is shown in Fig. 6c, d. It is clear that the presence of a carbon source is highly important for calcium precipitation. The CPA of H4 is less than 5 % with no lactate provided. However, if the initial concentration of lactate is 25 mM, a remarkable increase of CPA occurs, up to 80 %. Further increase in the lactate concentration from 25 mM only results in a gentle increase of CPA to approximately 90 % (Fig. 6c). However, an increase in the nitrate concentration from 0 to 18 mM gradually enhances the CPA, whereas a nitrate concentration greater than 25 mM leads to inhibition. Surprisingly, H4 shows a CPA of 50 % in the absence of nitrate, which is much higher than the 7 % obtained in the absence of lactate, implying that the carbon source might be more important than the nitrogen source in terms of calcium precipitation. The reason for this observation is still unknown, but we speculate that it might be due to the potential ability of certain strains to use nitrogen sources in the air (Bentzon-Tilia et al. 2014). However, determination of whether strain H4 has such N2-fixation ability requires further evidence.
a–d Carbon and nitrogen sources. a Na-for sodium formate, Sug sugar, Glc glucose, Stc starch, Na-ace sodium acetate, Na-lac sodium lactate. b YE yeast extract, BE beef extract, Tpt tryptone, Na-glt sodium glutamate. Different letters represent statistical significance, while same letters represent no statistical significance among the strains
pH
Due to the presence of a large amount of Ca(OH)2 in the inner pores, the microenvironment of concrete is highly alkaline with pH values possibly reaching 13.0 (Jonkers et al. 2010). However, ingress of water or moisture caused by the occurrence of cracks might decrease the pH to a varying extent. Therefore, it is important to evaluate the effect of pH on the ability of bacteria to induce calcium precipitation.
The result in Table 1 shows that the CPA of H4 was maintained at greater than 80 % in the range of pH 9.5 to 11.0 with the highest value of 96.3 % occurring at pH 10.5. However, as the pH dropped to 9.0, the CPA displayed a sharp decrease to approximately 30 %. In contrast, a pH higher than 11.0 also resulted in a noticeable decline of CPA, suggesting that H4 is not able to tolerate excessively high alkalinity, even if it is an alkaliphilic strain.
Spore concentration
If the amount of Ca2+ is sufficient, formation of CaCO3 induced by bacteria primarily depends on the production of CO3 2− via bacterial metabolism. Moreover, the availability of nucleation sites is also important for the growth of CaCO3 crystals. It was reported that certain macromolecules on the surface of bacterial cells, such as proteins and exopolysaccharides, could act as nucleation sites (Li and Qu 2015). From this point of view, an adequate amount of spores should be necessary to ensure high efficiency of self-healing. Our experimental result in Table 2 shows that if the initial spore concentration were lower than 1.0 × 107 spores/ml, the CPA of H4 was less than 40 %. However, an increase of the spore concentration from 2.0 × 107 to 4.0 × 107 spores/ml resulted in a significant increase of CPA up to 76 %. A spore concentration greater than 1.0 × 108 spores/ml could produce a further increase of CPA to 80 %, but this increase appeared to be economically ineffective, considering the cost of the production of bacterial spores. Therefore, 4.0 × 107 spores/ml is a suitable initial spore concentration for H4 to achieve a high level of CPA.
Ca2+ concentration
In our experiment, the effect of Ca2+ on microbial calcium precipitation was evaluated in terms of the ability of bacterium itself, i.e. CPA. The result disclosed that Ca2+ concentration had a great impact on bacterial calcium precipitation (Fig. 7). If the Ca2+ concentration were less than 30 mM, the CPA of H4 exceeded 85 %. However, an increase in the Ca2+ concentration from 30 to 60 mM produced a sharp decrease of CPA from 86 to 35 %. With Ca2+ concentrations greater than 120 mM, the CPA of H4 dropped to less than 10 %.
The decline of CPA at high Ca2+ concentration could be partially explained by the fact that the growth of bacterial cells was inhibited by high Ca2+ concentration. Figure 7 shows that H4 could grow well if the Ca2+ concentration was low, but with further increase of the Ca2+ concentration from 60 mM, the cell concentration dropped drastically. The inhibition of high Ca2+ concentration on cell growth can be expected because biologically, Ca2+ can change the permeability of the cellular membrane and consequently influence the metabolism of the cells. A pattern of cell concentration similar to that of CPA in Fig. 7 suggested that the decrease of cell concentration might lead to the decrease of CPA and thus reduce the overall efficiency of the self-healing process.
Discussion
High-throughput strategy for CPA detection
For screening of bacteria mineralization potential for self-healing of concrete cracks, CPA can reflect the ability to induce formation of CaCO3. However, due to the difficulty in collecting CaCO3 precipitates, weighing the CaCO3 crystals produced in the process is not a good approach for evaluating the CPA of bacteria. From this point of view, monitoring the change of free Ca2+ concentration in the solution might be more feasible. It has been proven that EDTA titration is a reliable and reproducible approach used to measure free Ca2+ concentration (Bang et al. 2010; Xu et al. 2013). However, titration can only treat one sample at a time, making the method unsuitable for screening microorganisms from the environment because the screening process usually involves large-scale measurements (Chaudhry and Nautiyal 2011). Therefore, we established a novel high-throughput CPA assay based on detection of free Ca2+ in the solution. Compared with EDTA titration, the high-throughput method established in this study allows detection of dozens of samples at a time, thus displaying promising potential for screening of self-healing bacteria with high calcium precipitation activity.
Screening and identification of bacteria with high CPA
Using the high-throughput method established in this study, a bacterium with the highest CPA of 94.8 %, designated as H4, was screened and identified as a Bacillus sp. Different types of calcium-precipitating bacteria were used for self-healing of concrete cracks in previous studies (Jonkers et al. 2010; Wiktor and Jonkers 2011; Wang et al. 2014b). However, it is difficult to compare the calcium precipitation abilities of various strains due to the absence of CPA data. In our study, the CPAs of two reported self-healing strains, B. pseudofirmus DSM8715 and B. cohnii DSM6307 (Sierra-Beltran et al. 2014), were evaluated together with other isolated strains under the same experimental conditions. Figure 2 shows that the CPAs of DSM8715 and DSM6307 were 76.5 and 38.3 %, respectively, which are much higher than that of M95 but fairly lower than H4.
Furthermore, several Bacillus species close to H4 in the phylogenetic tree (Fig. 3) were confirmed to have the ability to precipitate calcium and heal concrete cracks. For example, B. cohnii DSM6307 could produce 20–80 μm mineral precipitates on the crack surface of concrete (Jonkers et al. 2010). Xu and Yao (2014) found that B. cohnii was able to enclose a portion of the crack in concrete. In another work, B. alkalinitrilicus was used to heal 0.46-mm-wide cracks of concrete (Wiktor and Jonkers 2011). L. sphaericus, formerly Bacillus sphaericus, was able to heal cracks with widths ranging from 0.15 to 0.17 mm (Wang et al. 2012).
Although the efficiency of the bacterial-induced self-healing process depends on a variety of influential factors, bacterial strains with high CPA values should have greater potential for industrial application.
Morphology of calcium precipitate
The amount of CaCO3 formed is closely related to the Ca2+ concentration, CO2 or HCO3 − concentrations and ambient pH value (Qian et al. 2009). The high pH of CPM, together with the CO2 or HCO3 − produced by bacteria, provides the appropriate conditions for CaCO3 precipitation. However, the morphological variation in CaCO3 crystals is affected by factors such as bacterial species, microbial excretions, solution composition, pH, etc. (Qian et al. 2010; Hou et al. 2011; Ziegler 2008). Bang et al. (2010) found that compared with the spherical amorphous crystal shapes, cuboidal crystals were more frequent in CaCO3 precipitation induced by S. pasteurii cells and urease. However, in another work, CaCO3 crystals induced by S. pasteurii exhibited different morphologies at different Ca2+ concentrations, including spherical, rod-shaped and clumped particles (Qian et al. 2009). Wang et al. (2014c) confirmed that L. sphaericus induced cubic, rectangular and spherical particles with sizes of 10–50 μm. However, the effect of the morphology of induced CaCO3 crystals on the self-healing efficiency of concrete cracks remains elusive.
Optimization of the calcium precipitation process
Carbon and nitrogen sources
Our experimental results revealed that sodium lactate and sodium nitrate were the best carbon and nitrogen sources for H4. The advantageous effect of these carbon and nitrogen sources on bacterial-induced calcium precipitation was consistent with the findings of previous studies (Jonkers et al. 2010; Xu and Yao 2014). Furthermore, incorporation of lactate into concrete was found to result in a slight increase in the compressive strength of concrete (Jonkers et al. 2010), suggesting that lactate is a suitable carbon source. In addition, urea appeared to be a good nitrogen source in our experiment (Fig. 6b). However, use of urea might result in release of ammonia, which is harmful to the environment (Wiktor and Jonkers 2011). In contrast to glucose, which was not a good carbon source for calcium precipitation, yeast extract appeared to be a fairly good nitrogen source (Fig. 6a, b). Nevertheless, it has been confirmed that embedment of yeast extract or peptone into concrete might lead to an obvious decrease in the concrete strength (De Muynck et al. 2010). Given that nitrate is commonly used as a type of early strength agent for concrete and does not reduce the concrete strength (Karagöl et al. 2013), it could be concluded that lactate and nitrate are suitable carbon and nitrogen sources for H4 in the self-healing process.
pH
The crack self-healing ability of alkaliphilic bacteria in an alkaline environment has been confirmed (Sierra-Beltran et al. 2014; Xu et al. 2013). Moreover, certain researchers evaluated the microbial crack healing process under neutral pH conditions (Van Tittelboom et al. 2010; Wang et al. 2014a). Although comparison of the calcium precipitation ability of the strains under neutral and alkaline pH conditions is difficult due to the different experimental conditions used in those studies, De Belie stated that alkaline pH is more beneficial for dissolution of CO2 and the subsequent transformation of CO2 to CO3 2− and hence the formation of calcium carbonate (De Muynck et al. 2010). Qian et al. (2013) investigated the effect of pH on the time required by bacteria to activate the calcium precipitation process and found that when the pH was 5.0, bacterial cells required 36–48 h to activate the formation of CaCO3, but with an increase of pH, the activation time was remarkably reduced. If the initial pH reached 9.0, the formation of CaCO3 was observed in only 0–2 h.
It is clear that pH poses a significant impact on the bacteria-induced calcium precipitation, and H4 could effectively induce calcium precipitation in the pH range of 9.5 to 10.5. Although it is difficult to determine the actual pH inside a crack after ingress of water or moisture, the inner microenvironment of the concrete will be alkaline to a great extent due to the existence of Ca(OH)2. Therefore, H4 is a promising bacterium for self-healing of concrete cracks, considering that its CPA can remain at greater than 80 % even if the pH reaches 11.0.
Ca2+
Apparently, Ca2+ is indispensable for formation of calcium carbonate. However, whether excessive Ca2+ is favourable to the bacterial-induced calcium precipitation process is questionable. Qiu et al. (2014) found that microbial calcium precipitation first increased with the increase of Ca2+ concentration, but no more CaCO3 was produced when the Ca2+ concentration was increased to 150 mM. Okwadha and Li (2010) investigated ability of S. pasteurii to induce the formation of CaCO3 with Ca2+ concentrations ranging from 2.5 to 250 mM. Compared with no calcium precipitation observed at 2.5 mM Ca2+, 8.1 mg days−1 CaCO3 was produced as the Ca2+ concentration increased to 25 mM. However, as the Ca2+ concentration further increased to 250 mM, the strain could only induce 13.0 mg days−1 CaCO3, suggesting that excess Ca2+ did not necessarily facilitate the induction of more calcium precipitate. However, the authors did not give an explanation.
It could be concluded from our experiment that the presence of excessive Ca2+ not only inhibits the bacterial-induced calcium precipitation process but also results in waste of the Ca2+ resource, and maintenance of a Ca2+ concentration lower than 30 mM is a good strategy. Fortunately, due to the poor solubility of calcium hydroxide, the free Ca2+ concentration of the pore solution inside the concrete is normally less than 30 mM (Dong et al. 2015), making bacterial-induced calcium precipitation feasible. Furthermore, introduction of an extra Ca2+ source into concrete for the microbial self-healing process, as used in selected previous studies, might not be necessary from this point of view.
References
Alghamdi HS, Bosco R, Both SK, Iafisco M, Leeuwenburgh SC, Jansen JA, van den Beucken JJ (2014) Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomater 35:5482–5490
Bang SS, Lippert JJ, Yerra U, Mulukutla S, Ramakrishnan V (2010) Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int J Smart Nano Mater 1:28–39
Bentzon-Tilia M, Farnelid H, Jürgens K, Riemann L (2014) Cultivation and isolation of N2-fixing bacteria from suboxic waters in the Baltic Sea. FEMS Microbiol Ecol 88:358–371
Chaudhry V, Nautiyal CS (2011) A high throughput method and culture medium for rapid screening of phosphate accumulating microorganisms. Bioresour Technol 102:8057–8062
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136
Dong BQ, Wang Y, Fang G, Han NX, Xing F, Lu Y (2015) Smart releasing behavior of a chemical self-healing microcapsule in the stimulated concrete pore solution. Cem Concr Comp 56:46–50
Douglas S, Beveridge TJ (1998) Mineral formation by bacteria in natural microbial communities. FEMS Microbiol Ecol 26:79–88
Ghosh S, Korza G, Maciejewski M, Setlow P (2015) Analysis of metabolism in dormant spores of Bacillus species by 31P nuclear magnetic resonance analysis of low-molecular-weight compounds. J Bacteriol 197:992–1001
Hou W, Lian B, Zhang X (2011) CO2 mineralization induced by fungal nitrate assimilation. Bioresour Technol 102:1562–1566
Jonkers HM, Thijssen A, Muyzer G, Copuroglu O, Schlangen E (2010) Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng 36:230–235
Karagöl F, Demirboğa R, Kaygusuz MA, Yadollahi MM, Polat R (2013) The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg Sci Technol 89:30–35
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbial 62:716–721
Lee BD, Apel WA, Walton MR (2006) Calcium carbonate formation by Synechococcus sp. strain PCC 8806 and Synechococcus sp. strain PCC 8807. Bioresour Technol 97:2427–2434
Li P, Qu W (2015) Bacteria for concrete surface treatment. In: Torgal FP, Labrincha JA, Diamanti MV, Yu CP, Lee HK (eds) Biotechnologies and biomimetics for civil engineering, 1st edn. Springer International Publishing, Switzerland, pp 325–358
Neelamegam P, Jamaludeen A, Rajendran A (2010) Analysis of calcium in milk using an embedded system. Sens & Instrumen Food Qual 4:119–125
Nejadnik MR, Yang X, Bongio M, Alghamdi HS, Van den Beucken JJ, Huysmans MC, Leeuwenburgh SC (2014) Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomater 35:6918–6929
Okwadha G, Li J (2010) Optimum conditions for microbial carbonate precipitation. Chemosphere 81:1143–1148
Pfennig N (1974) Rhodopseudomonas globiformis, sp. n., a new species of the Rhodospirillaceae. Arch Microbiol 100:197–206
Qian CX, Wang JY, Wang RX (2009) Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater Sci Eng: C 29:1273–1280
Qian CX, Wang RX, Cheng L, Wang JY (2010) Theory of microbial carbonate precipitation and its application in restoration of cement-based materials defects. Chin J Chem 28:847–857
Qian CX, Luo M, Pan QF, Li RY (2013) Mechanism of microbially induced calcite precipitation in self-healing concrete. J Chin Ceram Soc 41:620–626
Qiu J, Tong QS, Yang EH (2014) Surface treatment of recycled concrete aggregates through microbial carbonate precipitation. Constr Build Mater 57:144–150
Ramirez-Peralta A, Stewart KAV, Thomas SK, Setlow B, Chen Z, Li YQ, Setlow P (2012) Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J Bacteriol 194:3417–3425
Sierra-Beltran MG, Jonkers HM, Schlangen E (2014) Characterization of sustainable bio-based mortar for concrete repair. Constr Build Mater 67:344–352
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Boil Evol 24:1596–1599
Van Tittelboom K, De Belie N, De Muynck W, Verstraete W (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40:157–166
Wang JY, De Belie N, Verstraete W (2012) Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J Ind Microbial Biotechnol 39:567–577
Wang JY, Snoeck D, Van Vlierberghe S, Verstraete W, De Belie N (2014a) Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr Build Mater 68:110–119
Wang JY, Soens H, Verstraete W, De Belie N (2014b) Self-healing concrete by use of microencapsulated bacterial spores. Cem Concr Res 56:139–152
Wang JY, Dewanckele J, Cnudde V, Van Vlierberghe S, Verstraete W, De Belie N (2014c) X-ray computed tomography proof of bacterial-based self-healing in concrete. Cem Concr Comp 53:289–304
Wiktor V, Jonkers HM (2011) Quantification of crack-healing in novel bacteria-basedself-healing concrete. Cem Concr Comp 33:763–770
Xu J, Yao W (2014) Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem Concr Res 64:1–10
Xu J, Yao W, Jiang Z (2013) Non-ureolytic bacterial carbonate precipitation as a surface treatment strategy on cementitious materials. J Mater Civil Eng 26(5):983–991
Ziegler A (2008) The cationic composition and pH in the moulting fluid of Porcellio scaber (Crustacea, Isopoda) during calcium carbonate deposit formation and resorption. J Comp Physiol B 178:67–76
Acknowledgments
The authors acknowledge the financial support provided by National Natural Science Foundation of China (No. 51120185002, No. 51578339) and American Journal Experts for English improvement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All of the authors of this article (J.L. Zhang, R.S. Wu, Y.M. Li, J.Y. Zhong, X. Deng, B. Liu, N.X. Han and F. Xing) declare that they have no conflicts of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhang, J.L., Wu, R.S., Li, Y.M. et al. Screening of bacteria for self-healing of concrete cracks and optimization of the microbial calcium precipitation process. Appl Microbiol Biotechnol 100, 6661–6670 (2016). https://doi.org/10.1007/s00253-016-7382-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7382-2